Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 3
Gas Hydrates: Recent Development and Commercial Approaches
Anupama Kumari* and C.B. MajumderReceived: 31-Dec-2019, Manuscript No. JPEB-24-3034; Editor assigned: 03-Jan-2020, Pre QC No. JPEB-24-3034 (PQ); Reviewed: 17-Jan-2020, QC No. JPEB-24-3034; Revised: 01-Jul-2024, Manuscript No. JPEB-24-3034 (R); Published: 29-Jul-2024, DOI: 10.35248/2157-7463.24.15.574
Abstract
Gas hydrates have the ability to become a vast energy source in the future and can fulfill natural gas demand in the future. All over the world, there are huge sized deposits are present. At present demand for energy is growing, but currently these are not present commercially. There are large deposits of gas hydrates, but safe and economic production of gas from gas hydrates are not known yet. Hence, the development of new and economical, suitable technologies and methods are developed for the dissociation of gas from gas hydrates. Currently the simplest and economical dissociation technique is geothermal dissociation. The production method of natural gas from gas hydrates is being developed such as depressurization, thermal stimulation and chemical injection. In this study, we reviewed several articles to compare the different dissociation techniques of gas hydrates and also studied new acquired techniques for dissociation of gas hydrates till now.
Keywords
Gas hydrates; Depressurization; Thermal simulation; Clathrates; CO2 sequestration
Introduction
Gas hydartes is a crytal structure of ice which can formed naturally in the environment by trapping gas inside it. It is a crystalline solid compounds which will form when a gas molecules are encaged by water molecules at low temperature and high pressure and resembles the cage like structure. At ambient temperature and pressure, can be burned and hence called as combustible gas. Gas hydrates can be formed by methane, ethane, hydrogen sulfide, carbon dioxide, nitrogen, propane, isobutane, n-butane, etc. but these are predominantly called as methane gas hydrates. At equilibrium conditions, 1 m3 of natural gas hydrates contain 160 m3-180 m3 of natural gas [1]. Methane formed either by organic decomposition microbial activity and thermogenic gas laeking beneath the sea level or permafrost region. Then it combines with water at sufficiently high pressure and low temperatures and formed methane gas hydrates naturally.
The gas hydrates were discovered first time by sir Humphrey Davy in 1810 but there is also a possibility that it was discovered by Priestley in 1778.
Natural gas hydrates can be found in the high altitudes/ latitudes of permafrost or in the layers of sediment which occurs in deep water on the continental margins [2]. Small parts of red lakes i.e., the continental land, seas of the Caspian Sea, Lake Baikal and Black Sea also contain natural gas hydrates. The infinite reserves of natural gas hydrates have been discovered in mountain permafrost regions or deep water, such as the Gulf of Mexico, South China Sea, Indian Ocean, Siberia, Arctic Alaska, multiple regions in Qilian and Mainland China. The curiosity for scientific research in gas hydrates has increased exponentially when it became responsible for blockage in oil and gas pipelines. Hence various researches are going on for the production of gas from gas hydrates because it can solve the fulfill crisis of energy and become a potential source of energy in the world [3]. The proposed methods for the decomposition of gas hydrates are thermal stimulation, depressurization and chemical injection. The combined mode of depressurization method and thermal stimulation is referred as a most favorable method for gas hydrates decomposition (Table 1).
| S. No | Decomposition methods | Description |
|---|---|---|
| 1 | Thermal stimulation | If the temperature of the system increases at a specified pressure then it can disturb the stability of hydrate |
| 2 | Depressurization | If pressure of the system decreases at a specified temperature then it can disturb the stability of hydrate |
| 3 | Chemical injection | Inject chemical inhibitors, which disturb the equilibrium condition of gas hydrate |
| 4 | CO2 sequestration | Sequestering or substituting carbon dioxide with gas entrapped in gas hydrates |
| 5 | Microwave heating | Disturb the stability of gas hydrates by increasing the temperature of the system using microwave (rays) |
| 6 | Bacterial decomposition | Bacterial decomposition is exothermic in nature and liberates heat |
Table 1: Different dissociation techniques of gas hydrates.
Materials and Methods
Indian scenario
In India natural deposits of gas hydrates are found in various areas of Kerala-Konkan Basin, Krishna-Godavari Basin, Mahanai Basin and Andaman Offshore. Till now, two phases of Natural Gas Hydrate Program (NGHP-01 and NGHP-02) have been conducted to find the location of these deposits and third phase (NGHP-03) will be initiated soon for the exploration and dissociation of gas hydrates. The following figure shows the locations of gas hydrates deposits in India (Figure 1) [4].
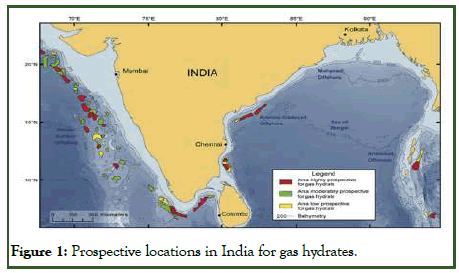
Figure 1: Prospective locations in India for gas hydrates.
The first objective of this paper is to review the various methods available for the dissociation of gas from gas hydrates and compare them on the basis of different parameters such as cost, suitable for the environment and stability conditions. We also compare these methods by focusing on their importance and problems arise by using them and also investigate the stability parameters, i.e., temperature and pressure on which gas is dissociated [5]. We will highlight the drawbacks of various dissociation techniques and introduce some new techniques which can be used. Also studied the implementation conditions for these methods. At last a section of conclusions, final remarks, comparisons, issues and directions for future research has been discussed for all dissociation techniques of gas from gas hydrates.
Stability conditions of gas hydrates
Gas hydrates are stable at certain conditions, i.e., low temperature and high pressure. The stability conditions of gas hydrates in pure water and seawater will be different because of the existence of ions and salts in sea water. Gas hydrates are stable at high pressure in sea water as compared to pure water, but the temperature can be lower for seawater as compared to pure water [6]. The co-existence of all dissolved ions in sea water depresses the dissociation temperature for the stability of methane hydrate ranges between pressures of 2.75 MPa-10 MPa. For sea water with the salinity of 33.5%, the observed offset in dissociation temperature was 274.25 K as compared to pure water (Figure 2 and Table 2).
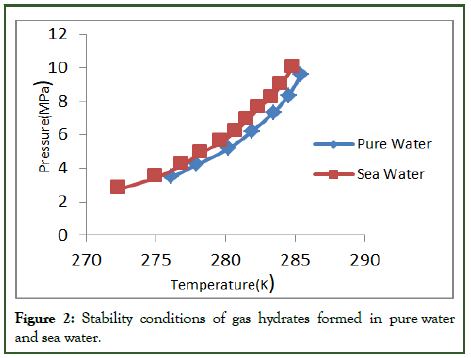
Figure 2: Stability conditions of gas hydrates formed in pure water and sea water.
| Assignee | Description of patent |
|---|---|
| Mobil oil corporation, Fairfax, VA (US) | Electromagnetic radiation can be employed in the recovery of gas from gas hydrates |
| Conoco Philips company, Houston, TX (US) | A more stable structure of hydrate has been formed by the substitution of the gas with the releasing agent without melting the gas hydrate |
| New Mexico tech research foundation, Socorr, N.M. | Apparatus and methods for the transportation, generation and the dissociation of hydrates |
| Conoco Philips company, Houston, TX(US) | By the application of electromagnetic radiation, methane can be recovered |
| The university of Tulsa, Tulsa, OK (US) | Electric heater was used in the opening of hydrate rich subset sediment and then the released gas is collected in an overhead receiver |
| Chevron U.S.A. Inc., San Ramon, CA (US) | A two stage method has been employed to produce fluids from a class 3 reservoirs |
| Halliburton energy services, Inc., ss Duncan, Okla | The method or the decomposition of hydrates is to combine the acidic liquid with basic liquid to form a exothermic chemical reaction and form a salt solution |
Table 2: List of patents filed on new techniques for the dissociation of gas hydrate
Results and Discussion
Thermal stimulation
The temperature increase in the gas hydrates during the thermal stimulation method can disturb the stability condition of gas hydrate system. Hydrate will destabilized due to the heat developed in the reservoir. The thermal energy in form of hot fluids like brine, water or steam has been injected from the upper surface or may be by applying down hole process like as electromagnetic heating and electric or in situ combustion.
Various novel ideas has been developed due to the relatively high rate of production of gas such as fire flooding, nuclear waste burial, electromagnetic heating, microwaves, downhole combustion but these methods has not been used yet [7].
Schicks et, al. designed a counter current heat exchange reactor to produce the heat by the catalytic oxidation of methane. About 15% of the dissociated methane gas can be used to produce heat by the catalytic oxidation process. Su et, al. investigated the production potential of Huff and Puff method for the production of gas from the deposits of gas hydrates in Shenu area on the northern continental slope of south China sea. They dissociated hydrate by producing fluids and injecting hot water alternatively.
They suggested that the Huff and Puff method for the gas production through vertical wall is not favorable and has not industrial potential. Ning et, al. proposed a technique to use renewable solar energy to provide energy for the dissociation of marine gas hydrates by the use of thermal stimulation method [8].
Depressurization
The main aim of this technique is to decrease the stability zone of the hydrate to decompose the hydrate at the bottom of the stability zone of hydrate and free gas will move upward in the direction of the well bore. This method is the first production method which has been applied outside the laboratory. The thermal stimulation method can also be combined with the depressurization method to dissociate the gas hydrates and various studies have been reported to explore the effects of this combined method. The stability of gas hydrates is disturbed by exposing the hydrates to a low pressure domain where the hydrates become unstable and then dissociated into water and gas. The released gas can be recovered by applying conventional technology. The uninterrupted recovery of gas is supposed to assist this applied pressure influenced dissociation of the hydrate zone at the bottom. The depressurization method is most favorable for those natural deposits where inescapable gas found in a closure that forms the hydrate cap above it. A well is extended and drilled into the hydrate layer and formed the zone of free gas. The dissociated gas from this hydrate layer causes the reduction in pressure and dissociation of the hydrate. The dissociation of hydrates in the Messoyakha fields has been done by the depressurization method. There is no need of expensive continuous stimulation for the depressurization method, hence it is an effective method for the future gas hydrate dissociation [9].
Chemical injection
In the chemical injection method, the equilibrium condition of hydrates has to be displaced by the injection of liquid inhibitor or chemical beside the hydrate.
This technique is also expensive, but it is less expensive than the thermal stimulation. The thermo inhibitors injection is most favorable for the development of ocean reservoir.
In this method various chemicals, for example glycerin, methanol, glycol and ethanol are injected which shifted the thermodynamic equilibrium stability curve of hydrates. The above techniques have been applied only in permafrost areas and till now have not been applied in the dissociation of gas hydrates of marine areas.
Sequestration
The shortcomings of thermal stimulation methods, depressurization and chemical injection is the low efficiency of heat transfer in a reservoir which controls the rate of decomposition of hydrates [10]. The thermal conductivity of formation is relatively poor hence the efficiency of the decomposition process cannot be compensated. The temperature in the hydrate decomposition region declines rapidly due to the huge absorption of heat. These above methods will decrease the strength of reservoir and further causes the slope instability, seabed destruction because these methods depend on the principle of decomposition of hydrate. The CO2 replacement method appears to be more profitable as it permit both recovery of gas from hydrates and sequestration of CO2 as hydrates in the region of deep sea sediments. This method is applicable where the floor of the ocean remains stable during the process and after the injection of CO2. Several researchers have done work on this method for the decomposition of gas hydrates before and after the experiment. Studies done by Yoon et, al. showed that the surface covering of CO2 hydrate in the outer most layer is taken as an important part for de-accelerating the dissociation of methane hydrate within the core. But the water phase generated during the sequestration process allows the process to move forward rapidly by increasing the diffusion of carbon dioxide and methane which results in absolute and comparatively fast production of methane gas. Lee et, al. suggested that various factors such as reaction kinetics, environment of sediment, size of hydrate particle and mass transfer should have to be consider for the replacement of CO2 in natural gas hydrates deposits. Xu, et al. worked for the replacement of different gas mixtures to produce methane gas from hydrates by the replacement method. Small molecules sized gases enhanced the rate of replacement by the stronger diffusivities in gas hydrates. Ota, et al. observed that the mole fraction of CH4 and CO2 in the hydrate zone increases and decreases with time respectively [11].
Microwave heating
The interaction characteristics between electromagnetic waves and disperse systems, the high frequency energy and microwave electromagnetic fields can be used for the decomposition of gas hydrates. A heat source has been generated because of the interaction between high frequency electromagnetic radiation and medium and the energy of propagating electromagnetic wave converges to heat. This heat source distributed over the gas hydrate volume and then phase transition occurs in the absence of temperature gradient. In the case of large zones of phase transition can occur, due to which the decomposition temperature of gas hydrate is attained and the energy of the electromagnetic field has been extended to the dissociation of gas hydrates.
Bacterial decomposition
The extraction of methane from hydrates cannot be done by bacterial decomposition but this method may convert water and carbon dioxide to methane which are present in hydrates. For the implication of this method anaerobic archae bacterial can be used for the conversion of carbon dioxide and water to methane. This reaction has exothermic nature which liberates heat and it can increase the temperature of the system above the stability condition of hydrate to dissociate methane from hydrates.
The above methods are not favourable for the dissociation of gas hydrates in marine sediments due to their limitations and disadvantages (Table 3) [12].
| Technique | Advantages | Disadvantages |
|---|---|---|
| Depressurization | Can reduce energy consumption | Favorable for the widespread gas deposits in a closure |
| Thermal stimulation | Can overcome the problems associated with low gas production by depressurization | High heat loss |
| Low efficiency | ||
| High energy consumption | ||
| Chemical injection | Less expensive than other methods | Not suitable for the ocean area |
| Very expensive environmental pollution | ||
| CO2 replacement | Highly profitable | Cost of CO2 supply is high |
| Efficiency and replacement speed is low |
Table 3: Advantages and disadvantages of different dissociation techniques of gas hydrates.
Economics
The production of gas from gas hydrates is seems to be more expensive as compared to the conventional natural gas. Because of the higher cost of current dissociation techniques, it becomes important to find the economically favorable dissociation techniques of gas hydrates based upon higher production rate, lower transportation cost to balance the higher wellhead cost as compared to the conventional gas production from gas fields. Depressurization has been suggested by many researchers as the most economical dissociation techniques for gas hydrates. Table 4 show the economic comparison of different dissociation techniques of gas hydrates.
| Parameter | Depressurization | Thermal stimulation | Conventional gas production |
|---|---|---|---|
| Investment (million Rs.) | 166000 | 254200 | 157500 |
| Annual cost (million Rs.) | 125500 | 160000 | 100000 |
| Total production (million m3/year) | 1557.43 | 1274.26 | 1557.43 |
| Production cost (Rs. million/m3) | 4025.9 | 6356.6 | 3213.6 |
| Breakeven wellhead price (Rs. million/m3) | 2532.3 | 7945.8 | 3972.9 |
Table 4: Comparison of different dissociation techniques of gas hydrates.
Conclusion
Natural gas hydrates are the major source of energy in all over the world. It can replace the various other sources of natural gas to fulfill the future energy requirement. Hence the recovery of natural gas from gas hydrates became a major research area in present days. There are various techniques are available for the dissociation of gas hydrates, but the economic production of gas from gas hydrates is a major issue now a days. This paper reviews the present dissociation techniques of gas hydrates and their advantages and disadvantages. Hence, to overcome these disadvantages, there is need to explore the new dissociation techniques for the economic production of natural gas from gas hydrates.
Acknowledgments
This work is supported by Gas Hydrate Research and Technology Centre (GHRTC) through the Oil and Natural Gas (ONGC), Panvel.
References
- Li XS, Wang Y, Li G, Zhang Y, Chen ZY. Experimental investigation into methane hydrate decomposition during three-dimensional thermal huff and puff. Energ Fuels. 2011;25(4):1650-1658.
- Zhou Y, Castaldi MJ, Yegulalp TM. Experimental investigation of methane gas production from methane hydrate. Ind Eng Chem Res. 2009;48(6):3142-3149.
- Ciotoli G, Procesi M, Etiope G, Fracassi U, Ventura G. Influence of tectonics on global scale distribution of geological methane emissions. Nat Commun. 2020;11(1):2305.
[Crossref] [Google Scholar] [PubMed]
- Kvenvolden KA. A primer on the geological occurrence of gas hydrate. Geol Soc London Spec Publ. 1998;137(1):9-30.
- Lu Z, Zhu Y, Zhang Y, Wen H, Li Y, Liu C. Gas hydrate occurrences in the Qilian Mountain permafrost, Qinghai province, China. Cold Reg Sci Technol. 2011;66(2-3):93-104.
- Koh CA, Sum AK, Sloan ED. Gas hydrates: Unlocking the energy from icy cages. J Appl Phys. 2009;106(6).
- Tang LG, Li XS, Feng ZP, Li G, Fan SS. Control mechanisms for gas hydrate production by depressurization in different scale hydrate reservoirs. Energ Fuels. 2007;21(1):227-233.
- Lee J. Experimental study on the dissociation behavior and productivity of gas hydrate by brine injection scheme in porous rock. Energ Fuels. 2010;24(1):456-463.
- Ota M, Abe Y, Watanabe M, Smith Jr RL, Inomata H. Methane recovery from methane hydrate using pressurized CO2. Fluid Ph Equilibria. 2005;228:553-559.
- Park Y, Kim DY, Lee JW, Huh DG, Park KP, Lee J, et al. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. Proc Natl Acad Sci. 2006;103(34):12690-12694.
[Crossref] [Google Scholar] [PubMed]
- Tishchenko P, Hensen C, Wallmann K, Wong CS. Calculation of the stability and solubility of methane hydrate in seawater. Chem Geol. 2005;219(1-4):37-52.
- Ning F, Wu N, Jiang G, Zhang L, Guan JA, Yu Y, et al. A method to use solar energy for the production of gas from marine hydrate-bearing sediments: A case study on the Shenhu area. Energies. 2010;3(12):1861-1879.
Citation: Kumari A, Majumder CB (2024) Gas Hydrates: Recent Development and Commercial Approaches. J Pet Environ Biotechnol. 15:574.
Copyright: © 2024 Kumari A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

