Indexed In
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 18, Issue 1
Future Outlook for Developing Ex Vivo Models to Study Germline Developmentand Fertility Preservation
Swati Sharma*Received: 04-Dec-2020 Published: 25-Dec-2020, DOI: 10.35248/2090-7214.21.18.371
About the Study
Innovative technologies offer promising potential to transform the future of reproductive biology by developing specialized ex vivo models and tools for research by providing personalized therapeutic treatment options, which can be translated from bench to bedside [1-4]. Keeping future perspective in view, the potential of emerging tools need to be explored to gain a deeper insight in understanding the regenerative potential of germ cells, gonadal function and for developing clinical options for fertility preservation [5-7]. During germline development Primordial Germ Cells (PGCs) undergo sex specific differentiation and give rise to male and female germ cells during sex specific gonadal development [2]. PGC specification occurs during epiblast stage in humans, and is regulated by a tripartite network of transcription factors Prdm1, Prdm14 and Tfap2c.
A subpopulation of epiblast cells receive inductive signals from adjoining tissues and cells, and are specified as PGC precursors. Specified PGCs translocate to the developing gonadal ridges and undergo male or female sex specific differentiation. In males, PGCs give rise to gonocytes which migrate to the basement membrane of the seminiferous tubules colonizing and embarking the first wave of spermatogenesis with their transition into SSCs at puberty. Epigenetic reprogramming occurs by erasure of genomic imprints and Re-establishment of gamete specific imprints. Unlike rodents, where blimp1 and prdm14 influence germline reprogramming; in humans blimp1 and Sox17 are involved in germline development [8].
Underlying signaling mechanisms and the distinct role of functional pathways (like BMP, SMAD and WNT pathways) involved in human germline specification are largely unknown. Understanding regulatory mechanisms e.g. up regulation and down regulation of genes that influence germline development are significant for both biological and clinical purposes (for instance, to acquire a comprehensive understanding of germ cell biology, and to devise fertility preservation strategies). The characteristic features of germline stem cells, the evolutionary and developmental pathways (morphogenetic cascade and signaling events, the gene regulatory network controlling Germ -cell lineage development) and their role in clinical pathologies like Germ Cell Cancer pathogenesis (GCC) leading to infertility should be investigated In-depth.
Moreover, research on germline stem cells will provide an insight on their regenerative therapeutic potential and enable to bridge the existing research gaps in the field [9]. For instance, the mechanisms guiding clonal expansion need to be investigated in males to understand spermatogonial biology [10,11]. There is limited evidence in primates, if clonal expansion mechanisms are unidirectional or sequentially regulated, and if any cellular or molecular event triggers germ cell expansion or their differentiation process [12]. The role of the epithelial cycle in orchestrating the sequence of events and its influence on stem cell fate further needs to be elucidated [13]. In addition, the specific role of intrinsic and extrinsic cues and function of testicular niche in primate SSC regulation needs to be determined in future studies. It is not known, if any compensatory properties or mechanisms catalyze clonal dynamics. For instance, theories suggesting the presence of a crowding-feedback pathway (cells acting by gauging cell density) in SSCs in the seminiferous epithelium [14] to control testicular homeostasis have never been systematically investigated. The role of the undifferentiated SSC pool, or competing mitogens in maintaining total stem cell activity and regulation of testicular homeostasis needs to be determined [15].
Further studies are needed to understand the phenomenon of germ cell quiescence. It is not known what triggers specific SSCs to enter and stay in a quiescent state, and if any molecular or biochemical tools can be employed to identify these resting cell populations. Investigating quiescent cell populations and features of these resting cells will give us an insight on the cellular and molecular events triggering the activation of ‘’the state of quiescence’’ and ‘’restoration of self-renewal and differentiation potential’’. In addition, further research studies are needed to investigate the concept of male germ cell ageing, if germ cell lineages disappear on ageing or if these lineages are present within the testis in a quiescent state. There is a lot of speculation about the function of the testicular niche and the role of niche factors in directing germ cells to specific stem cell fate or to acquire a quiescent state. Future studies should explore whether the niche has a role in stimulating germ cells to acquire plasticity potential or if plasticity potential is inherent in these cells since early pluripotent stage.
The evidence from the reviewed literature on germ cell heterogeneity leads us to speculate if the existing stochastic heterogeneity in germ cells at single cell level ensures maintenance of homeostasis and balance between self-renewal and differentiation at population level [2]. The checkpoints regulating this conserved evolutionary mechanism across species need to be identified. Further studies are required to explore if active evolutionary checkpoints regulate integrity of the genome by clonal selection mechanisms, or if specific epigenetic mechanisms are involved in this process. It is not known if testicular changes during ageing influence spermatogonial selection, and why functional sperm produced from specific -SSC derived dominant clones contribute to offspring production.
What are the inherent characteristic profile and unique features, which distinguish these clones from the rest of the SSC population in the testis? Epigenetic studies are required to investigate and compare the characteristic distinctions in epigenetic expression patterns of competing clones, clones from younger and older fathers, and healthy men and men with fertility disorders. Data from such studies and a comparison with patients suffering from germ cell tumors will provide more insight whether specific epigenetic patterns are required for maintenance of germ cell homeostasis and normal fertility function. The association of epigenetic heterogeneity and specific response to particular chemotherapeutic treatments and dose regimes will be interesting to investigate in detail.
During germline development, most DNA damage is repaired or corrected by active DNA repair mechanisms. Those that skip can give rise to cell death or mutation. We do not know whether failure in repair is due to intrinsic mechanisms or DNA repair enzymes. It is not known, which of the subpopulations are error -prone. Are there any differences in the repair proteins where DNA repair mechanisms are not functionally robust? This can also be detected by expression analysis at single cell level by using single cell microfluidic approach. Besides this, single cell transcriptomic analysis will enable us to understand the molecular and cellular events involved and the function of various transcription factors within the testis. For characterization of single cells, combined multi-analysis platforms with cytometry, electrophoresis and spectroscopy tools can be developed.
Developing approaches for disease modeling and drug screening is necessary for identification of therapeutic targets. Identification of specific subpopulations, which can be used for therapeutic treatment, can be isolated using approaches like microRNA switch. This will enable compound screening and drug discovery for specific infertility disorders. Development of in vitro models will allow studies on specific infertility disease etiologies and pathologies, and development of personalized drugs and regenerative medicine for clinical purposes. Multi organ-on-chip platforms can be developed for multifactorial disease modeling, integrating and combining genomic and cellular tools to predict rare diseases and mimic complex patient pathologies in vitro. Multi-organ on chip models can be used to study endocrine function by developing models mimicking the HPG (Hypothalamic-Pituitary-Gonadal) axis using male and female gonadal tissue or cells [4].
In addition, to evaluate the synergistic effect of drug exposure or treatment on other organs (like liver), tissue fragments/slices or cell-based organoid samples from other organs can be cultured in parallel in such a system. In combination with bioengineering approaches like microfluidics, these tools can be employed to develop a BTB (Blood-Testis-Barrier) model to study BTB function and a BEB (Blood-Epididymal-Barrier) model to study epididymal function. Such barrier systems can be used to test the effect of exposure to environmental toxicant/plasticizer, endocrine disruptors or pharmaceutical drugs on testis and epididymis.
Development of germ cell tumor organoids will allow analyzing histopathological functions and genetic characterization of tumor heterogeneity, and to test whether reprogramming tumor cells is a feasible option in the future. Reprogrammed iPS cells or seminoma cell lines like T-cam2 can be used to develop these models for future research. Such models will enable us to understand the role of niche factors in tumor genesis [16]. In addition, testicular organoid models using PGCs and testicular cell fractions can be used to enhance our understanding on early stages of germ cell differentiation [17].
Tissue engineering and Bio-engineering strategies like 3D printing can be used to generate and print special bio -constructs like 3D scaffolds (composed of nanofiber materials implants) or to establish cell culture on 3D matrices. Generation of 3D printed scaffolds will allow cell-seeded tissue engineered grafts carrying testicular cells to support testicular or tubular regeneration. Depending on specific objectives, specialized biomaterials and systems can be used, as distinct architecture and components can influence cell proliferation and development to fulfill specific needs. 3D matrices and nanoparticle cues will direct reassembly and aggregation of testicular cells. Personalized cellular implants can be generated using these tools to restore and stimulate body’s signaling pathways and restoration of normal reproductive function.
Genome-Editing Tools
Potential of therapeutic genome editing technologies like CRISPR/Cas9 to edit germline mutations in humans in order to repair genetic disorders need to be explored [18]. Genome editing tools skip exons that bear a genetic mutation resulting in mutated gene expression. Cell therapy and epigenetic remodeling by CRISPR/Cas9 can potentially be used for development of genome-edited clones. Generating cell lines of genome edited cell population for disease modeling and resurrection can serve as a useful biological (for understanding signaling pathways involved) and clinical tool (for infertility treatment).
Ssc Culture Approaches
Findings and observations from SSC culture studies provide strong evidence, new perspective and ideas (to further optimize culture conditions and strategies) which might prove to be very useful for designing future studies to establish in vitro SSC culture systems for primate species [3]. In order to stimulate germ cells to propagate, expand and differentiate, additional factors (somatic feeder cells, factors secreted by testicular somatic cells) might be required by germ cells in the dish. One possible strategy to stimulate propagation of cells can be to introduce species-specific proliferation-inactivated somatic feeder cells in culture; so that somatic cells do not overgrow germ cells but provide necessary factors to stimulate germ cells to proliferate.
Another option can be to explore alternate isolation, sorting or separation strategies reported in previous studies. Instead of separation (germ cells and somatic cells) after overnight plating, germ cells can be separated at a later time point and pure germ cell population can be isolated using sequential differential plating steps or MACS. Further studies will be required in the future to optimize, improve and devise efficient separation strategies and culture protocols for primate spermatogonial propagation. Further development of non-static culture approaches will prove to be more efficient in establishing SSC culture systems. It will be necessary to devise stringent approaches to analyze the cultured cells. It might be misleading to predict the effect of different culture conditions based on only molecular evidence. Therefore, additional analytical approaches will be employed to validate the presence of germ cells in culture. Sequencing approaches have opened further avenues to determine the molecular profile and momentary hierarchical state of the cell.
Adding on the same note, we also need to challenge our way of thinking, if specific factors, markers, receptors modulating different aspects of testis function and if innate heterogeneity at genomic and phenotypic level ever allow identification of novel general and exclusive germ cell markers. Future studies should explore the efficiency of non-static culture systems for establishing primate testicular cultures and if using intact tissue will prove to be more useful for future clinical applications.
For clinical applications, efficient cryopreservation protocols for long-term storage of testicular biopsies and samples to preserve structural integrity and cellular function need to be established. Further it needs to be determined if species-specific culture systems and protocols are necessary to be established.
Multi-lineage Tracing
Multi-lineage tracing approach can be exploited to study cellular dynamics (clonal kinetics, tumor development), by tracking of progenitor cell populations in 2D and 3D cultures. Employing fluorescent markers can help in analyzing non -integrative somatic cell reprogramming. Novel biocompatible fluorescent nanoparticles can be used to trace viable, proliferating and differentiating cell populations.
In vitro cell culture approaches with live imaging enable tracking of clonal behavior, germ cell-somatic cell interactions and effect of niche factors. Similar studies using GFP-labeled cells can be performed using in vitro cell culture approaches. Clonal expansion mechanisms and differentiation patterns can be studied in vitro. Specific developmental and epithelial stages can be studied in-depth and clonal dynamics can be tracked by employing live imaging approaches. Soma-germline interactions, role of BTB (Blood-Testis-Barrier) components in influencing spermatogenic function can be studied comprehensively by employing some of these approaches. Trans illumination studies with primate seminiferous tubules can be performed to understand clonal expansion patterns during different epithelial stages and topographical arrangement in the tubules.
To investigate the role of testicular niche in influencing stem cell fate decisions.
Clonal Tracking of Transplanted Transgenic Pluripotent Stem Cells in Non-Human Primate Models
Transplantation of transgenic pluripotent cell line in primate testis, and tracking the cellular behavior periodically will allow us to evaluate the role of testicular microenvironment in defining cellular fate decisions. It will give us an insight whether testicular microenvironment of a primate directs these cells towards differentiation pathway.
Transplantation of Transgenic or Pulse-Labeled Pgcs in Male and Female Gonads
Transplanting PGCs into male and female mouse gonads and tracking their fate will allow us to investigate how PGC respond in different microenvironments and if male and female microenvironments provide sex-specific cues.
Transplantation and Lineage Tracing to Study Clonal Dynamics
NHP’s are used to study primate spermatogenesis as stem cell systems of primates are comparable and distinct from rodents. Transgenic modifications in NHPs have not been performed so far therefore rodents are extensively used to study various aspects of spermatogenesis [19]. Such studies need to be performed in primate models [10,11]. Using transplantation, lineage tracing studies with dual-GFP labeled germ cells can be performed. Labeled clones and their progenitors can be tracked until they differentiate further into spermatozoa. Spermatogenic turnover rates from undifferentiated cell types to differentiated cells can be estimated. Another aspect to investigate will be whether the transplanted lineages colonize the testis, and for how long do they remain active functionally and participate in differentiation. This may answer many questions related to germ cell stochasticity (Figure 1).
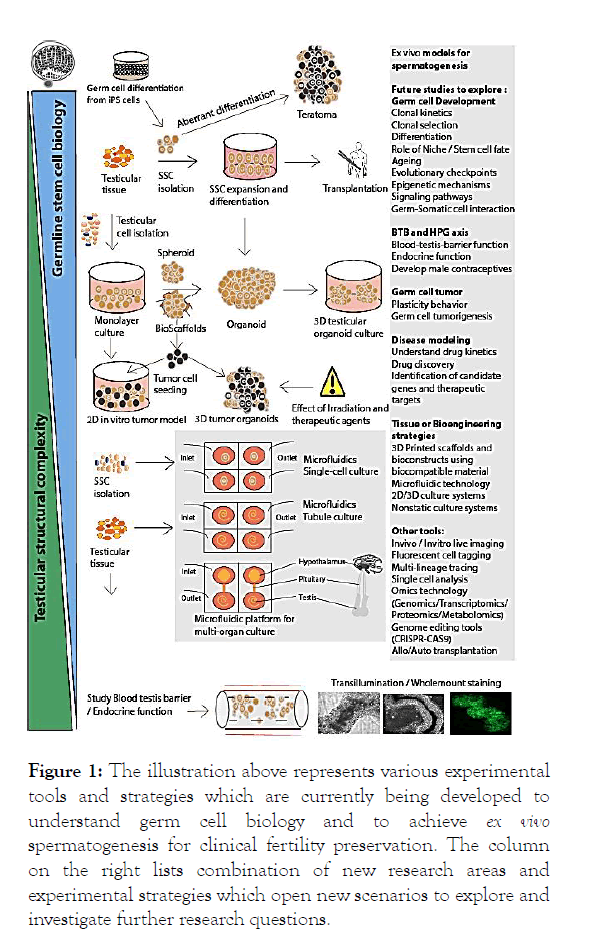
Figure 1: The illustration above represents various experimental tools and strategies which are currently being developed to understand germ cell biology and to achieve ex vivo spermatogenesis for clinical fertility preservation. The column on the right lists combination of new research areas and experimental strategies which open new scenarios to explore and investigate further research questions.
Discussion and Conclusion
Research efforts to establish novel ex vivo models, in combination with development of exciting technologies have revolutionized the field of germ cell biology and reproductive medicine. This opens new scenarios for conducting biological and translational research, and for exploring germ cell function to devise advanced treatment strategies for infertile patients.
Acknowledgements
I would like to thank my mentor Prof. Stefan Schlatt for his impeccable mentoring, guidance and supervision during every step of my doctoral and post-doctoral research. I would like to thank him for stimulating discussions, for sharing his immense knowledge, expertise, ideas and suggestions from the vast experience of over 30 years of working as a leading expert scientist in the field. The day-to-day deliberations with him during the past 6 years were instrumental in shaping up my perspective presented in this mini review.
I would like to thank Joachim Wistuba and Nina Neuhaus for sharing their insights and knowledge on testicular physiology, histology and spermatogonial biology. I would like to thank all the members (PIs and fellows) of the Grow sperm consortium for their vibrant support, strong research collaboration, their research inputs and valuable suggestions during the past years.
REFERENCES
- Sharma S, Klaverkamp RS, Schlatt S. Differentiation of testis xenografts in the prepubertal marmoset depends on the sex and status of the mouse host. Front Endocrinol (Lausanne). 2018;9:467
- Sharma S, Wistuba J, Pock T, Schlatt S, Neuhaus N. Spermatogonial Stem cells: Updates from specification to clinical relevance. Human Reprod Update. 2019;25(3):275-297.
- Sharma S, Schlatt S, Pelt AV, Neuhaus N. Characterization and population dynamics of germ cells in adult macaque testicular cultures. PLOS One. 2019;14(6):e0218194.
- Sharma S, Venzac B, Burgers T, Le Gac S, Schlatt S. Microfluidics in male reproduction: is ex vivo culture of primate testis tissue a future strategy for ART or toxicology research? Mol Hum Rep. 2020;26(3):179-192.
- Reuter K, Schlatt S, Ehmcke J, Wistuba J. Fact or Fiction: In vitro spermatogenesis. Spermatogenesis. 2012;2(4):245-252.
- Neuhaus N, Schlatt S. Stem cell-based options to preserve male fertility. Science. 2019;363(6433):1283-1284.
- Goossens E, Jahnukainen K, Mitchell RT, Van Pelt AMM, Pennings G, Rives N, et al. Fertility preservation in boys: Recent developments and new insights. Hum Reprod Open. 2020;2020(3):hoaa016.
- Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160(1-2):253-268.
- Sharma S, Portela JMD, Röwer LD, Wistuba J, Neuhaus N, Schlatt S. Male germline stem cells in non-human primates. Primate Biol. 2017;4(2):173-184.
- Ehmcke J, Simorangkir DR, Schlatt S. Identification of the starting point for spermatogenesis and characterization of the testicular stem cell in adult male rhesus monkeys. Human Reproduction. 2005; 20(5):1185-1193.
- Ehmcke J, Luetjens CM, Schlatt S. Clonal organization of proliferating spermatogonial stem cells in adult males of two species of non-human primates, Macaca mulatta and Callithrix jacchus. Biol Reprod. 2005;72(2):293-300.
- Simorangkir DR, Marshall GR, Ehmcke J, Schlatt S, Plant TM. Pubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73(6):1109-1115.
- Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist’s perspective. Semin Cell Dev Biol. 2014;29:2-16.
- Greulich P, Simons BD. Dynamic heterogeneity as a strategy of stem cell self-renewal. Proc Natl Acad Sci. 2016;113(27):7509-7514.
- Kitadate Y, Jörg DJ, Tokue M, Maruyama A, Ichikawa R, Tschuiya S, et al. Competition for mitogens regulates spermatogenic stem cell homeostasis in an open niche. Cell Stem Cell. 2019;24(1):79-92.
- Skakkebaek NE, Meyts E, Louis BGM, Toppari J, Andersson AM, Eisenberg ML, et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol Rev. 2016;96(1):55-97.
- Mall EM, Rotte N, Yoon J. Klaverkamp RS, Röpke A, Wistuba J, et al. A novel xeno-organoid approach: Exploring the crosstalk between human iPSC-derived PGC-like and rat testicular cells. Mol Hum Reprod. 2020;26(12):879-893.
- Mulder CL, Zheng Y, Jan SZ, Struijk RB, Repping S, Hamer G, et al. Spermatogonial stem cell cell autotransplantation and germline genomic editing: A future cure for spermatogenic failure and prevention of transmission of genomic diseases. Hum Reprod Update. 2016;22(5):561-573.
- Hara K, Nakagawa T, Enomoto H, Mikiko S, Yamamoto M, Simons BD, et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14(5):658-672.
Citation: Sharma S (2020) Future Outlook for Developing Ex Vivo Models to Study Germline Development and Fertility Preservation. Clinics Mother Child Health. 17:371.
Copyright: © 2020 Sharma S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

