Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 13, Issue 1
Frequency, Pattern and Risk Factors for Red Blood Cell Alloimmunization in Multiply Transfused Cancer Patients in a Nigerian Tertiary Healthcare Facility in Nigeria
Ologbo Thompson Onoriode1, Nwogoh Benedict2* and Enosolease Mathew Ebose22Department of Haematology and Blood Transfusion, University of Benin Teaching Hospital, Benin City, Edo State, Nigeria
Received: 04-Jan-2022, Manuscript No. JB DT-22-15391; Editor assigned: 06-Jan-2022, Pre QC No. JB DT-22-15391; Reviewed: 18-Jan-2022, QC No. JB DT-22-15391; Revised: 21-Jan-2022, Manuscript No. JB DT-22-15391; Published: 25-Jan-2022, DOI: 10.4172/2155-9864.22.13.485
Abstract
Background: Anaemia is a common complication of cancer and its treatment. Red blood cell transfusion is an important supportive care element for the treatment of anaemia in cancer patients. Red cell alloimmunization and its associated effect can increase disease morbidity and outcome in cancer patients. This study is aimed at determining the frequency, pattern and risk factors associated alloimmunization in multiply transfused cancer patients.
Methodology: This was a hospital-based cross-sectional study at the University of Benin Teaching Hospital, Benin- City, Edo State. Seventy-five cancer patients including 15 with haematological malignancies and 60 with solid organ cancers who have been multiply transfused were recruited consecutively into the study. A structured questionnaire was used to obtain subject demographics, information on the type of cancer, transfusion history. Blood sample was collected from consenting participants for blood counts, blood group determination, alloantibody screening and identification. All serological tests were performed using a standard protocol. Data was analyzed using Statistical Package for Social Sciences (SPSS) version 22.
Results: The frequency of red cell alloimmunization was 13.3%. Thirteen alloantibodies were detected in 10 patients. The majority 8 (61.5%) were against the Rhesus blood group antigens, 3 (23.1%) anti-Kell, 1 (7.7%), anti-Lewis and one (7.7%). There were no significant associated risk factors in the study population.
Conclusion: There a high rate of red cell alloimmunization in multiply transfused cancer patients in our environment Age below 40 years, female gender, non-O ABO blood group status, and initiation of chemotherapy were associated with increased odds of alloimmunization.
Keywords
RBC alloimmunization; Cancer; Multiply-transfused
Introduction
Cancer is the second leading cause of death globally, and was responsible for an estimated 9.6 millon deaths in 2018 [1]. Its burden has continued to rise. An estimated 18.1 million new cases were recorded in 2018. About 1 in 6 deaths globally is attributed to cancer. In Nigeria, about 115,950 new cases and 70,327 cancer associated deaths were recorded in 2018 [2,3].
Cancer is an abnormal proliferation cells and have the ability to invade surrounding normal tissues, spread to distant sites including the bone marrow to cause disease and death [4]. invasion of the bone marrow may lead to bone marrow suppression causing anaemia. Other mechanisms of anaemia in cancer patients include bleeding from the tumor, poor nutrition, infection, inflammatory disorders, and chemotherapy/radiation therapies. The prevalence of anaemia in cancer patients at different stages of treatment is estimated to range from 39-68% and may be severe enough to require blood transfusions [5]. Red cell alloimmunization is a complication of RBC transfusion support.
Alloimmunization is an immunological response by an immunocompetent host against donor or non-self-antigens, resulting in various clinical consequences depending on the type of blood cells and specific antigens involved [6]. Red cell alloimmunization is the formation of alloantibodies, against non-self- antigens on Red Blood Cells (RBCs) [7].
RBCs express self-antigens on its surface which determines its group specificity. Antibodies against RBC antigens are either naturally occurring or immune-based [8]. Naturally occurring antibodies appear in the serum at about 3-6 months of age and are implicated in some cases of alloimmunization. They include Anti A, Anti B, Anti P and Anti Le which are antibodies into the ABO, P, and Le blood group systems.8. Immune antibodies are elicited following exposure to non-self-antigens following transfusion, pregnancy and birth. The blood group systems associated with like Rh, Kell, Duffy, Kidd, Lutheran and MNS and they are mainly implicated in RBC alloimmunization [9]. Alloimmunization is one of the most relevant post-transfusion complications as it is associated with transfusion delays, shortened in vivo survival of donor blood, and hemolytic transfusion reactions which can be fatal in some cases. RBC alloimmunization was recently identified as an independent predictor of HLA alloimmunization in HSCT with possible clinically significant adverse consequences [10].
In Nigeria, there is a paucity of data on the frequency and pattern of RBC alloimmunization in multiply transfused cancer patients. However, studies abound on RBC alloimmunization in multiply transfused sickle cell disease patients [11,12]. Other non-Nigerian based studies have estimated the risk of alloimmunization to range from 0.3%-29% [13-16].
The objective of this study is to the prevalence and specificities of immune red cell alloantibodies in multiply transfused cancer patients, to identify possibly associated risk factors.
Materials and Methods
Study design
This was a hospital-based cross-sectional study.
Study area
The study was conducted at the University of Benin Teaching Hospital, Benin–City, Edo State. The hospital is a tertiary health facility located in Benin City, the state capital of Edo State. The hospital is an 800 bed capacity facility and serves as a regional referral centre for oncology diseases. It serves the neighbouring states (Delta, Kogi, Ondo and Bayelsa states). It has a well establish oncology unit.
Study population
The study population consisted of adult (18 years and above) patients on treatment for malignancies who have been multiplytransfused. Multiply transfused was defined as patients who have been transfused with ten or more units of blood [17]. The patients were recruited from the oncology ward of the hospital. Patients with autoimmune diseases such as systemic lupus erythematosus and those known circulating RBC antibodies unrelated to transfusion were excluded.
Sample size estimation
The sample size was calculated using the formula for crosssectional study. The estimated sample size was later adjusted for the population size using the formula:
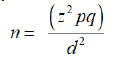 [18].
[18].
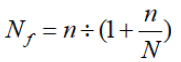
Where Nf = the desired sample size when the population is less than 10,000;
N=the estimate of the population size (about 100 patients per year).
n=desired sample size when population is more than 10,000 and calculated using the formula:
z=standard normal deviation (1.96)
Using 17.4% prevalence of haematological malignancies in UBTH [19]
q=1-p, 0.826
d=degree of precision to be used (0.05)
n therefore is 267.3=267
The adjusted sample size was 72.7=73
The working sample size for the study was 75 participants.
Sampling technique: consenting patients who met the study inclusion were recruited.
Study procedure
Each participant was interviewed with a structured, intervieweradministered questionnaire to obtain and document relevant biodata including age, sex and ethnic group. ABO and Rh (D) blood groups of the participants were retrieved from their hospital file records and confirmed in the course of the study.
Five millilitres (ml) of blood was collected from each participant by antecubital venipuncture after obtaining due consent. Three ml of the blood was dispensed into a plain bottle, while the remaining two millilitres was dispensed into an EDTA anticoagulant bottle. The EDTA anti-coagulated blood samples were used for ABO/ Rh D blood group phenotyping and haematological blood count test. The serum was used for alloantibody studies. Samples in the plain tube were allowed to clot at room temperature, centrifuged at 3000 rpm for 5 minutes and the serum was separated into another plain tube and stored frozen at -20°C. Maximum storage time was 14 days for each sample. All specimens were labelled with a personally generated identification number and recorded in stored in datasheet.
Test procedures
Tests were carried out in the following order: Basic haematological count determination, alloantibody screening test, and alloantibody identification test.
Blood count estimation: Complete blood count which includes haematocrit, haemoglobin concentration, total white cell count and platelet count was carried out from the EDTA sample, using an automated blood cell counter (Sysmex Haematology Autoanlyzer model KN21). The basic principles underlying these techniques are electronic impedance and light scatter. This was done in the main haematology laboratory, UBTH.
Alloantibody screening and identification
Screening cells and panel of cells (Albacete reagent red cells) for detection and identification of unexpected alloantibodies (procured from Lorne Laboratories UK) was used to screen patients’ sera for RBC alloantibodies and identification test using standard protocol [20].
Data analysis: Data was analyzed with the Statistical Package for Social Sciences (SPSS) version 22. Normally distributed continuous variables including the age of participants, haematological parameters, number of blood units received by study subjects was summarized using mean, standard deviation and ranges while the skewed continuous variable duration of illness was summarized as median and interquartile range. Categorical variables including age group, sex, ethnicity, occupation, comorbidity, type of malignancy, blood group of participants, frequency of alloimmunization, the pattern of alloantibody were summarized using simple frequencies and percentages.
The difference in mean age and number of units transfused between alloimmunized and non-alloimmunized was tested using the Student t-test. Chi-square statistics and odds ratio was used to estimate the association between alloimmunization with age group, gender, parity, blood group, comorbidity, cancer type and treatment status for underlying malignancy. Statistical significance was set at 0.05.
Ethical consideration: The study was approved the Institutional Ethics Committee All information given by participants was kept confidential.
Results
Seventy five patients with a history of multiple blood transfusions including 15 (20%) patients with haematological malignancies and 60 (80%) with solid organ cancers participated in the study.
Demographic and clinical characteristics of the study population
The mean age of the subjects was 52.0 ± 15.2 years with a range of 22.0-84.0 years. The mean ages of subjects with haematological and non-haematologic malignancies were 55.4 ± 16.0 and 51.1 ± 15.1 years respectively. The difference in their mean age was not statistically significant (p=0.332). The peak ages of the subjects were 50–59 years.
There were 24 (32.0%) males and 51 (68.0%) females. There were more males than females with haematological malignancies (7, (46.7%) vs. 8 (53.3%)) but those with non-haematologic cancers were predominantly females. The difference in gender distribution of subjects was statistically significant (p=0.048). Table 1 shows the sociodemographic and clinical characteristics of subjects based on the type of malignancy.
| Haematological malignancy | Non-haematologic cancer | Total | Statistics | P-value | |
|---|---|---|---|---|---|
| n (%) | 15 (20%) | 60 (80%) | |||
| Age (yrs.) | |||||
| Mean ± SD Range |
55.4 ± 16.0 25.0-76.0 |
51.1 ± 15.1 22.0-84.0 |
52.0 ± 15.2 22.0- 84.0 |
t=0.977 | 0.332 |
| DOI (yrs.) | |||||
| Median (yrs) Interquartile range |
1.0 1.0–2.0 |
1.0 1.0-2.0 |
1.0 1.0-2.0 |
Man Whitney U | 0.966 |
| Age group | |||||
| <40 | 2 (13.3) | 13 (21.7) | 15 (20.0) | ||
| 40-49 | 2 (13.3) | 12 (20.0) | 14 (18.7) | ||
| 50-59 | 5 (33.3) | 19 (31.7) | 24 (32.0) | *χ2=0.329 | 0.987 |
| 60-69 | 3 (20.0) | 9 (15.0) | 12 (16.0) | ||
| 70 and above | 3 (20.0) | 7 (11.7) | 10 (13.3) | ||
| Sex | |||||
| Female | 7 (46.7) | 44 (73.3) | 51 (68.0) | χ2=3.922 | 0.048 |
| Male | 8 (53.3) | 16 (26.7) | 24 (32.0) | ||
| Occupation | |||||
| Professional | 1 (6.7) | 0 (0.0) | 1 (1.3) | ||
| Skilled worker | 8 (53.3) | 21 (35.0) | 29 ((38.7) | ||
| Unskilled worker | 3 (20.0) | 23 (38.3) | 26 (34.7) | *χ2=1.939 | 0.747 |
| Retiree | 2 (13.3) | 9 (15.0) | 11 ((14.7) | ||
| Dependent | 1 (6.7) | 7 (11.7) | 8 ((10.7) | ||
| Comorbidity | |||||
| Diabetes | 3 (20.0) | 15 (25.0) | 18 (24.0) | *χ2=0.005 | 0.943 |
| Number on therapy | 13 (86.7) | 33 (55.0) | 46 (61.3) | *χ2=3.827 | 0.050 |
Table 1: Demographic and clinical characteristics of study subjects.
Duration of illness
The median duration of illness of subjects in both groups was 1 year with an interquartile range of 1.0-2.0 years.
Comorbidity
Eighteen subjects including 3 (20.0%) with haematological malignancies and 15 (25.0%) with solid organ cancer were diabetic. The difference in the proportion of diabetics in both groups was not statistically significant.
Treatment status
13 (86.7%) of subjects with haematological malignancies compared to 33 (55.0%) of those with non-haematologic cancers had commenced therapy for the underlying malignancy (p=0.050).
Distribution of haematologic and solid organ malignancies
The most common haematological malignancy was multiple myeloma which accounted for 7 (46.7%) followed by Non- Hodgkin’s lymphoma 4 (26.7%), Chronic Lymphocytic Leukaemia (CLL) 2 (13.3%), chronic granulocytic leukaemia 1 (6.7%) and acute myeloid leukaemia n (6.7%) (Figure 1). Non-haematologic cancers had the following distribution: breast (17, 28.3%), colorectal (7, 11.7%), ovarian (6, 10.0%), cervical/vulva and sarcoma (5, 8.3%) each, pancreatic and gastric (4, 6.7%) each, endometrial (3, 5.0%) among others (Figure 2).
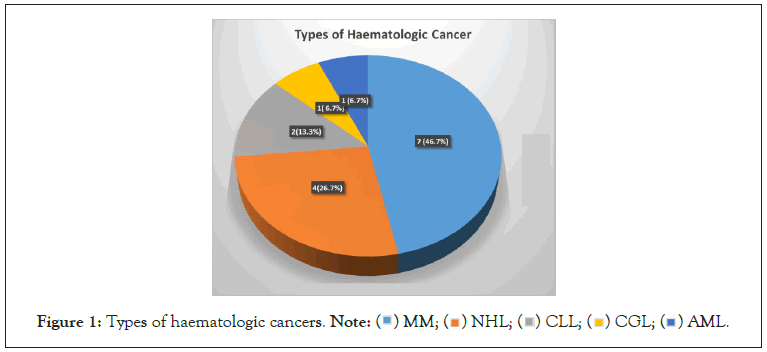
Figure 1: Types of haematologic cancers. Note:  AML.
AML.
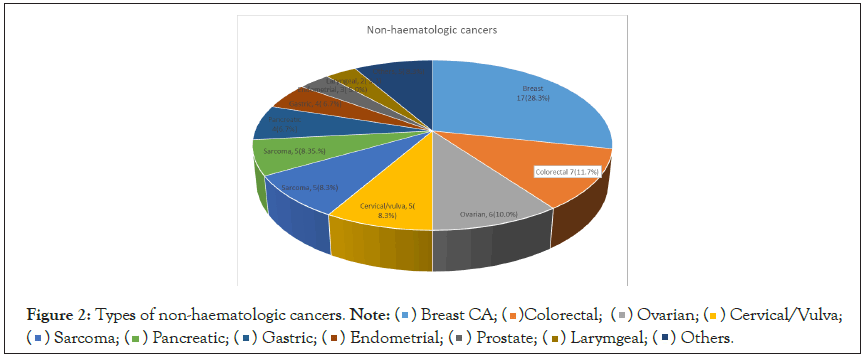
Figure 2: Types of non-haematologic cancers. Note: Cervical/Vulva;
Cervical/Vulva; Others.
Others.
Haematologic parameters of the study population
The mean haematocrit of the study population was 27.0 ± 5.7% with a range of 9.0-41.7%. The haematocrit did not differ significantly between patients with haematological malignancies and those with non-haematologic cancer (24.7 ± 7.1 vs. 27.6 ± 5.2%; p=0.988).
The mean White Blood Cell Count (WBC) was 7.6 ± 4.3 (range, 1.50-28.0). The WBC count did not differ significantly between those with haematologic and non-haematologic cancers (7.6 ± 2.5 vs. 7.6 ± 3.8, p=0.079).
The mean platelet count of the study population was 243 ± 127.2 with a range of 24.0–558.0 × 109/L (Table 2). The difference in mean platelet count was significantly increased in subjects with non-haematologic cancers compared to those with haematological malignancies (266.7 ± 127.3 vs. 151.7 ± 76.5, p=0.001).
| Haematological parameters | Haematological malignancy | Non-haematologic cancer | Total | T-test | P-value |
|---|---|---|---|---|---|
| Haematocrit | |||||
| Mean ± SD | 24.7 ± 7.1 | 27.6 ± 5.2 | 27.0 ± 5.7 | -0 | 0.988 |
| Range | 9.0-35.0 | 15.9-41.7 | 9.0-41.7 | ||
| WBC | |||||
| Mean ± SD | 7.6 ± 2.5 | 7.6 ± 3.8 | 7.6 ± 4.3 | -2 | 0.079 |
| Range | 2.3-28.0 | 1.5-18.6 | 1.50-28.0 | ||
| Platelets | |||||
| Mean ± SD | 151.7 ± 76.5 | 266.7 ± 127.3 | 243.7 ± 127.2 | -3 | 0.001 |
| Range | 24.0-303.0 | 74.0-558.0 | 24.0-558.0 | ||
Table 2: Haematologic parameters of the study population.
Blood group distribution of study population
Regarding blood group distribution, the observed blood group antigen frequencies were O (66.7%), A (17.3%), B (10.7%), AB (5.3%) of the ABO blood group system. Seventy-two (96.0%) were positive for the Rhesus D antigen while 4% were negative (Table 3).
| Haematological malignancy | Non-haematologic cancer | Total | Statistics test | P-value | |
|---|---|---|---|---|---|
| Blood group | |||||
| ABO | |||||
| O | 9 (60.0) | 41 (68.3) | 50 (66.7) | χ2=5.136 | 0.162 |
| A | 1 (6.7) | 12 (20.0) | 13 (17.3) | ||
| B | 2 (13.3) | 6 (10.0) | 8 (10.7) | ||
| AB | 3 (20.0) | 1 (1.7) | 4 (5.3) | ||
| Rhesus D | |||||
| Positive | 15 (100.0) | 57 (95.0) | 72 (96.0) | χ2=0.022 | 0.882 |
| Negative | 0 (0.0) | 3 (5.0) | 3 (4.0) | ||
| No of units transfused | |||||
| Mean ± SD | 12.0 ± 3.5 | 10.5 ± 1.7 | 10.8 ± 2.2 | t=2.358 | 0.021 |
| Range | 10-21 | 10-22 | 10-22 | ||
Table 3: ABO and Rhesus D blood group pattern and average unit of blood transfused.
Number of blood units transfused
The average number of blood units received by the study subjects was 10.8 ± 2.2 units with a range of 10-22 units. Subjects with haematological malignancies received a significantly higher number of blood units compared to subjects with non-haematologic cancers (12.0 ± 3.5 vs. 10.5 ± 1.7, p=0.021).
Alloimmunization status of the study population
A total of 10 (13.3%) of the study subjects had a positive alloantibody test. These include 2 (13.3%) of subjects with haematological malignancies and 8 (13.3%) of those with nonhaematologic malignancies. There was no significant difference in the proportion of alloantibody seropositivity between subjects with haematological and those with solid organ cancers (p=1.000).
Three subjects (30.0%) had double alloantibodies detected and 7 (70.0%) had single alloantibodies. In one of the subjects with a single antibody, the antibody specificity could not be established.
A total of 13 alloantibodies were detected in 10 subjects. The majority 8 (61.5%) were against the Rhesus blood group antigen, 3 (23.1%) against Kell, 1 (7.7%) against Lewis and the remaining 1 (7.7%) could not be identified. The most prevalent alloantibody is anti-Cw with 4 (30.8%) anti-E with 3 (23.1%), anti-Kpa had 2 (15.3%), anti-C, anti-K and anti-Lea were 1 (7.7%) each (Figure 3).
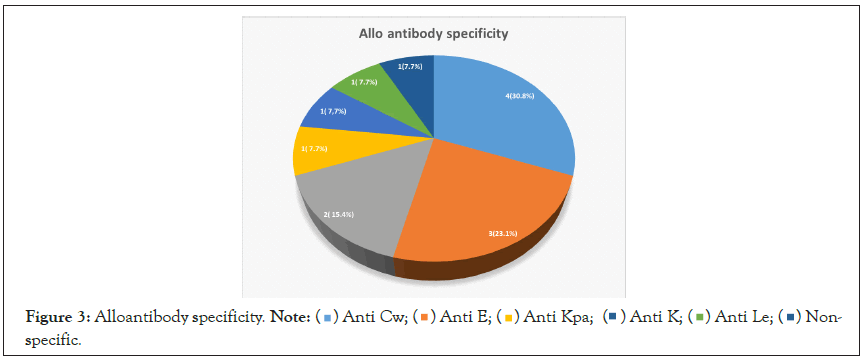
Figure 3: Alloantibody specificity. Note: Non-specific.
Non-specific.
Risk factors for RBC alloimmunization
The association between alloimmunization and potential risk factors including the age group of subjects, number of transfusions, sex, parity, ABO blood group, Rhesus antigen, diabetes, nature of cancer and use of chemotherapy were tested (Table 4).
| Risk factors | Allo-immunized | Non-alloimmunized | Statistics | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| n | 10 | 65 | |||
| Age | 46.0 ± 16.1 | 52.9 ± 15.0 | t=-1.336 | 0 | |
| No of transfusion | 10.7 ± 1.1 | 10.8 ± 2.4 | t=-0.193 | 1 | |
| Age group | |||||
| <40 | 4 (40.0) | 11 (16.9) | χ2=1.632 | 1 | 1 |
| 40-49 | 2 (20.0) | 12 (18.5) | 0.45 (0.06-3.01) | ||
| 50-59 | 2 (20.0) | 22 (33.8) | 0.25 (0.04-1.58) | ||
| 60 and above | 2 (20.0) | 20 (30.8) | 0.28 (0.04-1.14) | ||
| Sex | |||||
| Female | 7 (30.0) | 44 (67.7) | χ2=0.048 | 1 | 1.11 (0.26-4.74) |
| Male | 3 (70.0) | 21 (32.3) | |||
| Parity | |||||
| ≤ 3 | 4 (66.7) | 7 (18.9) | χ2=3.929 | 0 | 0.12 (0.02-0.77) |
| >3 | 2 (33.3) | 30 (81.1) | |||
| Blood group | |||||
| ABO | |||||
| Non-O | 6 (60.0) | 19 (29.2) | χ2=2.437 | 0 | 3.63 (0.92-14.34) |
| O | 4 (40.0) | 46 (70.8) | |||
| Rhesus | |||||
| Positive | 10 (100.0) | 62 (95.4) | χ2=0.030 | 1 | |
| Negative | 0 (0.0) | 3 (4.6) | |||
| Diabetes | |||||
| Yes | 2 (20.0) | 16 (24.6) | χ2=0.006 | 1 | 0.77 (0.15-3.98) |
| No | 8 (80.0) | 49 (75.4) | |||
| Nature of CA | |||||
| Haematologic | 2 (20.0) | 13 (20.0) | χ2=0.180 | 1 | 1.00 (0.19-5.28) |
| Non-haematologic | 8 (80.0) | 52 (80.0) | |||
| Use of chemotherapy | |||||
| Yes | 7 (70.0) | 39 (60.0) | χ2=0.065 | 1 | 1.56 (0.37-6.57) |
| No | 3 (30.0) | 26 (40.0) | |||
Table 4: Factors associated with alloimmunization.
Female gender was associated with increased odds of alloimmunization but this was not statistically significant. Odds ratio (OR; 1.11, 95% CI, 0.26 – 4.74). Parity (, 3) was associated with reduced risk of alloimmunization OR=0.12 (0.02-0.77). Non-O blood had a high Odds of alloimmunization (OR=3.63, 95%CI: 0.92- 14.34). The use of chemotherapy use was associated with higher odds of alloimmunization but this did not reach statistical significance 1.56 (0.37–6.57).
Discussion
The overall prevalence of alloantibodies in multiply transfused cancer patients was observed to be 13.3%. The prevalence observed in this study falls within the range reported in some studies on the subject.15, 16 El Fetouh et al. [15] reported a rate of 9.16% amongst multiply transfused Egyptians with various cancers including haematological and non-haematological cancers. In another related study, Ahmed Ali et al. [16] reported a rate of 29% among Sudanese patients with cancers. The prevalence of alloantibody in a population may vary depending on the definition of multiply transfused used in a study, the local transfusion policy and practice especially concerning extended blood group typing amongst others. The index study used a value of at least 10 units for the definition of multiply transfused.
The majority of alloantibodies identified in this study were those against the Rhesus and Kell blood group systems which accounted for 65.1% and 23.1% respectively. Others were of the Lewis blood group with 7.7%. This is consistent with existing studies which reported alloantibodies predominantly against Rhesus and Kell blood group systems. These include studies by Elrahman et al. and Mohsin et al. [21,22] Their high prevalence may be as a result of the diverse and highly immunogenic nature of antigens of these blood group systems leading to the production of clinically significant alloantibodies. Anti-Lewis antibody was identified in one patient and it not clinically significant.
The relationship between the number of blood transfusions and the rate of alloimmunization was not significant. Some studies reported that increasing number of alloimmunized patients dependent on the number of RBC units transfused yet others did not confirm this association [23-26] Our findings is in keeping with a study by Zalpuri et al. [23] which showed no significant difference in the risk of alloimmunization in intensively transfused patients and non-intensively transfused patients. However studies by Papay et al. [24] and Schonnewille et al. [25] have shown otherwise. The conflicting results can be explained by the number of transfusions, the interval between transfusion events, the frequency of antibody testing, the specificity of the antibodies (whether clinically significant versus non-significant and low-incidence) and the practice of extended red blood cell phenotyping by different centers involved in the different studies. Besides, for most studies all transfusions administered to the patients were considered until antibody was detected. After transfusion with an incompatible antigen, a primary immune response needs time to result in a serological detectable antibody and additional transfusions can be given during this period. As a consequence, the number of transfusions needed to elicit an antibody response can be overestimated. Based on the frequency of common RBC antigens in the Caucasian population, most patients will theoretically encounter alloantigen during the first 3-4 transfusions. Blumberg et al. on the other hand showed that the rate of antibody formation per transfusion decreases with increasing numbers of transfusions (e.g. 7.9 per 1000 transfusions when less than 15 units were transfused compared to 2.5 when more than 44 units were transfused). This is in agreement with studies who reported that the majority of alloimmunized patients have made the antibodies early during the transfusion course and probably after the first few encounters with the foreign antigen [26,27].
The age of the patients was not significantly associated with the risk of alloimmunization. This study in keeping with reports by Schonnewille et al.26 However, studies by Murao et al. [28] and Samarah et al. [29] revealed a significant association between age and alloimmunization. In the index study patients in the age group less than 40 years had a higher rate of alloimmunization than the other age groups. This is similar to the observation by Karaffin et al. [30]. The reason for this is not entirely clear however it may be that patients in this age group are more immunocompetent compared to the older age groups. They may also lack comorbidities and other age-related factors that may reduce immunocompetence.
There was no statistically significant association between gender and risk of alloimmunization in this study despite a slightly higher odd in females. This is in keeping with the findings of Samarah et al. [29]. However, studies by Verduin et al. [31] and Karafin et al. [30] showed that the female sex is more at risk of alloimmunization compared to men. It is biologically plausible that women having been exposed to several sensitization episodes especially through pregnancy and/or transfusion should be more alloimmunized however, sensitization through pregnancy is dependent on the degree of antigen compatibility between mothers and their fetuses. Zalpuri found that the alloimmunization rate was comparable for males and females over the age of 45 years and lower alloimmunization frequency among women below 45 years of age [32]. Increasing age-related factors may have played a role in affecting the alloimmunization rate as the majority of the study population were of the older age group in this study even there was a significantly higher number of females compared to males.
Blood group O is the dominant ABO blood group in the study population. The distribution of ABO blood group in the study population is consistent with the blood group pattern in the region as described by Enosolease et al. and Egesie et al. [33,34] There was no significant association between risk of alloimmunization and ABO/Rh D blood group systems, however non-O ABO blood groups had higher odds of alloimmunization. This is consistent with the observation of Al-Joudi who found that there was no significant association between alloimmunization and ABO and Rh (D) blood groups [35] The possibility of subjects with blood group ‘O’ having reduced highly immunogenic antigens or having genetic factors that may make them resistant to alloimmunization may account for their low rate of alloimmunization despite accounting for a higher number of subjects in this study.
There was an inverse relationship between parity of the female patients and the rate of alloimmunization. Female patients with parity of 3 or less had higher risk of alloimmunization. This is inconsistent with the observations of Patel et al. that reported increased risk of alloimmunization with increasing parity [36] Pregnancy supposedly exposes mothers to fetal RBCs during pregnancy and around the time of delivery, however, only a few get alloimmunized. Several factors including the immunogenicity of the antigen they were exposed to and possibly other genetic and/or environmental factors play some roles in pregnancy related risk of alloimmunization [7].
The risk of alloimmunization was similar between patients with haematologic malignancies and those with solid organ cancers. Some studies have reported a higher risk of alloimmunization in patients with solid organ cancers including studies by Arora et al. and Hendrickson et al. [37]. The role of the nature of the underlying disease in alloimmunization have been highlighted perhaps via associated inflammation or genetic polymorphisms [38,39]. The heterogeneity of these disease conditions involved in this study may contribute to the inability to establish any associations.
Patient with comorbidity such diabetes has reduced risk of alloimmunization. This is consistent with a study by Higgins et al. [40]. However, on the contrary, Bauer et al. reported diabetes mellitus as a risk factor for red cell alloimmunization [41] Generally diabetes may impact negatively on the immunity of affected individuals and thus impair immunological response associated with the process of alloimmunization.
The use of chemotherapy was not associated with significant risk of alloimmunization, however the use of chemotherapy was associated with increased odds of alloimmunization. Different studies have shown varying reports. Studies by Sanz et al. [42] and Mangwana et al. [14] showed diminished immune responses most likely reflect the intensity of treatment-associated immunosuppression while that by Leisch et al. [43] revealed two-fold higher alloimmunization rates for patients treated with immunomodulating therapies compared to patients not receiving immunomodulating therapies [14,42,43]. It is also possible that the use of steroids as part of the chemotherapy regimen may have contributed to reduced immunity and the rate of alloimmunization found in this study.
The study has some strengths and limitations. This is among a few studies on alloimmunization in multiply transfused cancer patients in the region. The definition of multiply transfused patients for this study was a reception of at least 10 units of blood. It is a strength for the study as a high benchmark provides a better platform to evaluate the association of transfusion with alloimmunization risk. This is unlike some studies where reception of at least two units of blood was used to define alloimmunization [11]. The study had a fair representation of different types of common malignancies prevalent in the region. However, the study is limited by the fact that the proportion of various malignancies represented was small and thus limited the capacity to test the association of specific malignancies with the risk of alloimmunization. Though a majority of the alloantibodies detected, were identified, however, the identification of one of the alloantibody was not possible. This may be attributed to the limited capacity to specify all detectable alloantibodies with the panel of cells available. However, it is also commonplace in some studies not to be able to specify all detectable alloantibody [23].
Conclusion
In conclusion, there is a high rate of RBC alloimmunization in multiply transfused cancer patients in our environment. The rate of alloimmunization is similar for patients with haematological and non-haematological cancers. Alloantibodies against the Rhesus and Kell blood group antigen systems were the most prevalent alloantibodies. Age below 40 years, female gender, non-O ABO blood group status, and initiation of chemotherapy were associated with increased odds of alloimmunization.
REFERENCES
- World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. International agency for research on Cancer. Geneva: World Health Organization. 2018.
- Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F, et al. Cancer incidence in Nigeria: A report from population-based cancer registries. Cancer Epidemiology. 2012; 36(5):e271-278.
[Crossref], [Google Scholar]
- International agency for research on cancer. Globocan 2018: Nigeria [Internet]. WHO. 2019 [accessed 2019 Aug 8].
- Kumar V, Abbas A, Aster JC. In: Robbins and cotran pathologic basis of disease. 9th edition. Philadelphia: Elsevier saunders; 2015; 266-270.
- Aapro M, Osterborg A, Gascon P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of iv iron. Ann Oncol. 2012; 23(8):1954-1962.
[Crossref], [Google Scholar], [PubMed]
- Blackall D. Alloimmunization from transfusions. [Internet]. Medscape. 2017 [accessed 2019 Aug 8].
- Hendrickson JE, Tormey CA. Understanding red blood cell alloimmunization triggers. Hematology. 2016; 2016(1):446-451.
[Crossref], [Google Scholar], [PubMed]
- Daniels G, Contreras M, Allard S. Red cell immune haematology. Postgraduate Haematology. 2015; 195-213.
- Westhoff CM. Red cell immunology and compatibility testing. Rossi's Principles of Transfusion Medicine. 2016; 193-205.
[Crossref], [Google Scholar]
- El-Beshlawy A, Salama AA, El-Masry MR, El Husseiny NM, Abdelhameed AM. A study of red blood cell alloimmunization and autoimmunization among 200 multi transfused Egyptian β thalassemia patients. Scientific Reports. 2020; 10(1):1-8.
[Crossref], [Google Scholar], [PubMed]
- Ugwu NI, Awodu OA, Bazuaye GN, Okoye AE. Red cell alloimmunization in multi‑transfused patients with sickle cell anemia in Benin City, Nigeria. Niger J Clin Pract. 2015; 18(4):522-526.
[Crossref], [Google Scholar], [PubMed]
- Kangiwa U, Ibegbulam O, Ocheni S, Madu A, Mohammed N. Pattern and prevalence of alloimmunization in multiply transfused patients with sickle cell disease in Nigeria. Biomarker Res. 2015; 3(1):1-6.
[Crossref], [Google Scholar], [PubMed]
- Mohsin S. Red cell alloimmunization in repeatedly transfused cancer patients. J Rawalpindi Med Coll. 2013; 17(2): 219-222.
[Crossref], [Google Scholar]
- Mangwana S, Kacker A, Simon N. Red cell alloimmunization in multi-transfused, oncology patients: Risks and management. Global J Transfusion Med. 2019; 4(1):74.
- El Fetouh RM, Abd Elmoniem GM, Allam RM, Sobeih ME, Kamel MM, Radwan SM. Frequency and specificity of Red blood cell alloantibodies in multitransfused Egyptian patients with hematological and nonhematological malignancies. Transfus Apher Sci. 2020; 59(6):102909.
- Ali AA. Determination of red cell alloimmunization among sudanese cancer patients with multi-blood transfusion-Khartoum State (Doctoral dissertation, Sudan University of Science and Technology). 2014.
- Philip J, Biswas AK, Hiregoudar S, Kushwaha N. Red blood cell alloimmunization in multitransfused patients in a tertiary care center in Western India. Laboratory Med. 2014; 45(4):324-330.
[Crossref], [Google Scholar], [PubMed]
- Araoye MO. Sample size determination. Research methodology with statistics for health and social sciences. Ilorin: Nathadex Publishers. 2004; 115-121.
- Nwannadi IA. The epidemiology of haematological malignancies at the university of Benin teaching hospital: A ten year retrospective study. The International J Epidemiol. 2010; 9(2).
- Roback JD, Combs MR. Technical Manual, 16th edition. American Association of Blood Banks. 2008.
- Mohsin S. Red cell alloimmunization in repeatedly transfused cancer patients. J Rawalpindi Med Coll. 2013; 17(2):219-222.
- Erahman S, Mirghani L. Alloimmunization in Sudanese leukaemic patients with multiple blood transfusions. IOSR-JDMS. 2017; 11(16):61–65.
- Zalpuri S, Zwaginga JJ, Le Cessie S, Elshuis J, Schonewille H, Van der Bom JG. Red‐blood‐cell alloimmunization and number of red‐blood‐cell transfusions. Vox sanguinis. 2012; 102(2):144-149.
[Crossref], [Google Scholar], [PubMed]
- Papay P, Hackner K, Vogelsang H, Novacek G, Primas C, Reinisch W, et al. High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. Am J Med. 2012; 125(7):717.e1-717.e8.
[Crossref], [Google Scholar], [PubMed]
- Schonewille H, Haak HL, Van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999; 39(7):763-771.
[Crossref], [Google Scholar], [PubMed]
- Schonewille H. Review of the literature on red cell alloimmunization. Red blood cell alloimmunization after blood transfusion. 2008;18-42.
- Blumberg N, Heal JM. Blood transfusion immunomodulation: the silent epidemic. Arch Pathol laboratory Med. 1998; 122(2):117.
[Google Scholar], [PubMed]
- Murao M, Viana MB. Risk factors for alloimmunization by patients with sickle cell disease. Braz J Med Biol Res. 2005; 38(5): 675-682.
[Crossref], [Google Scholar], [PubMed]
- Samarah F, Srour MA, Yaseen D, Dumaidi K. Frequency of red blood cell alloimmunization in patients with sickle cell disease in Palestine. Adv Hematol. 2018; 2018:1-7.
[Crossref], [Google Scholar], [PubMed]
- Karafin MS, Westlake M, Hauser RG, Tormey CA, Norris PJ, Roubinian NH, et al. Risk factors for red blood cell alloimmunization in the Recipient Epidemiology and Donor Evaluation Study (REDS‐III) database. Br J Haematol. 2018; 181(5):672-681.
[Crossref], [Google Scholar], [PubMed]
- Verduin EP, Brand A, Middelburg RA, Schonewille H. Female sex of older patients is an independent risk factor for red blood cell alloimmunization after transfusion. Transfusion. 2015; 55(6pt2):1478-1485.
[Google Scholar], [PubMed]
- Zalpuri S, Zwaginga JJ, Le Cessie S, Elshuis J, Schonewille H, Van der Bom JG. Red‐blood‐cell alloimmunization and number of red‐blood‐cell transfusions. Vox Sanguinis. 2012; 102(2):144-149.
[Crossref], [Google Scholar], [PubMed]
- Enosolease ME, Bazuaye GN. Distribution of ABO and Rh-D blood groups in the Benin area of Niger-Delta: Implication for regional blood transfusion. Asian J Transfus Sci. 2008; 2(1):3.
[Crossref], [Google Scholar], [PubMed]
- Egesie UG, Egesie OJ, Usar I, Johnbull TO. Distribution of ABO, Rhesus blood groups and haemoglobin electrophoresis among the undergraduate students of Niger Delta University Nigeria. Niger J Physiol Sci. 2008; 23(1-2).
[Crossref], [Google Scholar], [PubMed]
- Al-Joudi F, Ali AB, Majdan Bin Ramli SA, Ismail M. Prevalence and specificities of red cell alloantibodies among blood recipients in the Malaysian state of Kelantan. Asian J Transfus Sci. 2011; 5(1):42.
[Crossref], [Google Scholar], [PubMed]
- Patel J, Shukla R, Gupte S. Red cell alloimmunization in multitransfused patients and multiparous women. Indian J Hematol Blood Transfus. 2009; 25(2):49-52.
[Crossref], [Google Scholar], [PubMed]
- Arora K, Kelley J, Sui D, Ning J, Martinez F, Lichtiger B, et al. Cancer type predicts alloimmunization following RhD‐incompatible RBC transfusions. Transfusion. 2017; 57(4):952-958.
[Crossref], [Google Scholar], [PubMed]
- Hendrickson JE, Tormey CA. Red blood cell antibodies in hematology/oncology patients: Interpretation of immunohematologic tests and clinical significance of detected antibodies. Hematol/Oncol Clin. 2016; 30(3): 635-651.
[Crossref], [Google Scholar], [PubMed]
- Gehrie EA, Tormey CA. The influence of clinical and biological factors on transfusion-associated non-ABO antigen alloimmunization: responders, hyper-responders, and non-responders. Transfus Med Hemother. 2014; 41(6):420-429.
[Crossref], [Google Scholar], [PubMed]
- Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: Evidence for a distinct population of immunologic responders. Blood. J Am Soc Hematol. 2008; 112(6):2546-2553.
[Crossref], [Google Scholar], [PubMed]
- Bauer MP, Wiersum‐Osselton J, Schipperus M, Vandenbroucke JP, Briet E. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007; 47(11):2066-2071.
[Crossref], [Google Scholar], [PubMed]
- Sanz C, Nomdedeu M, Belkaid M, Martinez I, Nomdedeu B, Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013; 53(4):710-715.
[Crossref], [Google Scholar], [PubMed]
- Leisch M, Weiss L, Lindlbauer N, Jungbauer C, Egle A. Red blood cell alloimmunization in 184 patients with myeloid neoplasms treated with azacitidine–A retrospective single center experience. Leukemia Res. 2017; 59:12-9.
[Crossref], [Google Scholar], [PubMed]
Citation: Onoriode OT, Benedict N, Ebose EM (2022) Frequency, Pattern and Risk Factors for Red Blood Cell Alloimmunization in Multiply Transfused Cancer Patients in a Nigerian Tertiary Healthcare Facility in Nigeria. J Blood Disord Transfus. 13:485.
Copyright: © 2022 Onoriode OT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

