Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 4
Formulation Development & Characterization of Nintedanib loaded Micellar Drug Delivery System for improved therapy of Idiopathic Pulmonary Fibrosis
Rajesh Palva*, Ahmedraza R. Memon, Rajnikant Suthar and Musaratafrin SaiyedReceived: 01-Jul-2024, Manuscript No. jnmnt-24-26444; Editor assigned: 04-Jul-2024, Pre QC No. jnmnt-24-26444(PQ); Reviewed: 18-Jul-2024, QC No. jnmnt-24-26444(QC); Revised: 25-Jul-2024, Manuscript No. jnmnt-24-26444(R); Published: 31-Jul-2024, DOI: 10.35248/2157-7439.24.15.738
Abstract
Aim: The study aims to create a formulation of Nintedanib Esylate loaded Polymeric Micelles for treating Idiopathic pulmonary fibrosis (IPF) by administering the drug via the pulmonary route, preventing adverse side effects from oral administration. Experimental work: The study aimed to optimize polymeric micelles preparation using various polymers and drugs. The optimal polymer ratio was selected using a Central composite design, and the impact of polymer concentration on drug entry efficiency was evaluated. The optimized batch was used for drug release and Dry Powder Inhaler (DPI) preparation. Result and Discussion: Solvent emulsification yielded polymeric micelles with high encapsulation efficiency (%EE) increasing with polymer concentration. Particle size (10-100 nm) was confirmed. Central composite design optimized Solutol-HS 15 (400 mg): Soluplus (300 mg) ratio for maximum %EE (85.36%) and desired particle size (78.1 nm). TEM confirmed spherical micelles with core-shell structure. Micelles showed significantly higher drug release than pure drug, indicating improved dissolution. They were stable at lower temperatures (2-8°C) with minimal changes in %EE, size, and zeta potential over four weeks. The DPI formulation achieved high drug content (94.1%) with spherical micelles and showed greater lung binding affinity compared to pure API. Conclusion: The present study reports the development of Nintedanib Esylate-loaded DPI Polymeric Micelles may be a promising alternative to overcome the problem of conventional oral delivery of Nintedanib Esylate.
Keywords
Nintedanib esylate; Polymeric micelles; Dry powder inhaler; IPF
INTRODUCTION
Researchers worldwide are increasingly focusing on nanotechnology to improve the effectiveness, specificity, tolerability, and therapeutic index of medications. Polymeric Micelles (PMs) have gained interest as a multifunctional drug delivery method for medications with poor water solubility [1]. PMs are smaller, easier to prepare and sterilize, and have better solubilization capabilities than other nanocarriers [2]. They can bypass limitations of free pharmaceuticals and therapeutic compounds, such as low solubility, non-selective action, poor biodistribution, toxicity, and multi-drug resistance [3]. Researchers are exploring ways to load pharmaceuticals into polymeric micelles physically and chemically, with the potential to function as drug carriers by evading host defenses, maintaining extended circulation, and extravasating from the circulatory system [4]. Polymeric micelles are spherical nanoparticles formed by the self-assembly of amphiphilic polymers in water. They have a hydrophilic shell for stability and a hydrophobic core for carrying poorly soluble drugs, ensuring long circulation times. The manufacturing process and polymer chemistry crucially affect their final properties, safety, and effectiveness [5]. Above the Critical Micelle Concentration (CMC), amphiphilic polymers self-assemble into spherical micelles with a hydrophilic shell and a hydrophobic core. The hydrophobic core houses hydrophobic drugs, while the hydrophilic shell interacts with water, stabilizing the micelles in solution. Self-assembly is a natural process seen in structures like honeycombs, butterfly wings, and phospholipid cell walls. In scientific research, small molecules self-assemble into stable structures due to their mechanical and physical properties. Polymers, including graft, cyclic, and amphiphilic block copolymers, can self-assemble into micelles at a specific concentration known as the Critical Micelle Concentration (CMC). Micelles often use amphiphilic polymers, but polyelectrolytes and phospholipids can also form micelles [6].
Polymeric micelles can be prepared using various methods [7].
Dialysis Method: Dissolve polymer and drug in organic solvent, dialyze in water for 24 hours, filter to remove precipitates, and dilute with water [8,9].
O/W emulsion method: Dissolve drug-free polymers in water, add drug dissolved in organic solvent, stir to evaporate organic solvent, ultrafiltrate, and lyophilize [9,10].
Solvent evaporation method: Dissolve polymer and drug in organic solvent, evaporate to form a thin film, hydrate with water, and disperse using ultrasound.
Co-solvent evaporation method: Dissolve polymer in an organic solvent, mix with water, evaporate solvent, and centrifuge to remove precipitates.
Freeze-drying method: Dissolve polymers and drug in organic solvent, vortex in water, freezing, lyophilize, rehydrate, and centrifuge.
Polymeric micelles enhance solubility and bioavailability of hydrophobic drugs by incorporating them into their hydrophobic core. Their nano size improves gastrointestinal absorption. Targeted micelles accumulate in specific body compartments or diseased areas, enhancing drug efficacy. They utilize the Enhanced Permeability and Retention (EPR) effect to accumulate at pathological sites with leaky capillaries, reducing side effects. Stimuli-sensitive micelles release drugs in response to external or internal triggers. Acid-sensitive micelles exploit pH differences in the body for targeted drug delivery. Thermo-sensitive micelles release drugs with temperature changes, improving cellular absorption.
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by scar tissue in the lungs, leading to breathlessness and dry cough. It affects 10.7 per 100,000 men and 7.4 per 100,000 women, with higher prevalence in older adults and smokers. Risk factors include genetics, aging, male sex, lung Microbiome, cigarette smoke, and environmental exposures. IPF results from abnormal alveolar epithelial repair, causing fibrosis. Diagnosis involves imaging, biopsies, and multidisciplinary evaluation. Treatments include surgery, radiotherapy, chemotherapy, targeted therapy, immunotherapy, and supportive care. Drug therapies include N-acetyl-cysteine, pirfenidone, and nintedanib, with future potential treatments like simtuzumab, lebrikizumab, and STX-100.
The pulmonary drug delivery system offers a non-invasive route with rapid onset and targeted action in the lungs, using devices like MDIs, DPIs, and nebulizers. Advantages include a large absorption area, avoidance of gastrointestinal issues, and suitability for various drugs. Challenges include low efficiency, limited drug mass per dose, and dosing reproducibility issues. Drug transport across the lung epithelium varies by region, with the lower airways providing better absorption. Aerosol deposition is influenced by particle size and ventilation patterns. Clearance mechanisms include mucociliary and alveolar clearance, with device characteristics affecting particle size and uniformity. Nintedanib Esylate is a small molecule kinase inhibitor used to treat non-small cell lung cancer (NSCLC), interstitial lung disease linked to systemic sclerosis, and pulmonary fibrosis. It is one of the two approved therapies for idiopathic pulmonary fibrosis (IPF), primarily to slow lung function loss. Marketed as Ofev®, the oral bioavailability is low (~5%) due to poor solubility and first-pass metabolism. Nintedanib is a triple angiokinase inhibitor with the molecular formula C33H39N5O7S and was approved on October 15, 2014. It appears as a yellow crystalline solid and works by inhibiting multiple tyrosine kinases implicated in fibrosis and angiogenesis. It has low bioavailability (~4.7%), extensive tissue distribution, and is metabolized via esterases and UGT enzymes. Side effects include liver issues, gastrointestinal problems, and cardiovascular risks. The dosage is 300mg/day, taken twice daily.
Marketed formulations in India include Nintena, Nintib, Nindanib, Idofnib, and others in 100mg and 150mg capsule forms. Worldwide brand names include Cyendiv (100-150mg) and Ofev (100mg) capsules.
Koliphor HS 15® is a solubilizer and emulsifier, with low histamine release and suitable for sterilization, used in parenteral and ophthalmic formulations. Soluplus® is a novel solubilizer and crystallization inhibitor, enhancing bioavailability, ideal for hot melt extrusion, and used in solid oral dosage forms. Both excipients have low toxicity and regulatory approval.
Idiopathic pulmonary fibrosis (IPF) is a severe lung disease with limited treatments. Nintedanib esylate, an antifibrotic drug, shows promise but suffers from poor solubility and gastrointestinal side effects, reducing its effectiveness. Micellar drug delivery systems (MDDS) can potentially address these issues by enhancing solubility, improving lung targeting, providing sustained release, minimizing side effects, facilitating cellular uptake, and increasing stability. The aim is to develop a Nintedanib esylate-loaded MDDS to improve therapeutic efficacy and reduce side effects for IPF treatment. Objectives include selecting suitable excipients, developing and optimizing preparation methods, characterizing the MDDS, investigating in vitro release profiles, achieving targeted lung delivery with dry powder inhalers, evaluating stability, and studying drug deposition in rat lungs compared to the micelle formulation. This research could significantly improve IPF treatment outcomes.
METHODS AND MATERIALS
Nintedanib Esylate was received as a drug gift sample from Sun Pharmaceuticals Ltd., Vadodara;
Solutol® HS 15 and Soluplus® were also received as complimentary samples from BASF India Ltd., Mumbai; Chloroform, Ethanol, and n-hexane were purchased from Anachem Co. Ltd., Ahmedabad; Methanol was purchased from Astron Chemicals (India), Ahmedabad; Hydrochloric acid was purchased from S D Fine Chem Limited, Mumbai; Potassium dihydrogen phosphate and Sodium hydroxide were purchased from Molychem, Mumbai; and Triton X 100 was purchased from Loba Chemie Pvt. Ltd.
Preparation of nintedanib esylate loaded polymeric micelles
Solvent emulsification method was used for preparation of Polymeric Micelles. 200 mg of drug-free polymers were dissolved in 20 mL of distilled water, and the resulting mixture was homogenized by sonication for 30 seconds. In different beaker 5 mL of chloroform, the drug (5.0 mg) was dissolved. The organic solution was gradually added drop by drop to the polymer solution in distilled water while rotating vigorously at room temperature. In an open air system, the reaction mixture was stirred in order to evaporate the chloroform. Subsequently, the solution undergoes ultra filtration to remove low molecular weight contaminants and unbound medication before being lyophilized.
Optimization of polymeric micelles using central composite design (CCD)
The optimization of Nintedanib-loaded polymeric micelles was conducted using a Central Composite Design (CCD) and the Design-Expert-13 software. The formulation aimed to optimize particle size, polydispersity index (PDI), and percentage entrapment efficiency (%EE) using a 3-level, 2-factor CCD. Soluplus and Solutol HS 15 concentrations were chosen as the independent variables. The CCD design included center points and factorial design, allowing for the prediction of the second-order response surface and curvature.
The experiment followed a 3² factorial design with high, medium, and low levels coded as +1, 0, and -1, respectively, resulting in 9 runs. Two replicates at the center point, coded as 0, were performed to estimate experimental error, totaling 11 runs. The experimental design and statistical analysis were carried out using the trial version of Design-Expert-13.
The variables for CCD included low, medium, and high concentrations of Solutol HS 15 and Soluplus, set at 200, 300, and 400 mg respectively. The 11 batches as described prepared were evaluated based on their composition and were characterized for various parameters. Constant parameters included using chloroform as the solvent, 5 ml solvent volume, 800 rpm rotation speed, 6 hours rotation time, 5 mg drug weight, distilled water as the continuous media, and a final volume of 20 ml.
Characterization of optimized batch of polymeric micelles
Morphological evaluation
The Digital microscope (Teraview LCD Digital Microscope, Celestron) was used to observe the shape of the prepared Polymeric Micelles formulation.
Particle size, polydispersibility index (PDI)
The dynamic light scattering (DLS) method was utilized to determine the particle size and size distribution through the use of a computerized inspection system (Zetasizer, HAS 3000; Malvern Instruments, Malvern, United Kingdom).
Estimation of percentage of entrapment efficiency
A certain volume of Nintedanib loaded Polymeric Micelles was isolated by centrifugation at 17,000 rpm for 15 min using a micro centrifugation (RM-12C, REMI Sales & Engineering ltd.). Take 1 ml Micellar formulation and make up to volume 10 ml with methanol. After that obtain sample was dilute if required. Nintedanib was estimated by UV spectrophotometer (Shimadzu 1800 UV Visible, Japan) at λmax 390.5 nm.
Transmission electron microscopy (TEM)
Using Transmission electron microscopy (TEM), the surface morphology of the prepared Micelles was assessed. For TEM, a thin liquid layer was left behind when a drop of the final micelle dispersion was applied on a copper grid covered with carbon. After that, the films were examined and captured on camera using a TEM (JEM-100CX, Jeol, Tokyo, Japan).
In-vitro release study
The In-vitro release of Nintedanib dispersion and the Micellar formulation was compared by the dialysis method. 0.50 mg of Nintedanib esylate was precisely weighed, and dispersed in distilled water, and the Micellar formulation containing the same quantity (0.50 mg/ml) of Nintedanib esylate was placed in dialysis bags (Molecular weight cut-off MWCO 3000–4000 kDa), seal with thread, and placed in a 50 mL pH 7.4 phosphate buffer saline (PBS) release medium. Because the drug is lipid-soluble, 1 % Triton X-100 was added to the PBS to meet the sink condition. In-vitro release characteristics were tested at 37°C ± 2 °C & 100 rotations per minute (rpm) on magnetic stirrer. 2 mL solution was absorbed at time points 0.5, 1, 2, 3, 4, 5, 6, 24, 48 and 72 h, respectively, while the fresh pH 7.4 PBS was added. The solution was detected by UV spectrophotometer (Shimadzu 1800 UV Visible, Japan) at λmax 392.5 nm and release amounts at different time points were calculated according to the standard calibration curve.
In-vitro cell line study
The in-vitro cell line study evaluated the cytotoxicity of different formulation samples on the lung cancer cell line A549 using the MTT assay. A549 cells were cultured in complete media containing F12K medium, fetal bovine serum, antibiotics, and HEPES buffer. After reaching 80-90% confluence, cells were trypsinized, counted using a haemocytometer, and seeded at 5000-10000 cells per well in a 96-well plate, incubated for 24 hours.
For drug treatment, media was replaced with various concentrations (5, 10, 20, 40, 80, and 160 µg/ml) of formulation samples in triplicate, alongside untreated control wells. After 24 hours of incubation, the MTT assay was performed by adding freshly prepared MTT solution (5 mg/ml in PBS), incubating for 2-3 hours, and then solubilizing formazan crystals with DMSO overnight at 37°C. Absorbance at 570 nm was measured using a microplate spectrophotometer.
Cell viability was calculated based on absorbance values from treated and control wells, and the concentration inhibiting cell growth by 50% (IC50) for each formulation sample was determined through data analysis.
Stability study of polymeric micelles
A stability study of the Polymeric Micelles formulation was carried out under different circumstances. For the preliminary stability investigation, the optimized Micellar formulation was maintained under three distinct conditions.
Temperature: 2-8°C
Temperature: 25 °C ± 2°C
Temperature: 40 °C ± 2°C
Particle size determination, PDI, and percentage entrapment efficiency were examined in the sample. Another crucial stability metric to research is the zeta potential. It is well knowledge that the stability of a distributed system may be obtained by measuring the zeta potential. Zeta potential was so measured.
Thermocycling study
A Thermocycling stability study was performed to investigate the behaviour of the Micellar formulation under temperature variations. During the preliminary assessment, the optimized formulation was subjected to three cycles. Each cycle consisted of storing the formulation at 2-8°C ± 2°C for 48 hours, then at 25°C ± 2°C for 48 hours, and finally at 40°C ± 2°C for 48 hours.
Preparation & characterization of dry powder inhaler of polymeric micelles by lyophilizer
Nintedanib esylate loaded Optimized batch (F6) of polymeric micelles was lyophilized using mannitol as a carrier. For pre freezing 5 ml formulation containing Nintedanib esylate and 5 % of mannitol as a carrier were mixed and frozen at -70 °C in deep freezer (Quick Freezer, REMI Instrument) in Petridish. Frozen cake was loaded in lyophillizer (Martin CHRIST, alpha 1-2) by applying vacuum. Process parameter (Temperature -55 °C and Pressure 0.025 mbar) were fixed. After 7 hours free flowing lyophilized polymeric micelles were obtained. Lyophilized product was weighed.
Estimation of percentage of drug content
The total drug loading in the freeze-dried particle was analysed by dissolving the 10 mg formulation in methanol and mixed properly. After that the sample was filtered using syringe filter and absorbance taken at 390.5 nm using UV-Visible spectroscopy. Concentration of drug in the solution was calculated by:
% Drug content = (Amount of drug in the micelles) / (Total weight of drug)×100
Scanning electron microscopy of dry powder of polymeric micelles
The surface morphology of optimized DPI formulation was investigated by scanning electron microscopy (Navonanosem 450). Double sided carbon tape was affixed on aluminium stube. The powder sample of DPI formulation was sprinkled onto the tap. The aluminium stube were placed in the vacuum chamber of scanning electron microscope.
Liquid chromatography-mass spectrometry (LC-MS) method development of nintedanib esylate
Stock solutions of Nintedanib esylate (10 mg/mL) was created in methanol and stored at 4°C for a week. Fresh working standard solutions (Blank, 0.1, 0.2, 0.5, 1.0 & 10.0 ng/ml) were prepared using a mixture of acetonitrile: water (20:80 v/v) with 0.1% formic acid.
The unknown sample used in the chromatography separation, were formulated with methanol, acetonitrile, and water. Formic acid was also added to each eluent. These eluents were stored at 4°C.
A calibration curve of concentration vs. analyte peak area was prepared from the 6 standard solutions. Then the unknown analyte was run and an analyte peak area was found, by placing it in the calibration curve the concentration of the unknown sample was found.
In- vivo study
A drug tissue binding study was conducted to compare the DPI Micellar formulation and API of Nintedanib esylate, and it was tested using male Wistar rats. The Institutional Animal Ethics Committee (IAEC) of A. R. College of Pharmacy, Vallabh Vidyanagar, permitted the study protocol(protocolNo.: CCSEA/IAEC/ARCP/2023-24/06). A total of 12 male Wistar rats were procured from Torrent research centre, Ahmedabad. In accordance with CCSEA (The Committee for the Purpose of Control and Supervision of Experiments on Animals) Guidelines, animals were kept in normal conditions. Rats were kept in propylene cages (38 cm x 23 cm x 10 cm) filled with corn-cab as bedding material at the Animal House of A.R.CP. in a controlled environment of temperature 25°C ±2°C and 60% ± 5% relative humidity. During animal housing, animals had unrestricted access to food (pellet diet) and water (ad libitum). Four rats per cage were fed ad libitum with rodent chow and given free access to drinking water. The animals were acclimatized to the laboratory conditions for 5 days prior to the start of the real study. The study was carried out in the Department of Pharmacology, A. R. College of Pharmacy, Vallabh Vidyanagar. A total of 12 male Wistar rats weighing 270 ± 30 grams were split into two groups. Group 1 animals got 0.375 mg/kg of API (Nintedanib), whereas Group 2 received DPI Micellar formulation of Nintedanib via pulmonary route of administration by intratracheal administration under anesthesized condition (Ketamine: Xylazine: 50mg/kg: 5mg/kg). After 30 minutes of medication delivery, animals were sacrificed with a Ketamine overdose. Following that, the lung was isolated, filled with phosphate buffer (pH 7.4), and collected after 5 minutes. The collected solution was used to estimate the free drug concentration. After that, the lung homogenate was produced and centrifuged at 5000 rpm for 15 minutes. The supernatant layer of homogenate was collected and tested for tissue-bound drug concentrations using the LC-MS technique. The results of the tissue-bound drug concentration and free drug concentration of DPI Micellar Formulation (Batch no. F6) were compared with API.
Drug and excipient compatibility study
Drug and excipient compatibility studies are crucial for designing effective dosage forms, considering the physical, chemical, and biological properties of both components. These studies help in selecting excipients that are compatible with the drug during formulation. Fourier Transform Infrared Spectroscopy (FTIR) was employed to evaluate compatibility by comparing spectra of pure Nintedanib esylate drug, micellar solution, freeze-dried formulation, and placebo solution of polymeric micelles against the drug alone. This analysis aimed to identify any interactions between the drug and excipients that could affect formulation stability or efficacy.
RESULTS
Preparation of nintedanib esylate loaded polymeric micelles
Nintedanib esylate loaded polymeric micelles were successfully developed using the solvent emulsification method as shown in [Figure 1].
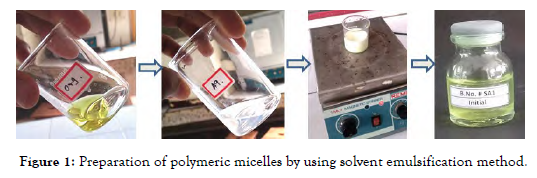
Figure 1: Preparation of polymeric micelles by using solvent emulsification method.
Optimization of polymeric micelles using central composite design (CCD)
CCD batches of Polymeric Micelles were successfully developed as shown in [Figure 2]. The combination of particle size, PDI, and entrapment efficiency in Batch F6 demonstrated superior characteristics compared to other batches in the CCD series. These attributes collectively contribute to improved stability, uniformity, and drug delivery efficiency of the Polymeric Micelles.

Figure 2: Pictures of CCD batches (F1- F11). Figure 3: Microscopic image of batch F6. Figure 4: Particle size & PDI value of batch F6. Particle size,
Characterization of optimized batch of polymeric micelles
Morphological evaluation:
The Digital microscope (Teraview LCD Digital Microscope, Celestron) was used to observe the shape of the optimized Polymeric Micelles formulation [Figure 3].

Figure 3: Microscopic image of batch F6.
Particle size, polydispersibility index (PDI):
Thedynamic light scattering (DLS) method was utilized to determine the particlesize and size distribution through the use of a computerized inspection system (Zetasizer, HAS 3000; Malvern Instruments, Malvern, United Kingdom). As shown in [Figure 4] Particle size & PDI of Batch F6 were found to be 78.10 nm & 0.357 respectively.
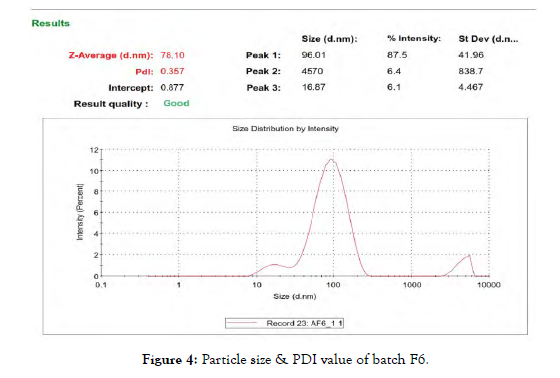
Figure 4: Particle size & PDI value of batch F6.
Estimation of percentage of entrapment efficiency:
A certain volume of Nintedanib loaded Polymeric Micelles was isolated by centrifugation at 17,000 rpm for 15 min using a micro centrifugation (RM-12C, REMI Sales & Engineering ltd.). Take 1 ml Micellar formulation and make up to volume 10 ml with methanol. After that obtain sample was dilute if required. Nintedanib was estimated by UV spectrophotometer (Shimadzu 1800 UV Visible, Japan) at λmax 390.5 nm. The % EE of Batch F6 was found to be 85.36 %.
Transmission electron microscopy (TEM):
Transmission electron microscopy (TEM) at high magnification revealed spherical Micelles as shown in [Figure 5] this morphology perfectly aligned with the self-assembled structure of Polymeric amphiphiles. The images showed a core region with low electron density enveloped by a shell with higher electron density, indicating successful formation of core-shell Micelles. The darker contrast at the periphery was due to the shell composed of hydrophilic head segments of the polymers.
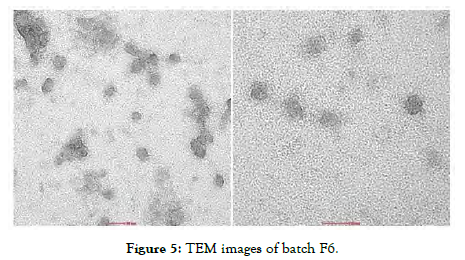
Figure 5: TEM images of batch F6.
In-vitro release study:
The results as shown in Tab.3 demonstrate that the Micellar formulation achieved a significantly higher release of Nintedanib esylate compared to the pure drug dispersion. Notably, Micellar formulation released 94.81% of the Nintedanib esylate within 72 hours. This release shows an enhanced dissolution rate as shown in [Figure 6] which suggests improved bioavailability of the drug by Micellar formulation compared to pure drug dispersion.
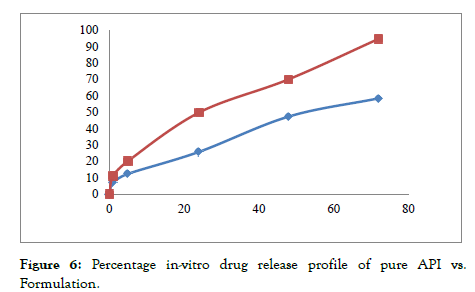
Figure 6: Percentage in-vitro drug release profile of pure API vs. Formulation.
In-vitro cell line study:
The Half Maximal Inhibitory Concentration (IC50) of Nintedanib API obtained in this study was 15.54 µg/ml and Nintedanib loaded Micellar formulation was 5.77 µg/ml. Results obtained were indicating that Nintedanib Micellar formulation was more toxic for cell line compared to Nintedanib API. It could also be seen from the results of A549 cell image as shown in [Figure 7, Figure 8 and Figure 9] using inverted microscope after being given pure Nintedanib treatment and Nintedanib Micellar formulation, it was seen that A549 cells with Nintedanib Micellar formulation treatment appeared to have more deaths than compared with Nintedanib API treatment.
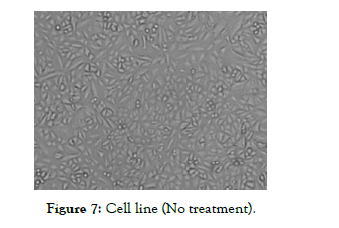
Figure 7: Cell line (No treatment).
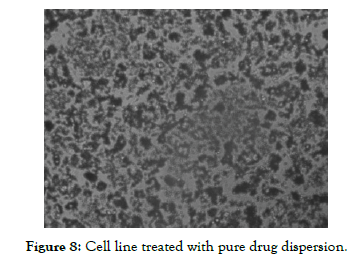
Figure 8: Cell line treated with pure drug dispersion.
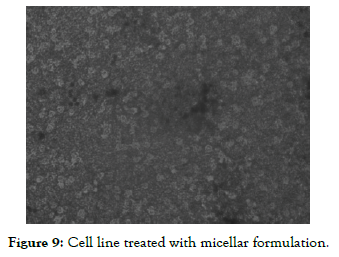
Figure 9: Cell line treated with micellar formulation.
Stability study of polymeric micelles:
Particle size, size distribution (PDI), zeta potential and % entrapment efficiency were monitored after completion of 1st week, 2nd week and 4th week.
Thermocycling study:
Based on the stability data, it is evident that the product is not remaining stable over time and is degrading. Therefore, we will lyophilize it to convert it into a powder form, which will enhance its stability.
Preparation & characterization of dry powder inhaler of polymeric micelles by freeze drying instrument:
The image shown in [Figure 10] is a dry powder inhaler prepared in which a mannitol was used as a carrier. The image shows the Micellar solution being formed into a Micellar dry cake.

Figure 10: Freeze dried formulation.
Estimation of percentage of drug content:
The drug content of DPI Polymeric Micelles was performed. The drug content of DPI formulation was found to be 94.1%.
Scanning electron microscopy of Dry powder of polymeric micelles:
The SEM micrography of optimize Nintedanib esylate-loaded Micellar formulation F6 are given in Figure 11.

Figure 11: SEM images of freeze dried powder.
Liquid chromatography-mass spectrometry (LC-MS) method development of nintedanib esylate for in-vivo study:
Use the equation for the calibration curve as shown in Figure 12 to calculate the concentration of the unknown sample based on its peak area.
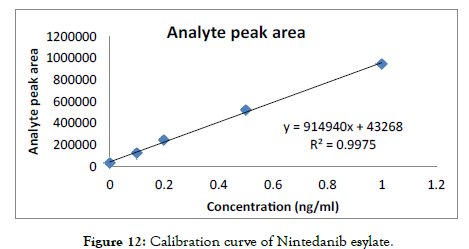
Figure 12: Calibration curve of Nintedanib esylate.
In- vivo study:
The images shown in Figure 13 describe administration of Polymeric micelles by intra tracheal route of administration.
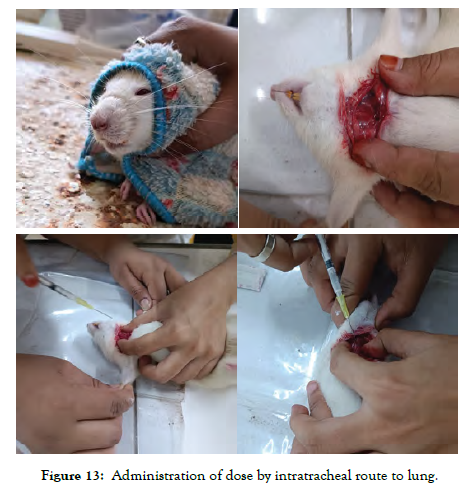
Figure 13: Administration of dose by intratracheal route to lung.
The Lungs shown in Figure 14 and Figure 15] describes comparison of lungs of rats after administration of Pure API vs. Formulation:
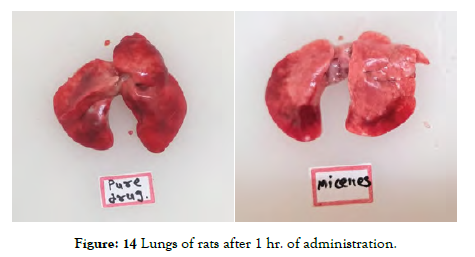
Figure 14: Lungs of rats after 1 hr. of administration.
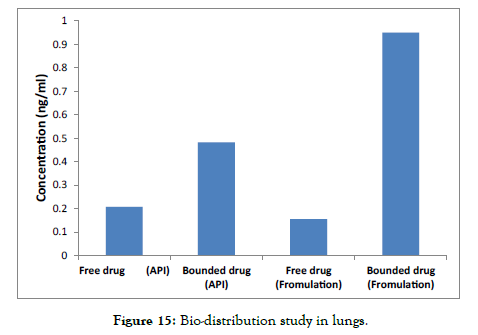
Figure 15: Bio-distribution study in lungs.
Preliminary visual observations indicated that Polymeric Micelles exhibited superior distribution in the lungs compared to the pure drug.
The data revealed a higher concentration of free drug in the pure API form, whereas the Formulation showed a lower concentration of free drug.
Additionally, the concentration of tissue-bound drug was significantly higher in the Formulation and lower in the pure drug.
These findings suggest that the drug binding affinity to tissue is enhanced in the Micellar Formulation compared to the pure drug.
Drug and excipient compatibility study:
In order to investigate the possible interaction between drug and excipients, FTIR studies were carried out. Spectrum of pure drug was compared with spectra of Micellar solution, freeze dried product and placebo of all excipients. Spectra are as follow [Figure 16].
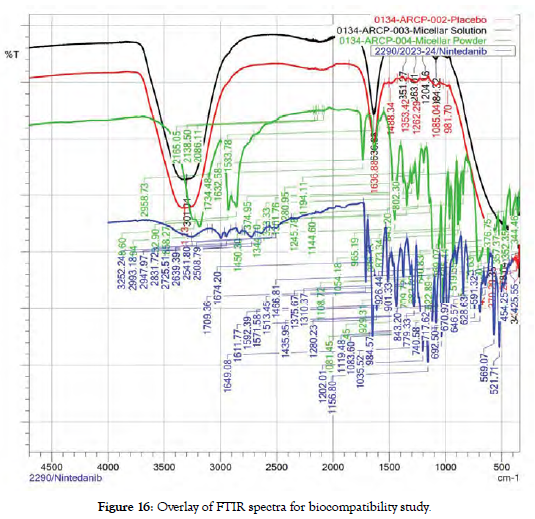
Figure 16: Overlay of FTIR spectra for biocompatibility study.
Placebo physical mixture: The absence of characteristic peaks of Nintedanib esylate in placebo suggests no interaction between the drug and excipients, indicating compatibility between Nintedanib esylate and the formulation components.
Freeze-dried formulation: The presence of majority peaks matching those of Nintedanib esylate, along with the observation of characteristic peaks of the drug, indicates that Nintedanib esylate is compatible with the formulation. This suggests that the formulation process does not significantly alter the chemical structure of Nintedanib esylate, and it remains stable within the formulation.
CONCLUSION
Improved drug delivery: Nintedanib esylate-loaded Polymeric Micelles were successfully developed to address the limitations of the original drug, including low bioavailability and undesirable side effects.
Enhanced solubility: The formulation utilizes Solutol HS 15 and Soluplus to overcome low solubility of Nintedanib esylate, potentially improving bioavailability.
Targeted drug delivery: Polymeric Micelles offer targeted delivery of Nintedanib esylate to lung cancer cells, potentially reducing side effects associated with non-targeted distribution.
Increased cytotoxicity: In-vitro cell line studies demonstrated that the Micellar formulation is more toxic to lung cancer cells compared to pure Nintedanib esylate drug.
Optimized formulation: Central composite design was employed to optimize the Micelles for particle size, polydispersity index (PDI), and entrapment efficiency. The final formulation exhibited a particle size of 78.1 nm, PDI of 0.357, and entrapment efficiency of 85.36%.
Stable formulation: Stability studies indicate that the formulation meets
pre-determined specifications, ensuring consistent drug delivery over time.
Improved tissue binding: In-vivo data suggests that the Micellar formulation achieves higher tissue binding compared to pure Nintedanib esylate, potentially leading to more effective treatment.
Dry powder inhaler compatibility: The formulation was successfully incorporated into a dry powder inhaler, potentially offering a convenient and efficient delivery method for lung cancer treatment.
Overall, this research demonstrates the successful development of a Nintedanib esylate loaded polymeric micelles formulation with improved therapeutic potential for lung cancer treatment.
REFERENCES
- Shi Y, Zhao R, Zhang Q, Wei Y, Zhao Y. Molecular Dynamics Simulation-Guided Rational Design of Polymer Nanocarriers for Drug Delivery. Nanoscale Res Lett. 2019; 14(1): 372.
- Zhang Y, Zhang R, Su H. Interaction of ligand-bound nanoparticles with cell membranes: from specific binding to nonspecific penetration. Langmuir. 2019; 35(3): 677-688.
- Sarvi F, Mahmoodzadeh A, Kiani M. A novel mathematical model for the effective drug administration and the drug concentration in brain tumor. J Drug Deliv Sci Technol. 2021; 61: 102275.
- Ranade A R, Zhang H, Drueckhammer D G. 3D-QSAR Models for Arylcarboxamides as Potent and Selective Human Beta 3 Adrenergic Receptor Agonists. J Chem Inf Model. 2018; 58(7): 1476-1487.
- Jiang H, Du L, Li L. Multiscale modeling and experimental study on the self-assembly of telo-dendrimers. J Mol Model. 2021; 27(1): 1-13.
- Bai Y, Li Y, Zhang G, Hou X. Understanding the mechanisms of graphene-based nanomaterials as drug carriers: a theoretical perspective. Phys Chem Chem Phys. 2020; 22(29): 16544-16556.
- Garg S, Liao W, El-Kadi A O. Understanding the interaction of dietary polyphenols with mammalian drug metabolizing enzymes. Curr Drug Metab. 2020; 21(6): 416-429.
- Zhang S, Bellinger A, Genshaft A. A blood–brain barrier–penetrating gut-microbe-derived molecule that enhances amyloid beta deposition and cognitive impairment in a mouse model. Science Advances. 2021; 7(23): eabf1773.
- Tong R, Chiang H H, Kohane D S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc Natl Acad Sci. 2020; 110(46): 19048-19053.
- Li C, Li Q, Zeng Z, Zhou X. Dispersions of hydrophobic magnetite nanoparticles in nonpolar solvents with different surfactants: the effect of surfactant chain length and surfactant concentration. J Colloid Interface Sci. 2019; 539: 567-577.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Rajesh P (2024) Formulation Development & Characterization of Nintedanib loaded Micellar Drug Delivery System for Improved Therapy of Idiopathic Pulmonary Fibrosis. J Nanomed Nanotech. 15: 738.
Copyright: ©2024 Rajesh P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


