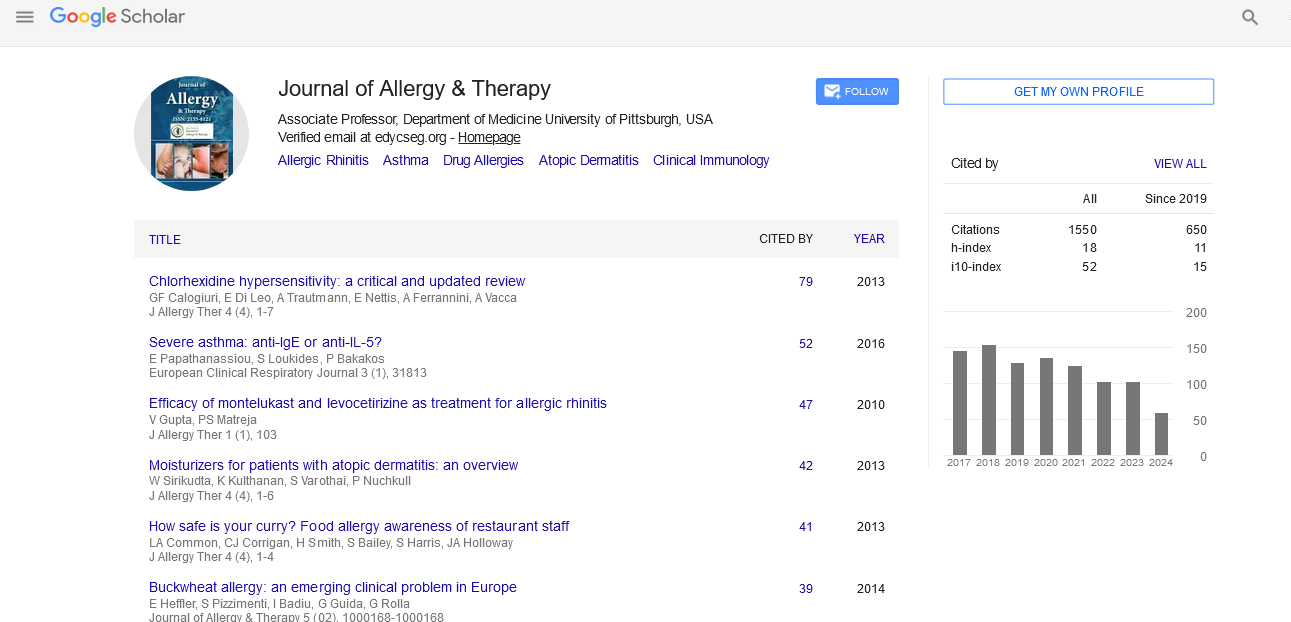
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2025) Volume 16, Issue 1
Food Safety: Labels and Eliciting Dose
Elena Galli*Received: 02-Jun-2020, Manuscript No. JAT-24-4703; Editor assigned: 05-Jun-2020, Pre QC No. JAT-24-4703 (PQ); Reviewed: 19-Jun-2020, QC No. JAT-24-4703; Revised: 15-Jul-2024, Manuscript No. JAT-24-4703 (R); Published: 12-Aug-2024
Abstract
Food Allergy (FA) has become an increasingly recognized global health concern, particularly in developing countries. It is a life-threatening condition with a world-wide rise in prevalence, estimated to be present in approximately 2%-10% of the population. It is important to note that reactions can be triggered not only by food ingestion but by inhalation and skin contact as well.
Keywords
Elicit dose; Food safety; Labels; Allergens
Introduction
Clinical presentation involves a large spectrum of symptoms ranging from dermatological and gastrointestinal to respiratory and circulatory. FA is also the primary cause of anaphylaxis. The clinical management of food allergies includes short-term interventions to manage acute reactions and long-term strategies to minimize the risk of further reactions [1]. Dietary restrictions should have the aim of eliminating the trigger food allergen(s) and need to be tailored to meet the individual’s specific allergic and nutritional needs. The strict avoidance of food allergens plays a key role in managing the symptoms. However, as inadvertent ingestion occurs frequently in allergic patients, with severe and potentially fatal reactions, this is a main source of anxiety with an obvious impact on the quality of life.
Literature Review
Indeed, allergens are ubiquitous elements in food manufacturing environments and a hidden allergen can be ingested inadvertently as a result of cross-contamination during processing, storage or transportation of food.
The allergen can be identified through molecular biology techniques, such as Enzyme-Linked Immunosorbent Assay (ELISA), which has a specific protein as a target or Polymerase Chain Reaction (PCR), with a specific nucleic acid as a target. The limitations of these techniques are numerous: The complexity of food matrix, the absence of animal and plant genome database, the presence of contaminants, the variability of the antibodies in any single commercial kit. A recent reliable technique known as mass spectrometry has proven helpful in overcoming these limits. As proteomics techniques are able to determine the exact amount of protein contained in food, the dose threshold can now be better assessed in even the smallest amount of food and therefore may be lower than the amount theoretically necessary to provoke an allergic reaction.
Most governments in the world have legislated food labeling to help allergic patients and to prevent inadvertent allergen ingestion.
Labeling is defined as "all the information, indications, trademarks or trademarks, images or symbols referring to the food product and appearing directly on the packaging or on a label affixed to it or on the closing device or on signs, rings, clamps tied to the product itself, or failing that, on the documents accompanying foodstuffs.
In the European Union (EU), the first labeling directive was issued in September 2001. The following Directives 2003/89/EC and 2006/142/EC require 12 food items to appear on the label of prepackaged foods: cereals containing gluten, crustaceans, egg, fish, peanut, soy, milk (including lactose), nuts, mustard, sesame seeds, celery and sulfite >10 mg/kg .
These directives ensure that all consumers receive comprehensive ingredient label information making it easier for people with food allergies to identify the ingredients they need to avoid. Allergen-labeling rules changed in December 2014 with the implementation of the Food Information for consumers regulation (EU) No.1169/2011 [2]. This new regulation requires that 14 food items be listed on the labels. Lupins and mollusks have also been identified as potential allergens.
This list is subject to constant revision by the European Food Safety Agency (EFSA), on the basis of the most recent data in scientific literature. Pine nuts don’t appear on the list, although they may be responsible for serious reactions.
This new regulation has further improved allergen food labeling by introducing the requirement that all allergen information be provided whether the food is prepackaged or not. Providing information on these substances with regard to non-prepackaged foods was also made obligatory. In addition, all restaurants, bakeries and supermarkets must furnish information on allergenic ingredients upon request [3].
Discussion
Moreover, in order to ensure that all potentially allergenic substances stand out so as to be easily identifiable by consumers, a minimum font size (larger than the other ingredients), style and color was established by EU No 1169/2011 as well as the obligation that the name of the substance be repeated whenever present as an ingredient in any product. Reading food labels is therefore an important element in the prevention strategy for the management of food allergies, with the caveat that one must bear in mind that the presence of an allergen in any given product cannot be completely ruled out solely based on the grounds that it is not mentioned on the label.
Although there is an obligation to declare any ingredient on the label that may cause allergies, a similar obligation has not yet been introduced with regard to the possible inadvertent contact of the product with allergens [4].
Unfortunately , legislation does not take into consideration the possibility of food allergens present from cross-contamination or cross-contact in the supply chain or due to shared production facilities. Allergens can inadvertently end up in food as a result of contamination during the transport, storage or processing of food.
To date, there is no obligation in the EU to label any allergens present in foods that are not part of the ingredients in the product, but which may be present due to cross-contamination.
Consequently, many food industries have introduced the use of Precautionary Allergen Labeling (PAL) to manage and alert the consumers to the possible inadvertent presence of allergens in food.
Precautionary Allergen Labeling (PAL)
Mandatory allergen labeling is used when the allergen is an intentionally added ingredient, but the risk to consumers of unintentional ingestion of food containing hidden allergens is still far too high and is not completely regulated by legislation. In a globalized economy, ensuring food safety is a growing challenge for manufacturers, especially given that ingredients and technologies are sourced worldwide from multiple business partners which increases the chance for errors and obvious consequences [5]. Therefore, voluntary precautionary allergen labeling is increasingly used by the food manufacturing industry to alert allergic consumers to the possible presence of allergens.
As PAL is not yet standardized the wording used by factories on their labels is often ambiguous and contains little clear information as to the presence of potential allergens, thus causing confusion in patients and discrediting the labels as disclaimers of liability. In Europe and in United States there are several variants.
We can say that to date these labels can actually be counterproductive for consumers. In fact, paradoxically they can lead to increased risk-taking. Consumers think that the different words used in PAL reflect a hierarchy of risk of reaction, for example “may contain” indicating a higher risk than “may contain traces”. So when a product with a PAL wording “may contain” does not cause a reaction, the patient may feel either the product is safe or consider himself less allergic and ignore PAL.
In particular, the term “traces” should be abandoned because the term fails to indicate the possibility of significant amounts of allergens. In fact, the potential for food allergen crosscontamination is not uniform across a food product line and when any single allergen risk management fails this may result in significant levels of unintended allergen presence. A recent French study has demonstrated that 39% of 17039 foods contain PALs but these statements are disregarded by a large majority of patients and parents of allergic children, including those with a past history of anaphylaxis. These data have been confirmed in numerous studies.
Further confusion may be caused by the practice of over labeling. As an example, the requirement of labeling lactose as allergenic for individuals allergic to cow's milk contradicts the fact that this sugar has never been reported to determine allergic reactions after ingestion among children allergic to cow milk.
Evaluated consumer preferences for different types of food labels using a survey (discrete choice experiment questionnaire design) in 1100 Canadian allergic patients. Consumers identified preferences for:
• Standardized precautionary and safety statements and
symbols.
• The use of symbols as opposed to statements.
• Little or no increase in cost for improved food allergen
labeling.
A small proportion of respondents appeared to be indifferent and were not likely to consider allergens when buying foods.
Followed an adult cohort with food allergies for one year to study frequency, severity and causes of accidental reactions [6]. A total of 157 patients completed the trial and 73/157 reported 151 accidental allergic reactions during the 1 year follow-up. Different ranges of food products were reported to cause accidental reactions. Patients attributed their reaction to a specific product (bread, cookies, chocolates, meat and meat products or fruits) in 78% (118/151 reactions) of the reported cases while 47% (56/118) was attributed to a wide range of other products. It is important to note that 59% (30/51) of the products analyzed did not have PAL. Furthermore, it was noted that some products which were labeled as containing peanut, egg and soy, contained none of these allergens, whereas the product in which peanut was detected did not have PAL statement.
Although cross-contamination may be the major source of undeclared allergens in food, research has shown that in products with a PAL statement, a large proportion of these may not contain sufficient allergens to cause an allergic reaction. It is evident that there is a poor relationship between the presence or absence of PAL and the actual risk of reaction.
Eliciting dose
In order to better develop PAL action directives, we need to address the lack of a definition of the threshold dose of food allergens above which a reaction is likely to occur.
The Minimal Eliciting Dose (MED) of allergen is defined as the minimal dose required to trigger symptoms in an allergic patient. On the other hand, the highest dose of allergens that doesn’t elicit an allergic reaction is called the no-observedadverse- effect level (NOAEL) [7].
The Eliciting Dose (ED) and in particular ED01, ED05 and ED10 are estimated population thresholds that are predicted to trigger a reaction in 1%, 5% and 10% of allergic subjects, respectively.
Standardization of Eliciting Threshold Doses (EDs) not only has the potential to improve risk management by identifying the most highly sensitive food allergic individuals, but has implications for public health measures and precautionary food allergen labeling as well [8]. By observing and recording data from OFC, the ED can be determined and used to estimate clinical thresholds for patients or the smallest amount of a food allergen to which a patient would react. After observation of a group of patients, an estimation of population-based thresholds can then be calculated.
EDs have been established for peanuts, hazelnuts, cashews, cow’s milk and hen’s eggs.
Immunotherapy and double blind placebo controlled food challenge studies have shown that baseline allergen thresholds can vary up to ten fold. Population thresholds have been estimated for numerous allergens and extremely low eliciting doses (as low as 0.2 mg of peanut protein) have been described. Numerous factors affect food allergen thresholds. Graham and Eigenmann considered the following: Nature of allergen, age, cofactors (alcohol, medication, drug, infections and exercise) and protocol used to determine threshold dose [9]. Studies have shown that a concomitant history of asthma increases the risk of having a severe reaction. Although many co-factors have been shown to affect the severity of the reaction, more data is needed to provide more accurate reproducibility of the eliciting dose.
The mere fact that a mathematical risk is not predictive of a reaction in an individual patient needs to be taken into consideration when treating patients in clinical practice. Clearly, even severely allergic patients can tolerate higher thresholds than previously expected, with the ED05 being sufficiently high of a threshold to avoid allergic reactions in even severely allergic individuals, as shown by the PATS study.
The VITAL'S effort
Australia was the first country that attempted to standardize PAL, by developing, through the Australian Food and Grocery Council, a system for risk assessment by industry called VITAL (Voluntary Incidental Trace Allergen Labelling). The first version was released in June 2007 with the aim of providing a risk-based assessment process to determine when a precautionary allergen labeling statement (May be present) is appropriate.
In 2010 the Allergen Bureau initiated a review of VITAL, building on a commitment to continue to invest in VITAL to ensure that it remains a relevant tool for industry. The Allergen Bureau recognized a need to form an expert scientific panel to review the science underpinning the VITAL action level grid that contains concentrations of cross contact allergens, called action levels which determine when it is appropriate to use a precautionary allergen statement [10]. The VITAL action level grid is a key component of this program and is incorporated into the VITAL calculator. The action level concentrations are determined using the reference dose information set by the VITAL.
The scientific review was a critical body of work to ensure that the action levels protect the allergic consumer by enabling industry to make appropriate precautionary labeling decisions and provide clear and consistent consumer communication through the use (or not) of the “may be present” which is the only statement that is allowed to be used to indicate that the VITAL 2.0 process has been used.
The authors of VITAL 2.0 established a baseline dose for 11 common food allergies by studying a statistical model of “dose distribution” of the thresholds of reaction (scientific evidence from reference doses and statistical dose-distribution modeling of individual thresholds for reactions among food allergic patients) during clinical oral food challenges, by combining the data of patients included in over 55 studies relative to “oral food challenges”. On the basis of the data collected, the identification of the threshold above which a warning on the food label must be displayed, was proposed.
If a product contains an allergen above a defined action level, then this particular product carries the PAL statement "may be present", which is the only statement that is used to indicate that the VITAL 2.0 process has been used, as we pointed out earlier.
Assessment of action levels for a particular product, however, does not involve actual quantification of an allergen present in the final commercial product.
The VITAL program is not applicable to foods specifically formulated for infants or other population groups that may have heightened sensitivity to the presence of allergens [11].
The major limitation of VITAL 2.0 is that the consumers are not aware whether or not a product has been assessed because any product lower than the defined action level does not receive a PAL label and even if the product has been assessed through the VITAL 2.0 process, any product that hasn’t undergone this process carries no information as to that effect.
Aromas
The use of the word "aromas" is another problem in the consultation of labels. This term refers to chemical substances which can be obtained by synthesis or extraction of natural products, in particular from aromatic plants [12]. It is a vast heterogeneous group of substances with characteristics quite different one from the other. Numbering in the thousands, the best known ones are those extracted from rosemary, parsley, fennel, anise, sage, etc. In view of the widespread use of flavouring substances, the European Union has entrusted EFSA with the task of assessing their safety on the basis of available scientific information. This study has made it possible to evaluate positively and therefore to consider “acceptable” more than 2000 flavouring substances which have been indicated. In any case, the presence or absence of natural and/or synthetic flavourings is indicated on food labels. Although this is a very important piece of information, it is practically impossible to verify the accuracy of the label since there are no control methods capable of precisely identifying the presence and concentration of the individual substances.
Reading and interpreting labels is therefore a fundamental practice in the management of food allergies and should consequently be taught to parents and at the right age, to young patients with food allergy, to provide advice on how to effectively decipher food labels.
Conclusion
It has been demonstrated that food allergies affect the quality of life of patients and their relatives. Patients and families coping with this problem often depend on others to ascertain the safety of foods. Parents of patients with food allergies often spend a considerable amount of time at the supermarket reading labels numerous times over to make sure they have understood them. Healthcare professionals play an important role in educating their patients on how to utilize PAL as part of their own risk management strategy. To date, interpreting food labels is the most important risk management tool in reducing exposure to allergens, but consumers find them difficult to understand and are often wary of their accuracy.
There is an urgent need for a comprehensive global approach to improving food labeling. The goal of regulating PAL at an international level must be set in order to give our patients and their families the peace of mind they deserve together with the ability to confidently and effectively manage their food allergies. We need to work towards enacting legislation to govern the standardization and application of PAL and the development of trials to establish recognized threshold levels to give patients a dependable and accurate source of information they can trust.
In the era of molecular diagnosis of food allergies, laboratory techniques allow for precise diagnoses, identifying with precision the protein that triggers allergic reactions. However, this does not yet go hand in hand with reliable information found on the packaging regarding the content of foods. International communities have made great strides in enacting increasingly stringent laws, but there is still more to be done. The inability to correctly interpret food labels is a problem experienced worldwide. These concerns are not only faced daily by consumers but also by employees in the food industry who must responsibly inform their clients as to what their food contains. The main goal for the future is to fill this gap in order to allow an improvement in the quality of life of patients with food allergies.
References
- Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers. 2018;4(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Sicherer SH, Allen K, Lack G, Taylor SL, Donovan SM, Oria M. Critical issues in food allergy: A national academies consensus report. Pediatrics. 2017;140(2):e20170194.
[Crossref] [Google Scholar] [PubMed]
- Kim JS, Sicherer SH. Living with food allergy: Allergen avoidance. Pediatr Clin North Am. 2011;58(2):459-470.
[Crossref] [Google Scholar] [PubMed]
- Alvarez-Perea A, Tanno LK, Baeza ML. How to manage anaphylaxis in primary care. Clin Transl Allergy. 2017;7:45.
[Crossref] [Google Scholar] [PubMed]
- Muraro A, Agache I, Clark A, Sheikh A, Roberts G, Akdis CA, et al. EAACI food allergy and anaphylaxis guidelines: Managing patients with food allergy in the community. Allergy. 2014;69(8):1046-1057.
[Crossref] [Google Scholar] [PubMed]
- Pascal M, Kamath SD, Faber M. Diagnosis and management of shellfish allergy: Current approach and future needs. Curr Treat Options Allergy. 2018;5:470-486.
- Pesek RD, Jones SM. Current and emerging therapies for IgE-mediated food allergy. Curr Allergy Asthma Rep. 2016;16:1-9.
[Crossref] [Google Scholar] [PubMed]
- Zurzolo GA, Allen KJ, Peters RL, Tang ML, Dharmage S, de Courten M, et al. Anaphylaxis to packaged foods in Australasia. J Paediatr Child Health. 2018;54(5):551-555.
[Crossref][Google Scholar] [PubMed]
- Antolín-Amerigo D, Manso L, Caminati M, de la Hoz Caballer B, Cerecedo I, Muriel A, et al. Quality of life in patients with food allergy. Clin Mol Allergy. 2016;14:1-0.
[Crossref] [Google Scholar] [PubMed]
- Blom WM, Michelsen-Huisman AD, van Os-Medendorp H, van Duijn G, Versluis A, Castenmiller JJ, et al. Accidental food allergy reactions: Products and undeclared ingredients. J Allergy Clin Immunol. 2018;142(3):865-875.
[Crossref] [Google Scholar] [PubMed]
- Di Girolamo F, Muraca M, Mazzina O, Lante I, Dahdah L. Proteomic applications in food allergy: Food allergenomics. Curr Opin Allergy Clin Immunol. 2015;15(3):259-266.
[Crossref] [Google Scholar] [PubMed]
- Fierro V, Di Girolamo F, Marzano V, Dahdah L, Mennini M. Food labeling issues in patients with severe food allergies: Solving a hamlet-like doubt. Curr Opin Allergy Clin Immunol. 2017;17(3):204-211.
[Crossref] [Google Scholar] [PubMed]
Citation: Galli E (2024) Food Safety: Labels and Eliciting Dose. J Allergy Ther. 15:398.
Copyright: © 2024 Galli E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.