Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 10
Field Evaluation of Bread Wheat (Triticum aestivum L.) Genotypes for Stripe Rust (Puccinia striiformis W.) Resistance in Arsi Highlands of Oromia Region, South -Eastern-Ethiopia
Getnet Muche Abebele1, Merkuz Abera Admasu2 and Bekele Hundie Agdu1*2Department of Plant Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Received: 04-Sep-2020 Published: 26-Oct-2020, DOI: 10.35248/2157-7471.20.11.521
Abstract
Wheat is one of the world's most important crops whose grain production is increasing year after year. However, the emerged virulent stripe rust races at one point of the world spread to the rest of wheat producing countries by wind as well as human travels and damaged popular resistant wheat cultivars thereby posed food insecurity. This study was carried out with the aim to identify possible sources of stripe rust resistance among Ethiopian bread wheat pipelines for durable resistance breeding. Twenty-eight advanced bread wheat pipelines, local susceptible and resistant check cultivars Kubsa and Wane respectively were field tested in randomized complete block design with three replications across two stripe rust hot-spot locations for their slow rusting characteristics. Slow rusting resistance at the adultplant stage was assessed through the determination of final rust severity (FRS), average coefficient of infection (ACI), and relative area under disease progressive curve (rAUDPC). Among the twenty-eight, 24, 2 and 2 genotypes displayed high, moderate and low level of slow rusting over two locations respectively. The results revealed that wheat lines, ETBW- 8858, ETBW-8870, ETBW-8583, ETBW-8668, ETBW-8595, ETBW-8684, ETBW-9548, ETBW-9549, ETBW-9552, ETBW-9554, ETBW-9558, ETBW-9559, ETBW-9560, ETBW-875, ETBW-8802, ETBW-8862, ETBW- 8804, ETBW-8896, ETBW-9556, ETBW-9557, ETBW-8991,ETBW-9486,ETBW-9556 and ETBW-9561 had low values of FRS, ACI and rAUDPC and were regarded as good slow rusting lines. Strong positive correlations were observed between different parameters of slow rusting. As compared with susceptible, resistant check variety and other test lines, three lines namely. ETBW-8684; ETBW-9558 and ETBW-8751 are high yielders and could be released for production. Twenty-four lines with high and moderate levels of slow rusting and expected to possess both major and minor resistance genes could be used for durable stripe rust resistance breeding in wheat. However, is advised to postulate inherent resistance genes in these lines and their seedling susceptibility for providing fruitful recommendations.
Keywords
FRS; Inherit resistant genes; Adult plat resistance; Slow rusting; Stripe rust
Introduction
Wheat (Triticum aestivum L.) is one of the most important food grain crops both in production and nutrition worldwide [1], being cultivated on 15.8% of the arable land. Ethiopia is the second largest wheat producer in sub-Saharan Africa next to Egypt [2]. The current total area devoted to wheat production in Ethiopia is estimated to be over 1.6 million hectare [3]. Despite the large area under wheat, average yield in Ethiopia is estimated around 2.97 t ha-1 that is far less than potential yields of 8 to 10 t ha-1 [3].
Worldwide, wheat production is hindered by several factors of these low productivity is partially attributed to the prevalence of wheat rust diseases, deficient in durable resistant variety. Of the rusts, stripe rustis a globally, in more than 60 countries [4,5] continue to cause losses, often major in various parts of the world [6] and are current threats to world food security [7].
Recently, stripe rust has become a serious threat to wheat, especially in case of highly susceptible varieties; losses may escalate as high as 80% [8]. In Ethiopia, stripe rust of wheat was first reported since the early 1940s, and the disease occurs regularly in highland areas – over 2000m above sea level where epidemic covered almost all wheat-growing regions in the country. However, gained importance with the expansion of high-yielding, semi-dwarf bread wheat cultivars in the mid-1980s [9]. As of other stripe rust vulnerable parts of the world, stripe rust of wheat is the major wheat production bottleneck in the highlands of Arsi and Bale areas, the wheat belts of Ethiopia [10,11]. Major stripe rust epidemics were experienced in Ethiopia in 1970’s, 1988s, 2010, 2017 and 2018 and resulted in significant grain yield losses of 30% to 69% [12], 58-100% [13], 96% [14-16] depending on the susceptibility of the cultivars and environmental conditions.
For instance in 2010, when Pst pathotypes overcame the resistance conferred by Yr27. During this epidemic year, many central and West Asian and North and East African countries were also suffered this epidemic and high yield losses were incurred in Syria, Morocco, Iran, and Turkey [17,18]. At the same time, more than US$3.2 million expended for purchasing fungicides alone, and food and nutritional security of 3.5 million Ethiopian smallholder farmers involved in wheat production was heavily threatened [19].
To reduce stripe rust-associated losses, developing and growing resistant cultivars is widely recognized as the most environmentally and economically feasible approach. Ethiopian national wheat breeding and improvement program with international help, ICARDA and CIMMYT have been developed high yielding, stable, disease resistant varieties with good grain quality and adaptable to the different agro-ecologies of the country [20]. However, the promising varieties developed were susceptible to breakdown and invariably short-lived. This was happened due to the resistance of many of these cultivars was based on single race-specific allstage resistance genes, which confer a high level of resistance to specific races throughout all growth stages. When used extensively over time and space, single resistance genes generally lose their effectiveness in a few years and the cultivars are vulnerable to stripe rust epidemics [5,21]. Therefore, characterization of genotypes for diverse sources of resistances partial (slow rusting), to yellow rust resistances in combination is imperative for developing and releasing durable rust resistant variety.
Materials and Methods
Descriptions of the study area
Kulumsa research center is located at 08o 01' 10''N, 39° 09' 11''E and at 2200 meters above sea level (m.a.s.l). It receives mean annual rainfall of 820 mm representing highland and high rainfall agro ecology. The monthly mean minimum and maximum temperature is 10.5 and 22.8°C respectively. The sites dominant soil type is loam type, which is fertile [22].
Meraro substation is located at 07° 24' 27''N, 39° 14' 56''E and 2990 m.a.s.l. Its average annual rainfall is 1196 mm representing extreme highland and frost prone agro ecology. The minimum and maximum temperature is 5.7 and 18.1°C respectively. The dominant soil type is clay soil (Nitosols) which is slightly acidic (pH=5.0). Both locations represent major wheat-growing and yellow rust prone areas in the highlands of Arsi [22].
Experimental materials
Twenty-eight advanced bread wheat genotypes with two commercial cultivars, Kubsa and Wane serve as susceptible and resistant control checks respectively were selected for evaluation against stripe rust under field conditions (Table 1).
| S. No | Name | Pedigree | Selection History |
|---|---|---|---|
| 1 | ETBW 8751 | SUP152//ND643/2*WBLL1 | CMSS08Y00274S-099Y-099M-099NJ-099NJ-7WGY-0B |
| 2 | ETBW 8858 | SWSR22T.B./2*BLOUK #1//WBLL1*2/KURUKU | CMSS08Y01116T-099M-099Y-099M-099NJ-099NJ-14WGY-0B |
| 3 | ETBW 8870 | WAXWING*2/TUKURU//KISKADEE #1/3/FRNCLN | CMSS08B00861T-099TOPY-099M-099NJ-099NJ-45WGY-0B |
| 4 | ETBW 8802 | CHAM-4/SHUHA'S'/6/2*SAKER/5/RBS/ANZA/3/KVZ/HYS//YMH/TOB/4/BOW'S" | ICW00-0634-6AP-0AP-0AP-23AP-0AP-0DZ/0AP-0DZ/0KUL/0SIN/0AP-0NJ/0AP-0ALK/0AP |
| 5 | ETBW 8991 | SUP152//ND643/2*WBLL1 | CMSS08Y00274S-099Y-099M-099NJ-099NJ-2WGY-0B |
| 6 | ETBW 8862 | C80.1/3*BATAVIA//2*WBLL1/3/C80.1/3*QT4522//2*PASTOR/4/WHEAR/SOKOLL | CMSS08B00337S-099M-099NJ-099NJ-29WGY-0B |
| 7 | ETBW 8804 | TURACO/CHIL/6/SERI 82/5/ALD'S'/4/BB/GLL//CNO67/7C/3/KVZ/TI | ICW99-0052-2AP-0AP-0AP-11AP-0AP-0DZ/0AP-0DZ/0KUL/0SIN/0AP-0NJ/0AP-0ALK/0AP |
| 8 | ETBW 8996 | FALCIN/AE.SQUARROSA (312)/3/THB/CEP7780//SHA4/LIRA/4/FRET2/5/DANPHE #1/11/CROC_1/AE.SQUARROSA (213)//PGO/10/ATTILA*2/9/KT/BAGE//FN/U/3/BZA/4/TRM/5/ALDAN/6/SERI/7/VEE#10/8/OPATA | CMSA08Y00096T-099B-050Y-040M-0NJ-8Y-0B |
| 9 | ETBW 8583 | MINO/898.97/4/PFAU/SERI.1B//AMAD/3/KRONSTAD F2004 | CMSS08B00391S-099M-099Y-10M-0WGY |
| 10 | ETBW 8668 | BAVIS*2/3/ATTILA/BAV92//PASTOR | CMSA08M00054T-050Y-040M-0NJ-5Y-0B |
| 11 | ETBW 8595 | BAVIS*2/3/ATTILA/BAV92//PASTOR | CMSA08M00054T-050Y-040M-0NJ-5Y-0B |
| 12 | ETBW 8684 | PASTOR//HXL7573/2*BAU/3/WBLL1/4/1447/PASTOR//KRICHAUFF | CMSA08M00406S-040ZTM-050Y-37ZTM-010Y-0B |
| 13 | ETBW 9486 | FRANCOLIN #1/3/PBW343*2/KUKUNA*2//YANAC/4/KINGBIRD #1//INQALAB 91*2/TUKURU | CMSS10B00779T-099TOPY-099M-099NJ-099NJ-5WGY-0B |
| 14 | ETBW 9547 | MUTUS*2/AKURI//MUTUS*2/TECUE #1 | CMSS11B00372S-099M-099NJ-099NJ-19WGY-0B |
| 15 | ETBW 9548 | REEDLING #1//KFA/2*KACHU | CMSS11B00167S-099M-0SY-70M-0WGY |
| 16 | ETBW 9549 | KFA/2*KACHU/3/KINGBIRD #1//INQALAB 91*2/TUKURU/4/KFA/2*KACHU | CMSS11B00958T-099TOPY-099M-099NJ-099NJ-8WGY-0B |
| 17 | ETBW 9550 | KFA/2*KACHU*2//WAXBI | CMSS11B00961T-099TOPY-099M-099NJ-099NJ-20WGY-0B |
| 18 | ETBW 9551 | KFA/2*KACHU/4/KACHU #1//PI 610750/SASIA/3/KACHU/5/KFA/2*KACHU | CMSS11B00957T-099TOPY-099M-099NJ-099NJ-8WGY-0B |
| 19 | ETBW 9552 | KACHU #1/4/CROC_1/AE.SQUARROSA (205)//BORL95/3/2*MILAN/5/KACHU/6/KFA/2*KACHU | CMSS11B00214S-099M-099NJ-099NJ-22WGY-0B |
| 20 | ETBW 9553 | MURGA/KRONSTAD F2004/3/KINGBIRD #1//INQALAB 91*2/TUKURU | CMSS11Y00116S-099Y-099M-0SY-4M-0WGY |
| 21 | ETBW 9554 | SAUAL/MUTUS/6/CNO79//PF70354/MUS/3/PASTOR/4/BAV92*2/5/FH6-1-7/7/CNO79//PF70354/MUS/3/PASTOR/4/BAV92*2/5/FH6-1-7 | CMSS11B00652T-099TOPY-099M-099NJ-099NJ-23WGY-0B |
| 22 | ETBW 9555 | KFA/2*KACHU/5/WBLL1*2/4/BABAX/LR42//BABAX/3/BABAX/LR42//BABAX/6/KFA/2*KACHU | CMSS11B00956T-099TOPY-099M-0SY-8M-0WGY |
| 23 | ETBW 9556 | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/PARUS/PASTOR | PTSA08M00046S-050ZTM-050Y-50ZTM-010Y-0B-020Y-0MXI |
| 24 | ETBW 9557 | SOKOLL/WBLL1/4/D67.2/PARANA 66.270//AE.SQUARROSA (320)/3/CUNNINGHAM | CMSA09M00466S-050ZTM-050Y-3WGY-0B |
| 25 | ETBW 9558 | BABAX/LR42//BABAX/3/ER2000/5/ATTILA/4/WEAVER/TSC//WEAVER/3/WEAVER/6/KA/NAC//TRCH | CMSA09Y00383T-099B-050Y-050ZTM-0NJ-099NJ-11WGY-0B |
| 26 | ETBW 9559 | CHIBIA//PRLII/CM65531/3/MISR 2*2/4/HUW234+LR34/PRINIA//PBW343*2/KUKUNA/3/ROLF07 | CMSS09Y00853T-099TOPM-099Y-099ZTM-099NJ-099NJ-24WGY-0B |
| 27 | ETBW 9560 | CHWINK/GRACKLE #1//FRNCLN | CMSS09Y00753T-099TOPM-099Y-099ZTM-099NJ-099NJ-13WGY-0B |
| 28 | ETBW 9561 | TRAP#1/BOW/3/VEE/PJN//2*TUI/4/BAV92/RAYON/5/KACHU #1*2/6/KINGBIRD #1 | CMSS10Y00842T-099TOPM-099Y-099M-1WGY-0B |
| 29 | Kubsa | THELIN/WAXWING//PATOR/ | ICW08-00270-4AP-0AP-040SD-4SD-OSD- |
| 30 | Wane | PASTOR//HXL7573/2*BAU/3/S OKOLL/WBLL1/4SAFI1//NS73 2/HER/3/SAADA |
ICW08-00214-7AP-0AP-040SD-7SD-0SD- |
| 31 | Morocco | Universal susceptible check/Cultivar( used in green house) | |
Source: Ethiopian national wheat research program and Ethiopian crop registration directory of 2016.
Table 1: List of advanced bread wheat lines employed in the study.
Kubsa carries stripe rust resistance gene, Yr27 that lost efficacy in 2010, however, still kept under production for its trait to give high yield in southeastern wheat belt agro ecologies. While wane is most recently released, 2016 variety and is relatively better in its genetic trait for yield and resistance against stripe rust. All 28 test wheat genotypes were obtained from Kulumsa Agricultural Research Center, a center coordinating national wheat-breeding program. These test lines have been under testing for yield performance at the national testing sites and currently under test in national variety trial stage.
Experimental method and design
To evaluate the level of adult plant stripe rust resistance in the field, test materials and checks were arranged in randomized complete block design having three replications. The treatments were planted in plots consisting of double rows of 1 m long with spacing of 0.2 m. intra row, 1 m between blocks and 0.5 m between plots. Plots were seeded in 150 kg ha-DAP and urea fertilizers were applied based on the recommended rate to the area. Weeds were managed by hand weeding. To ensure uniform spread of inoculums and for sufficient disease development during the trial period, the mixture of susceptible wheat cultivars ‘Morocco and Kubsa’ were planted a week earlier as infector row between blocks perpendicular to entries. The infector rows were sprayed and injected [23] with active uredinospores collected and maintained at Kulumsa wheat rust laboratory, south east Ethiopia.
Data collection and statistical analysis
Yellow rust disease severity notes were taken by estimating the approximate percentage of leaf area affected using modified Cobb scale [24]. Data recording was started from the first appearance of yellow rust on the susceptible check and continued every 14 days from all plants until the early dough stage [25].
Readings of disease severity and reaction were recorded together with severity first followed by infection type. And written like: TR=trace severity of resistant type infection; 10R-MR=10% severity of resistant to moderately resistant infection type; 20MR=20% severity of a moderately resistance infection type; 30MR-MS=30% severity of a moderately resistance to moderately susceptible; 40MS=40% severity of a moderately susceptible; 50MS-S=50% severity of a moderately susceptible to susceptible; and 70S =70% severity of Susceptible Infection Types.
Thousand Kernel Weight (TKW): The 1000 kernel weight was measured in grams by using electronic seed counter machine followed by automatically measuring the seed weight after moisture content was adjusted to 12% [26].
Grain yield plot-1: grain yield in grams obtained from both rows of each plot was weighed after the moisture content is adjusted to 12% [26]. The obtained grain yield was used to estimate grain yield in tons per hectare.
Average Coefficient of Infection (ACI%): calculated by multiplying the percentage severity and the constant value assigned to each reaction type. The constant values were considered as R (Resistant)=0.2, MR (moderately resistant)=0.4, MRMS (intermediate) =0.6, MS (moderately susceptible) =0.8, MSS=0.9 and S (susceptible) =1.
The relative area under the disease progress curve (rAUDPC(cycles/time) for each plot calculated from the multiple stripes rust severity readings using the formula suggested by Wilcoxson et al. [27].
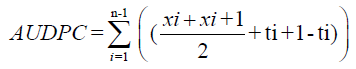
Where, xi=the average coefficient of infection of ith record, Xi+1=the average coefficient of infection of i+1th record and ti+1 - ti=Number of days between the ith record and i+1th record, and n=number of observations.
Cluster analysis: Clustering of the lines relay on grain yield weight was performed by average linkage method of SAS software SAS Institute [28]. The dendrogram was built using MINITAB 17.
Correlation analysis: Among the different disease parameters (FRS, AUDPC and ACI), grain yield and TKW were analyzed using the general linear model procedure of SAS version 9.0 statistical software [28].
ANOVA: Yield data was subjected to analysis of variance (ANOVA) using SAS computer software package version 9.0. Least significant difference (LSD, 0.05) values were used for mean separation [28].
Results and Discussion
Disease assessment under field conditions
Final rust severity: There was broad variation in the final stripe rust severity ranging from zero to 80 and 0 to 86% at Kulumsa and Meraro respectively. According to Parlevliet and Van Omeren [29], final rust severity signifies the collective result of all resistance factors during the progress of epidemics.
Formerly, many researchers like Ali et al. [30], Li et al. [31], Safavi [32], Tabassum [33] also used final severity as a parameter to assess slow rusting behavior of wheat lines. Rely on final rust severity, the tested bread wheat lines were categorized in to three groups of slow rusting resistance, which is high, moderate, and low level of adult plant resistance, having 1-30, 31-50% and above 50% respectively [32].
Although there was intense stripe rust disease pressure, twentyfour of tested bread wheat lines namely; ETBW-8858, ETBW-8870, ETBW-8583, ETBW-8668, ETBW-8595, ETBW-8684, ETBW- 9548, ETBW-9549, ETBW-9552, ETBW-9554, ETBW-9558, ETBW-9559, ETBW-9560, ETBW-8751, ETBW-8802, ETBW- 8862, ETBW-8804, ETBW-8896, ETBW-9557, ETBW-8991, ETBW-9560, ETBW-9556, ETBW-9486 and ETBW-9561 exhibited high level of adult plant rusting resistance (Figures 1 and 2) with complete resistant to moderately susceptible resistance response. Genotypes with slow rusting resistance are highly important to achieve effective breeding for durable resistance to stripe rust [34,35].
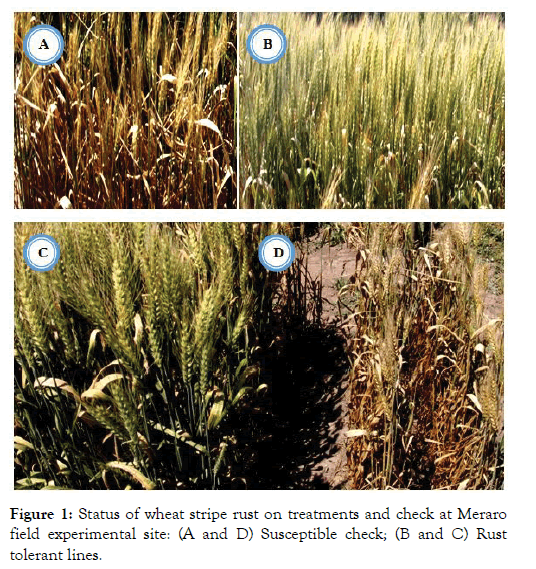
Figure 1: Status of wheat stripe rust on treatments and check at Meraro field experimental site: (A and D) Susceptible check; (B and C) Rust tolerant lines.
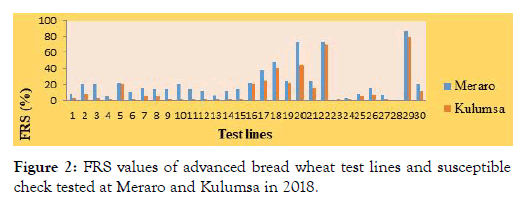
Figure 2: FRS values of advanced bread wheat test lines and susceptible check tested at Meraro and Kulumsa in 2018.
Based on the studies of Nzuve et al. [34], the availability of resistance genes in the genotypes overcome the prevailed virulence stripe rust pathogens in the field and led to statistically low disease severity, despite the compatible host pathogen reactions. Line, ETBW-9561 showed immune responses both at Meraro and at Kulumsa. The immune response on this line could be because of major genes; where resistance often breaks down due to the development of new races of the pathogen. A suitable breeding strategy like the use of inter-specific and remote crosses or even the direct transfer of these resistances through backcrosses could be used to improve the adopted but highly susceptible wheat varieties being grown in Ethiopia [36].
On the other hand, two lines showed final rust severities between 31% and 50% both at Meraro and Kulumsa testing sites were regarded as possessing moderate level of slow rusting resistance. The rest two lines exhibited above 50% final rust severities both at Meraro and at Kulumsa, which lacked adult plant rusting resistance. Similarly, the susceptible check Kubsa, displayed the highest disease severities of 80% and 86.7% with completely susceptible (S) responses both at Kulumsa and Meraro experimental testing sites respectively, indicating that an acceptable epidemic pressure was established over the locations and is in line with similar works of Ibrahim et al. [37].
Average coefficients of infections: The data acquired from disease severities and host reactions were combined to compute coefficient of infection (ACI). According to Ali et al. [30], genotypes with ACI values of 0-20, 21-40, 41-100 were regarded as possessing high, moderate and low levels of adult plant resistance, respectively.
In the present study, most of the tested lines, 85.7% (ETBW-8858, ETBW-8870, ETBW-8583, ETBW-8668, ETBW-8595, ETBW- 8684, ETBW-9548, ETBW-9549, ETBW-9552, ETBW-9554, ETBW-9558, ETBW-9559, ETBW-9560, ETBW-875, ETBW-8802, ETBW-8862, ETBW-8804, ETBW-8896, ETBW-9557, ETBW- 8991, ETBW-9486 and ETBW-9561) showed ACI values between 0 and 20 at both experimental locations and were designated as having a high level of slow rusting genes. Five lines; ETBW-9550, ETBW-9551, ETBW-9553 and ETBW-9555 had ACI values of 21 to 40, designated as having moderate levels of slow rusting resistance. However, none of the tested lines had showed above 41. However, the susceptible check Morocco displayed ACI above 41 (Figure 3).
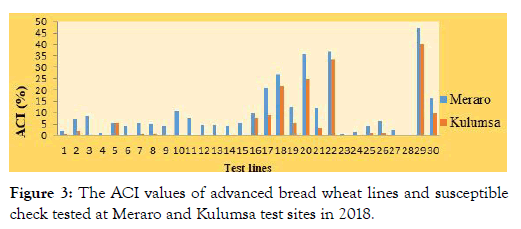
Figure 3: The ACI values of advanced bread wheat lines and susceptible check tested at Meraro and Kulumsa test sites in 2018.
Based on the results, common stripe rust pathotypes of testing sites were considered avirulent on most of the assessed lines (Figure 3). The lines; ETBW-8684, ETBW-9547, ETBW-8684, ETBW-8862, ETBW-8996, ETBW-9554, ETBW-9560, ETBW-8804 and ETBW- 8583 (with resistance reaction at both the seedling and adult plant stages) may probably carry major genes or combination of major gene-based resistance, effective against all virulence used [30,38]. However, the lines with race-specific resistance to the wheat rusts diseases often become susceptible within a few years after their commercial release, because of the rapid evolution of new virulent races of the wheat rust pathogens [39].
According to Singh et al. [40], lines that show high and moderate level of FRS and ACI could have durable resistance, which can serve as good parents for breeding. Thus, it is concluded that lines in both groups are potentially useful in wheat breeding.
Area under disease progress curve (AUDPC): Disease progress curve or its relative form (rAUDPC) is a better indicator of disease expression over time [41]. Therefore, selection of lines having lower rAUDPC value is acceptable for practical purposes. Based on the rAUDPC values, the tested wheat lines categorized in to three distinct groups for slow rusting resistance (Figure 4). Lines exhibited AUDPC values up to 30% of the check were grouped as having high level of partial resistance. While those lines having AUDPC values up to 70% of the check were grouped as moderately resistance lines and lines having above 70% of the check were grouped as susceptible lines [42].
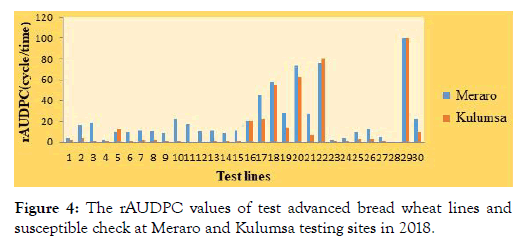
Figure 4: The rAUDPC values of test advanced bread wheat lines and susceptible check at Meraro and Kulumsa testing sites in 2018.
In this study among the twenty-eight lines tested at Meraro, 85.7% (twenty-four lines; ETBW-8751, ETBW-8858, ETBW-8870, ETBW- 8802, ETBW-8991, ETBW-8862, ETBW-8804, ETBW-8996, ETBW-8583, ETBW-8595, ETBW-8668, ETBW-8684, ETBW- 9486, ETBW-9547, ETBW-9548, ETBW-9549, ETBW-9552, ETBW-9554, ETBW-9556, ETBW-9557, ETBW-9558, ETBW- 9559, ETBW-9560 and ETBW-9561) showed AUDPC values lower than 30% of the check. These lines showed immune to MR-MS and MS-S types of infection in the field and were considered to have good levels of partial resistance.
According to Brown et al. [43], Kaur and Bariana [44] and Singh et al. [40], genotypes that had MS infection type and low rAUDPC might carry genes conferring durable resistance. These types of genotypes first shown rust infection characterized by chlorotic and necrotic lesions; subsequently the disease progression remained slower and highly retarded. Such partially resistant cultivars could highly delay evolution of new virulent races of the pathogen because multiple point mutations are extremely rare in such circumstance [45,46]. Two lines; ETBW-9550 and ETBW-9551) genotypes exhibited AUDPC values which lay in a range of 31% to 70% of check, Kubsa. These genotypes are considered possessing moderate level of slow rusting resistance. The rest two genotypes showed AUDPC value above 70% of the check and are considered as susceptible. Other researchers have also reported variation in AUDPC among different wheat cultivars and genotype with lower AUDPC values considered to have slow rusting resistance to stripe rust and is in agreement with works [47]. Data for wheat lines showing their rAUDPC values presented in Table 2.
| Lines | Meraro | Kulumsa | ||
|---|---|---|---|---|
| Grain yield tone ha-1 |
TKW(g) | Grain yield tone ha-1 |
TKW(g) | |
| ETBW-8751 | 8.3a | 36.8efgh | 8.6abc | 38.8hi |
| ETBW-8858 | 2.9op | 31.1hi | 7.5cdefg | 39.7fghi |
| ETBW-8870 | 4.5lm | 41.7bcde | 8.4bcd | 40.8defgh |
| ETBW-8802 | 8.3a | 39.6defg | 5.9ijk | 32.4l |
| ETBW-8991 | 6.2defg | 42.4bcde | 7.3defgh | 37.4ijk |
| ETBW-8862 | 5.3hijk | 46.7abcd | 8.1bcde | 42.2bcdef |
| ETBW-8804 | 4.9jkl | 41.7bcde | 5.1k | 35.6jk |
| ETBW-8996 | 6.7bcd | 43.4bcde | 7.3defgh | 41.7bcdefg |
| ETBW-8583 | 4.7klm | 40.6bcdefg | 7.2defgh | 39.9fghi |
| ETBW-8595 | 6.3cdef | 47.9ab | 8.5abc | 43.8ab |
| ETBW-8668 | 6.9bc | 47.7abc | 7.5cdefg | 45.2a |
| ETBW-8684 | 5.3hijk | 41.6bcde | 9.6a | 43.5abc |
| ETBW-9486 | 3.9mn | 42.5bcde | 7.1efgh | 43.0abcde |
| ETBW-9547 | 6.6bcde | 46.7abcd | 6.9efghi | 40.7defgh |
| ETBW-9548 | 5.0ijkl | 42.4bcde | 6.4ghij | 40.5efgh |
| ETBW-9549 | 4.4lm | 42.4bcde | 6.2hijk | 38.8hi |
| ETBW-9550 | 4.1mn | 36.6efgh | 6.6ghij | 37.6ijk |
| ETBW-9551 | 2.3pq | 32.7ghi | 6.7fghi | 38.3hij |
| ETBW-9552 | 5.6fghij | 43.5bcde | 8.1bcde | 39.0hi |
| ETBW-9553 | 1.5q | 33.7fghi | 5.1k | 42.8abcde |
| ETBW-9554 | 3.5no | 40.6bcdefg | 7.2defgh | 41.0cdefgh |
| ETBW-9555 | 4.0mn | 41.1bcdef | 5.3jk | 35.2k |
| ETBW-9556 | 4.5lm | 43.1bcde | 7.8cdef | 44.9a |
| ETBW-9557 | 7.2b | 47.9ab | 7.1efgh | 40.5efgh |
| ETBW-9558 | 5.4ghijk | 40.1bcdefg | 9.1ab | 43.1abcd |
| ETBW-9559 | 5.9efgh | 51.9a | 7.3defgh | 44.9a |
| ETBW-9560 | 7.1bc | 40.4bcdefg | 6.9efghi | 38.8hi |
| ETBW-9561 | 5.8fghi | 47.7abc | 7.1efgh | 43.8ab |
| Kubsa | 0.6r | 27.2i | 3.4l | 27.8m |
| Wane | 4.6klm | 39.83cdefg | 6.80fghi | 39.2ghi |
| CV | 9.75 | 11.71 | 10.44 | 3.79 |
| LSD | 0.99 | 7.93 | 1.21 | 2.81 |
| SEM | 0.40 | 3.96 | 0.60 | 1.40 |
Means within a column followed by the same letter are not significantly different at P=0.05.
Table 2: Grain yield and thousand-kernel weight of advanced bread wheat lines tested at Meraro and Kulumsa.
At Kulumsa experimental site, 89.3% or twenty-five of the tested wheat genotypes exhibited AUDPC values lower than 30% of Kubsa, locally susceptible check. These lines had variable responses; immune to MR-MS in the field evaluation. Two lines showed relative AUDPC values up 31% to 70% of the susceptible check with MS to S field responses. Despite high infection types (MS and S) exhibited on moderately slow rusting lines, stripe rust developed slowly as indicated by their AUDPC values. The remaining one-line along with susceptible check showed highest rAUDPC values and were considered as susceptible. Based on the AUDPC values, Ali et al. [42] wheat lines exhibiting AUDPC values up to 30% and 70% of the check were regarded as expressing good levels of slow rusting and moderate slow rusting resistance respectively. According to Parlevliet [35] wheat lines with variable field infection responses of MR-MS to S are expected to possess genes that confer partial resistance.
Grain yield and thousand kernel weight
The effect of stripe rust infection on the grain yield of all the tested wheat lines was estimated under field conditions at two experimental locations in 2018 main growing season to characterize and determine the capacity of wheat lines to tolerate these infections.
There was a highly significant difference (P<0.05) between entries for grain yield (Table 3). From the beginning, it should be underlined that the differences in grain yield between the entries could be explained not only by differences in the levels of disease attack, but also in the yield potential and environment of the lines [48,49].
| Location | Yield and Disease Parameters | ||||||
|---|---|---|---|---|---|---|---|
| FRS | ACI | AUDPC | rAUDPC | Gyld | TKW | ||
| Meraro | FRS | 1 | |||||
| ACI | 0.973** | 1 | |||||
| AUDPC | 0.963** | 0.965** | 1 | ||||
| rAUDPC | 0.995** | 0.973** | 0.958** | 1 | |||
| Gyld | -0.52** | -0.487** | -0.548** | -0.503** | 1 | ||
| TKW | -0.552** | -0.536** | -0.548** | -0.538** | 0.69** | 1 | |
| FRS | 1 | ||||||
| ACI | 0.985** | 1 | |||||
| AUDPC | 0.983** | 0.999** | 1 | ||||
| Kulumsa | rAUDPC | 0.983** | 0.999** | 1 | 1 | ||
| Gyld | -0.594** | -0.601** | -0.603** | -0.603** | 1 | ||
| TKW | -0.58** | -0.546** | -0.546** | -0.546** | 0.602** | 1 | |
Table 3: Linear correlation coefficients of stripe rust parameters along with yield variables of evaluated wheat lines at Meraro and Kulumsa test sites, 2018.
At Kulumsa testing site, the highest grain yield, 9.63 tha- was obtained from line ETBW-8684 whereas the lowest, 5.1 t ha-1, was displayed from lines of ETBW-8804 and ETBW-9553. At Meraro, the highest yield was displayed by line ETBW-8751 (8.3 t/ha) while the lowest (1.5 t ha-1) was from line ETBW-9553. The ranking of the genotypes for yield changed when exposed to higher disease pressure at Meraro. For instance, line ETBW-8751 had greater yield (8.3 tha-1) as compared to line ETBW-8684 (5.7 tha-1) at Meraro, relatively under higher disease pressure. This yield difference among lines over locations may be due to adaptation difference of varied agro ecologies and/or due to varying levels of tolerance to rust expressed by different lines [48,49].
Weather conditions such as temperature and moisture greatly affect disease expressions and consequently of yield. Many researchers have described stripe rust reducing grain yields of wheat cultivars [50,51]. The effect of rust on grain yield is due to the great injury to the photosynthetic surface of the plant and the energy expenses in plant defense mechanisms rather than for growth and grain formation [52]. According to Craigie [53] and Bushnell and Rowell [54], the fungus also reduces the food and water supply within the plants. The fungus desires food and water for spore production that would otherwise be used in the formation of well-developed kernels. Further, there is a loss of water by evaporation through the several bursts caused by the fungal pustules. The yield from heavily rusted plants was so much reduced and the quality of the grain is lowered.
Among the slow rusting lines identified, ETBW-8684, ETBW-9558 and ETBW-8751 had the highest yields at Kulumsa (>8.6 t/ha). This would be due to their higher genetic potential for yield and better tolerance against stripe rust. Twenty and twenty-three lines were showed yield exceeding resistant check, wane at Kulumsa and Meraro respectively. This finding is in agreement with previous works Kebede Temesgen et al. [55], their comparatively better yields make them superior candidates as donor parent for the incorporation of durable resistance in the bread wheat improvement program; and direct release for production to boost production and productivity in Ethiopia.
The wheat lines also showed significant variation in TKW (P<0.05) (Table 3). The highest TKW was recorded from line ETBW-8668 (45.20 g) and line ETBW-9557 (47.93 g) at Kulumsa and Meraro respectively. The lowest was obtained from line ETBW-8802 (32.4 g) at Kulumsa and from line ETBW-8858 (31.1 g) at Meraro. Nzuve et al. [34] also reported that wheat rusts significantly reduce TKW. It is well launched that the considerable effect of stripe rust on TKW is brought about by its effect on photosynthesis and later grain filling. According to Agrios [56], the competition of rust fungi for photosynthetic at grain filling would have increased importance in reduction of number and size of seeds on plants. Thus, among the slow rusting genotypes identified, ETBW- 8668, ETBW -9559 and ETBW-9561 had high TKW values suggested for future breeding purpose.
Cluster analysis
The combined average linkage analysis (two locations, Meraro and Kulumsa yield values) was made through squared Euclidean method. Numbers of clusters were determined by similarity level. At a 90% of similarity level, the cluster analysis grouped the 28 test lines and two check varieties into four distinct categories based on their grain yield obtained at Meraro and Kulumsa as illustrated in Figure 5. Members within a single cluster were considered as similar or as having more close relationships with each other than those in distant clusters. This finding is in agreement with similar works of Hailegiorgis et al. and Asmamaw [57,58]. The lines that lie in each cluster are indicated by dendogram below.
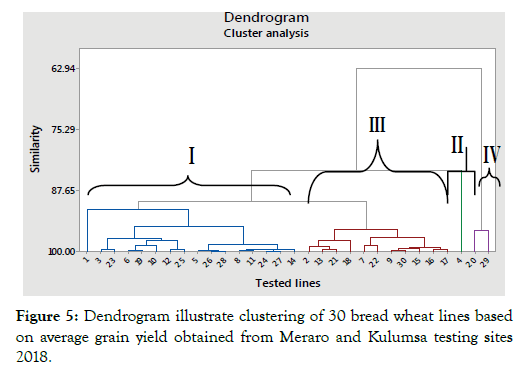
Figure 5: Dendrogram illustrate clustering of 30 bread wheat lines based on average grain yield obtained from Meraro and Kulumsa testing sites 2018.
From cluster mean values, lines in cluster I, II and III are suggested for direct release to production and/or deserve consideration to use as parents in hybridization programs with lines identified as resistant with disease parameters discussed above in this study. Thus, play vital role in the programs to develop high yielding stripe rust resistant wheat varieties.
Correlation between disease parameters along with yield variables
The correlations between the field based slow rusting diseases parameters, yield and thousand-kernel weight were significant (Table 3). A positive and highly significant correlation of rAUDPC with final rust severity (r=0.995) and ACI (r=0.973) was found at Meraro. Strong correlation coefficients of 0.983 and 0.999 also observed between rAUDPC with FRS and ACI at Kulumsa respectively. The high correlation coefficient was also observed between ACI and final rust severity at both locations'=0.973 at Meraro and r=0.985 at Kulumsa. These positive correlations between the parameters observed were in agreement with the results of other researchers on cereal rust pathosystems [32,55,59].
Therefore, selection of lines having low rAUDPC values (below 30% of the check), final disease severity scores of <30 MS and ACI between 0 and 20 provides a sound basis for identifying slow rusting resistance, which is one of the durable resistance breeding strategies [32]. Accordingly, twenty-four wheat lines, ETBW-8858, ETBW-8870, ETBW-8583, ETBW-8668, ETBW-8595, ETBW- 8684, ETBW-9548, ETBW-9549, ETBW-9552, ETBW-9554, ETBW-9558, ETBW-9559, ETBW-9560, ETBW-875, ETBW-8802, ETBW-8862, ETBW-8804, ETBW-8896, ETBW-9556 ETBW- 9557, ETBW-8991, ETBW-9486 and ETBW-9561 with highly slow rusting resistance characteristics rAUDPC <30%, ACI 0–20 and FRS 0–30% at both testing sites were selected for further resistance breeding.
Conversely, a negative and highly significant correlations of (r= -0.52,-0.487 and -0.548; and -0.594,-0.601 and -0.603 was observed between grain yield and FRS, ACI and AUDPC at Meraro and Kulumsa respectively. Similarly, all the slow rusting parameters showed negative correlations with yield variables and the result is in lined with works of Afzal et al., [60]; Kebede Temesgen et al., [55] suggesting that the rust disease reduced the grain yield and thousand kernel weight.
Conclusion
High stripe rust epidemic was built in the field at both trial screen sites and the true effect of stripe rust on yield variables has been revealed by negative correlation between diseases parameters and yield and kernel weight. Of the 28 test lines, 24 lines exhibited high level of adult plant resistance sustaining FRS (<30 MS-S), ACI (<20) and rAUDPC (<30). Therefore, the adult plant stripe rust resistant and seedling susceptible (true slow rusting) lines identified in this study which combined high yielders could be suggested directly for release to production while also could be used for durable stripe rust resistance breeding in Ethiopia.
REFERENCES
- USDA. United States Department of Agriculture. World Agricultural Production. Circular Series Report. 2018.
- FAO. Food and agriculture data. Explore Data. Crops: Wheat. 2018.
- CSA. Agricultural sample survey: Report on area and production of major crops, Central Statistical Agency, Addis Ababa, Ethiopia. 2018.
- Stubbs RW. Stripe rust. In: Roelfs AP, Bushnell WR (eds): The Cereal Rusts Cisease Methodology Manual, CIMMYT: Mexico, D.F. 46p.II. New York, USA. 1985;61–101.
- Chen XM. Epidemiology and control of stripe rust (Puccinia striiformis f. sp. tritici) on wheat. Can J Plant Pathol. 2005;27:314-337.
- Singh RP, Huerta-Espino J, Bhavani S, Herrera-Foessel SA, Singh D, Singh PK, et al. Race non-specific resistance to rust diseases in CIMMYT spring wheats. Euphytica. 2011;179(1):175-186.
- Abdulbagiyeva SA, Zamanov AA, Ahmadov NS. Effects of yellow rust on yield: 2nd International Wheat Stripe Rust Symposium: Regional Cereal Rust Research Center, Izmir, Turkey. 2014.
- Beard C, Jayasena K, Thomas G, Loughman R. Managing stripe rust and leaf rust of wheat. Department of Agriculture. Government of Western Australia. 2007.
- Gebre-Mariam H. Bread wheat breeding and genetics research in Ethiopia. Wheat research in Ethiopia: A historical perspective. 1991:73-93.
- Denbel W, Hundie B, Woldeab G, Hailu E, Kassa B, Abebe T, et al. Monitoring wheat stripe rust in Ethiopia: The 2011 and 2012 Trap Nurseries and Stripe Rust Virulence Surveys. 2nd International Wheat Stripe Rust Symposium: Regional Cereal Rust Research Center, Izmir, Turkey. 2014;1-27.
- Badebo A, Assefa S, Fehrmann H. Yellow rust resistance in advanced lines and commercial cultivars of bread wheat from Ethiopia. East Afr J Sci. 2008;2:29-34.
- Dawit W, Flath K, Weber WE, Schumann E, Röder MS, Chen X. Postulation and mapping of seedling stripe rust resistance genes in Ethiopian bread wheat cultivars. J Plant Pathol. 2012;1:403-409.
- Abebe T. Variability in stripe rust occurrence in Tigray, Ethiopia. 2nd International Wheat Stripe Rust Symposium: Regional Cereal Rust Research Center, Izmir, Turkey. 2014.
- Bekel E. Review of research on diseases of barley, tef and wheat in Ethiopia. In: Tsedeke Abate (ed). A review of crop protection research in Ethiopia. Proceedings of the First Crop Protection Symposium. IAR, Addis Ababa, Ethiopia. 1985;79-108.
- Hovmøller MS, Rodriguez-Algaba J, Thach T, Sørensen CK. Race Typing of P. striiformis on Wheat. In: Sambasivam Periyannan (ed.), Wheat Rust Diseases: Methods and Protocols, Methods in Molecular Biology. 2017;1659:29-40.
- Hovmøller M, Rodriguez-Algaba J, Thach T, Justesen AF, Hansen GH. Report for Puccinia striiformis race analyses and molecular genotyping 2018. Global Rust Reference Center (GRRC), Aarhus University, Flakkebjerg, DK-4200 Slagelse, Denmark, 2018.
- Solh M, Nazari K, Tadesse W, Wellings CR. The growing threat of stripe rust worldwide; In Proceedings of the Borlaug Global Rust Initiative (BGRI) Conference Beijing, China. 2012.
- Zegeye H, Rasheed A., Makdis F, Badebo A, Ogbonnaya FC. Genome-wide association mapping for seedling and adult plant resistance to stripe rust in synthetic hexaploid wheat. PLOSE online J. 2014;9:98.
- Abebe A, Bishaw Z, Eticha F, Gelalcha S, Tadesse Z, Aliye S, et al. Controlling wheat rusts and ensuring food security through deployment of resistant varieties in Ethiopia. 2nd International Wheat Stripe Rust Symposium: Regional Cereal Rust Research Center, Izmir, Turkey. 2014.
- ICARDA. Strategies to reduce the emerging wheat stripe rust disease: Synthesis of a dialog between policy makers and scientists from 31 countries at; International Wheat Stripe Rust Symposium, Aleppo, Syria. 2011;1-23.
- Line RF, Chen XM. Success in breeding for and managing durable resistance to wheat rusts. Plant Dis. 2005;79:1254-1255.
- Abdulkadir B. KARC stations distribution and website description (Unpublished data). 2011.
- Roelfs AP, Singh RP, Saari EE. Rust Diseases of Wheat: Concepts and Methods of Disease Management. CIMMYT, Mexico. 1992.
- Peterson RF, Campbell A, Hannah AE. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res. 1948;26:496–500.
- Large EC. Growth stages in cereals-illustration of the Feekes scale. Plant Pathol. 1954;3:128-129.
- AACC (American Association of Cereal Chemists). International Approved Methods of the American Association of Cereal Chemists.10th Ed. The Association: St. Paul, MN.U.S.A. 2000.
- Wilcoxson RD, Skovmand B, Atif AH. Evaluation of wheat cultivars ability to retard development of stem rust. Ann Appl Biol. 1974;80:275-281.
- SAS. SAS/STAT guide for personal computers, version 9.0 edition. SAS Institute Inc., Cary, North Carolina, USA. 2002
- Parlevliet JE, Van Ommeren A. Partial resistance of barely to leaf rust, Puccinia hordei. Relationship between field trials, micro plot tests, and latent period. Euphytica. 1975;24:293-303.
- Ali S, Shah SJA, Ibrahim M. Assessment of wheat breeding lines for slow yellow rusting (Puccinia striiformis West. tritici). Pak J Biol Sci. 2007;10:3440-3444.
- Li ZF, Xia XC, He ZH, Li X, Zhang LJ, Wang HY, et al. Seedling and slow rusting resistance to leaf rust in Chinese wheat cultivars. J Plant Dis. 2010;94:45–53.
- Safavi SA, Afshari F. Identification of resistance to Puccinia striiformis f. sp. tritici in some elite wheat lines. J Crop Prot. 2012;1:293-302.
- Tabassum S. Evaluation of advance wheat lines for slow yellow rusting (Puccinia striiformis f. sp. tritici). J Agri Sci. 2011;3:239-249.
- Nzuve FM, Bhavani S, Tusiime G, Njau P, Wanyera R. Evaluation of bread wheat for both seedling and adult plant resistance to stem rust. Afr J Plant Sci. 2012;6:426–432.
- Parlevliet JE. Strategies for the utilization of partial resistance for the control of cereal rust. In: Simmonds NW and Rajaram S. (eds). Breeding strategies for resistance to the rusts of wheat. CIMMYT Mexico. 1988;48-62
- Bartos PA, Sip V, Chrpová J, Vacke J, Stuchlíkova E, Blazkova V, et al. Achievements and prospects of wheat breeding for disease resistance. Czech J Genet Plant Breed. 2002;38(1):16-28.
- Draz IS, Abou-Elseoud MS, Kamara AE, Alaa-Eldein OA, El-Bebany AF. Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann Agri Sci. 2015;60(1):29-39.
- Johnson R. 1988. Durable resistance to yellow (stripe) rust in wheat and its implications in plant breeding.In: Simmonds, N. W. and Rajaram, S. (Eds), Breeding strategies for resistance to the rusts of wheat.Mexico: CIMMYT. 63-75.
- Wan AM, Chen XM. Virulence, frequency, and distribution of races of Puccinia striiformis f. sp. tritici and Puccinia striiformis f.sp. hordei identified in the United States in 2008 and 2009. Plant Dis. 2012;96:67-74.
- Singh RP, William HM, Huerta-Espino J, Rosewarne G. Wheat rust in Asia: meeting the challenges with old and new technologies. Proceedings of the 4thinternational crop science congress. September, Brisbane, Australia. 2004.
- Vanderplank JE. Plant Diseases: epidemics and control.Academic Press, New York. 1963;113.
- Ali S, Shah A, Khalil IH, Raman H, Maqbool K, Ullah W. Partial resistance to yellow rust in introduced winter wheat germplasm at the north of Pakistan. Aust J Crop Sci. 2009;3:37-43.
- Brown WMJ, Hill JP, Velasco VR. Barley yellow rust in North America. Annu Rev Phytopathol. 2001;39:367-384.
- Kaur J, Bariana HS. Inheritance of adult plant stripe rust resistance in wheat cultivars Kukri and Sunco. J Plant Pathol. 2010;92:391-394.
- Ali S, Shah A, Maqbool K. Field-based assessment of partial resistance to yellow rust in wheat germplasm. J Agri Rural Develop. 2008;6:99-106.
- Schafer JF, Roelfs AP. Estimated relation between numbers of urediniospores of Puccinia graminis tritici and rates of occurrence of virulence. Phytopathal. 1985;75:749-750.
- Draz S, Abou-Elseoud MS, Kamara AM, Alaa-Eldein OA, El-Bebany AF. Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann Agri Sci. 2015;60:29-39.
- Jifar H, Assefa K, Tesfaye K, Dagne K, Tadele Z. Genotype-by-Environment Interaction and Stability Analysis in Grain Yield of Improved Tef (Eragrostis tef) Varieties Evaluated in Ethiopia. J Exp Agri Int. 2019;7:1-3.
- Wu X, Chang X, Jing R. Genetic Insight into Yield-Associated Traits of Wheat Grown in Multiple Rain-Fed Environments. PLoS ONE. 2012.;7(2):31249.
- Pretorius ZA, Pakendori KW, Marais GF, Prins R, Komen JS. Challenges for sustainable cereals rust control in South Africa. Aust J Agric Res. 2007;58:593–601.
- Singh RP. Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, et al. Will stem rust destroy the world’s wheat crop? Adv Agron. 2008;98:271–309.
- Smedegaard-Petersen V, Tolstrup K. The limiting effect of disease resistance on yield.Annu. Rev. Phytopathol. 1985;23:475–490.
- Craigie JH. Epidemiology of Stem Rust of Cereals in Western. Can Sci Agri. 1957;25:285–401.
- Bushnell WR, Rowell JB. Premature death of adult rusted wheat plants in relation to carbon dioxide evolution by root system. Phytopathol. 1968;58:651–658.
- Muleta KT, Rouse MN, Rynearson S, Chen X, Buta BG, Pumphrey MO. Characterization of molecular diversity and genome-wide mapping of loci associated with resistance to stripe rust and stem rust in Ethiopian bread wheat accessions. BMC Plant Biology. 2017;17(1):134.
- Agrios GN. Plant Pathology. 3rd edition. London, New York, Academic Press. 1988;456.
- Hailegiorgis D, Mesfin M, Genet T. Genetic divergence analysis on some bread wheat genotypes grown in Ethiopia. J Cen Eur Agri. 2011;12(2):0-10.
- Asmamaw M. Genetic diversity and selection efficiency of Ethiopian Durum wheat (Triticum durum Desf) landrace collections. M.Sc. Thesis. Addis Ababa University, Ethiopia. 2016;1-23.
- Shah SJA., Imtiaz M, Hussain S. Phenotypic and molecular characterization of wheat for slow rusting resistance against Puccinia striiformis f.sp. tritici. J. Phytopathol. 2010;158:393–402.
- Afzal J, Ahmad M, Begume I. Vision for development of rangelands in Pakistan. A policy perspective: quarterly science vision. 2008;14:53-58.
Citation: Abebele GM, Admasu MA, Agdu BH (2020) Field Evaluation of Bread Wheat (Triticum aestivum L.) Genotypes for Stripe Rust (Puccinia striiformis W.) Resistance in Arsi Highlands of Oromia Region, South -Eastern-Ethiopia. J Plant Pathol Microbiol 11: 521. doi: 10.35248/2157-7471.20.11.521.
Copyright: © 2020 Abebele GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.