Indexed In
- Open J Gate
- Academic Keys
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- CABI full text
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2020) Volume 8, Issue 5
Favipiravir: Promising Therapy for COVID-19
Amit Kumar Das*Received: 17-Jul-2020 Published: 17-Aug-2020, DOI: 10.35248/2329-891X.20.8.356
Abstract
COVID 19 is become one of the most threatened pandemics in recent time and spreading rapidly. Favipiravir, an antiviral drug, has shown promising but yet unproven effect against COVID-19 infection. Drug Controller General of India (DCGI) has recently approved Favipiravir for treating moderate to severe COVID-19 infected patients. Favipiravir, a broad-spectrum antiviral drug that interferes with the viral replication and emerging as promising therapeutic potential as indicated by initial clinical studies. In this literature review author tries to summaries an overview of Favipiravir as a promising therapy for COVID-19 disease.
Keywords
Favipiravir; COVID-19; Antiviral drug
Introduction
Severe acute respiratory syndrome coronavirus 2 (COVID-19 or 2019 Novel Coronavirus, named by WHO on February 11, 2020) is a respiratory pathogen which caused recent outbreak of coronavirus disease [1]. Across the globe to control the current outbreak and to reduce person to person transmission of COVID-19, drastic confinement measures have been implemented. But in realty COVID-19 still poses a fetal and serious public health threat all over the world despite those measures.
Mechanism of Action of Favipiravir
Favipiravir has broad-spectrum antiviral activity against RNA viruses of different virus families and developed in Japan in 2002 [2]. Favipiravir, also known as Avigan or T-705. Favipiravir is a pyrazine derivative that acts as an inhibitor of viral RNAdependent RNA polymerase and as a result causing chain termination and preventing RNA elongation in cell. However, for both teratogenicity and embryotoxicity in humans it is a mutagen and has potential. Against DNA viruses it has no activity [3]. Of note is that favipiravir shows anti-viral activities against other RNA viruses such as arenaviruses, bunyaviruses and filoviruses, all of which are known to cause fatal hemorrhagic fever. These unique anti-viral profiles will make favipiravir a potentially promising drug for specifically untreatable RNA viral infections [4] (Figure 1).
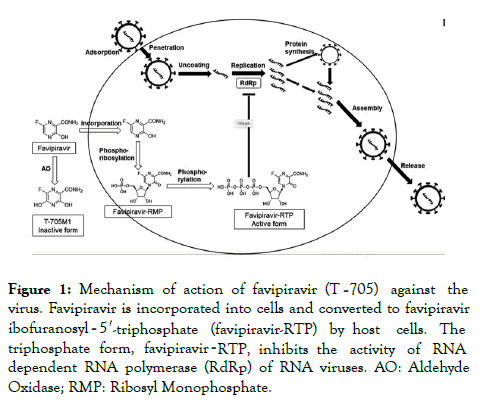
Figure 1. Mechanism of action of favipiravir (T‐705) against the virus. Favipiravir is incorporated into cells and converted to favipiravir ibofuranosyl‐5′-triphosphate (favipiravir-RTP) by host cells. The triphosphate form, favipiravir-RTP, inhibits the activity of RNA dependent RNA polymerase (RdRp) of RNA viruses. AO: Aldehyde Oxidase; RMP: Ribosyl Monophosphate.
Clinical Benefit of Favipiravir
Cai et al. had conducted an open-label, control, non-randomised trial to evaluate the effects of favipiravir versus lopinavir-ritonavir for the treatment of COVID-19 in the isolation ward of the national clinical research centre for infectious diseases in Shenzhen, China [5]. In this trial, on day 1 dose of 1600 mg twice-daily favipiravir was used and on days 2-14 favipiravir dose was 600 mg twice-daily. On contrast in same study 400 mg lopinavir and 100 mg ritonavir twice-daily was the dose of lopinavir-ritonavir. Both favipiravir and lopinavir-ritonavir were continued until 14 days had passed or until viral clearance was confirmed. In favipiravir (experimental) arm 35 patients were included and it was 45 in other arm of the study. A higher improvement rate in chest imaging and a faster viral clearance was observed in favipiravir arm compared to those in the control arm. No treatment discontinuation in favipiravir arm and much less adverse events observed as compared to control arm.
In a recent clinical trial favipiravir was compare with arbidol in 200 patients receiving conventional therapy. In this trial 120 patients were assigned to receive favipiravir where as other 120 were assign to receive arbidol for 10 days. Both pyrexia by 1.70 days (p<0.0001) and cough by 1.75 days (p<0.0001) were reduced or relieved by favipiravir as compare to other control arm [6].
A case reported in homepage of Japanese Association for Infectious Diseases indicate that COVID-19 pneumonia was alleviated by favipiravir [7].
In February 2020, in Shenzhen, an observational study, showed a significantly faster mean time to viral clearance of favipiravir than lopinavir/ritonavir {4 days vs 11 days (p<0.001)} [8]. In another RCT study than umefenovir favipiravir treatment led to a significantly greater recovery rate in non-critical COVID-19 patients (71.4% vs 55.9% {p<0.05}) [9]
Following Table 1 demonstrate the upcoming registered trial with favipiravir in recent future.
| Registration number | Design | Intervention | Outcomes |
|---|---|---|---|
| NCT04359615 | Randomized, double-blind, placebo-controlled trial | Experimental Interventions Drug: Favipiravir, Hydroxychloroquine Active comparator Hydroxychloroquine |
Mortality, oxygen saturation by pulse oximetry (SpO2) Improvement, Incidence of new mechanical ventilation use, Duration of hospitalization, Cumulative incidence of serious adverse events. |
| CTRI/2020/05/025114 | Randomized controlled trial | Intervention: Favipiravir 200 mg Comparator: Standard Supportive Care |
Time until cessation of oral shedding of SARS-CoV-2 virus, frequency of serious adverse event. |
| ChiCTR2000029544 | Randomized controlled trial | Group A (n=10): Current antiviral treatment plus Baloxavir Marboxil tablets. Group B (n=10): Current antiviral treatment plus favipiravir tablets. Group C (n=10): Current antiviral treatment. |
Time to viral negativity by RT-PCR. Time to clinical improvement. |
| ChiCTR2000029548 | Randomized, open-label, controlled trial | Group A (n=10): Baloxavir Marboxil: 80 mg on day 1, 80 mg on day 4; 80 mg on day 7 as necessary. No more than 3 times administration in total. Group B (n=10): Favipiravir: 600 mg t.i.d. with 1,600 mg first loading dosage, no more than 14 days. Group C (n=10): Lopinavir-Ritonavir: 200 mg/50 mg, twice daily, for 14 days |
• Time to viral negativity by RT-PCR • Time to clinical improvement: Time from start of study drug to hospital discharge or to NEWS<2 for 24 hours |
| ChiCTR2000029600 | Nonrandomized controlled trial | Group A (n=30): Alpha-interferon atomization. Group B (n=30): Lopinavir and Ritonavir plus alpha-interferon atomization. Group C (n=30): Favipiravir plus alpha-interferon atomization. |
• Declining speed of SARS-CoV-2 by PCR • Negative time of SARS-CoV-2 by PCR • Incidence rate of chest imaging. • Incidence rate of liver enzymes • Incidence rate of kidney damage |
| ChiCTR2000029996 | Randomized controlled trial | Group A (n=20): Favipiravir tablets; 200 mg; oral; twice a day. The adult dose is 1,600 mg per time on first day; the duration of treatment will be 10 days. Group B (n=20): Favipiravir tablets; 200 mg; oral; twice a day. The adult dose is 1,800 mg per time on the first day; the duration of treatment will be 10 days. Group C (n=20): Favipiravir tablets; 200 mg; oral; twice a day. The adult dose is 2,400 mg per time on first day; the duration of treatment will be 10 days. |
Time to clinical recovery. |
| ChiCTR2000030113 | Randomized controlled trial | Group A (n=15): Keep ritonavir/ritonavir treatment. Group B (n=15): Favipiravir. |
• Blood routine tests • Liver function examination • Renal function examination • Blood gas analysis • Chest CT examination |
| ChiCTR2000030254 | Randomized controlled trial | Group A (n=120): Favipiravir tablets Group B (n=120): Arbidol tablets |
Clinical recovery rate of day 7. |
| ChiCTR2000030894 | Randomized controlled trial | Group A (n=90): Favipiravir combined with Tocilizumab Group B (n=30): Favipiravir Group C (n=30): Tocilizumab |
Clinical cure rate. |
| ChiCTR2000030987 | Randomized controlled trial | Group A (n=50): The oral trial drug favipiravir tablets plus chloroquine phosphate tablets Group B (n=50): Oral trial drug favipiravir tablets Group C (n=50): Oral placebo treatment |
• Improvement or recovery of respiratory symptoms • Viral nucleic acid shedding |
Table 1: Registered clinical trials with favipiravir for the treatment of COVID-19.
Side Effect
Using an elevated dosing regimen of favipiravir, elevated liver enzyme levels and lipemia (abnormally high blood concentration of emulsified fat) and transient thrombocytopenia (abnormally low levels of platelets) was found in an animal study of Lassa virus infection [10]. Favipiravir may cause corrected QT (QTc) interval prolongation when administered at high doses as reported by Chinello et al. [11]. If pregnancy is confirmed or suspected favipiravir administration should be avoided in women as it is known to be teratogenic [12,13]. Favipiravir and paracetamol might have a drug interaction as suggested in one in vitro study [14]. In patients concomitantly taking favipiravir maximum daily paracetamol dosage to 3 g (rather than the conventional 4 g) to avoid clinically significant interaction between these two drugs as suggested by Zhao et al. [14]. Exposure of cephalexin, flucloxacillin and penicillin may potentially increase by favipiravir [15].
There is evidence to support the safety and tolerability of favipiravir in short-term use. However, more evidence is needed to assess the longer-term effects of treatment. Given the limits of the evidence and the remaining specific safety concerns, caution is warranted in the widespread use of favipiravir against pandemic COVID-19.
Conclusion
Existing studies has confirmed the clinical effectiveness of favipiravir to treat mild to moderate COVID-19 patients. Given the limitations of the evidence and unresolved efficacy and safety concerns, caution is warranted in the widespread use of favipiravir against pandemic COVID-19. Rather an overview of several relevant publications in this area of practice this review does not intend to be a comprehensive collation of all relevant literature following a basic literature search strategy.
REFERENCES
- Du AD. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18:123.
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad-spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys. Biol Sci. 2017;93:449-463.
- Mendenhall M, Russell A, Smee DF, Hall JO, Skirpstunas R, Furuta Y, et al. Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic fever. PLoS Negl Trop Dis. 2011;5:e1342.
- Safronetz D, Rosenke K, Westover JB, Martellaro C, Okumura A, Furuta Y, et al. The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci Rep. 2015;5:14775.
- Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;3:007.
- Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997-1005.
- http://www.kansensho.or.jp.
- Xuan Yu. Initial clinical results announced for favipiravir treatment of novel coronavirus pneumonia-viral clearance in four days. Biodiscover. 2020;1-39.
- Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. Med Rxiv. 2020;3:7432.
- Rosenke K, Feldmann H, Westover JB, Hanley PW, Martellaro C, Feldmann F, et al. Use of favipiravir to treat Lassa virus infection in macaques. Emerg Infect Dis. 2018;24:1696-1699.
- Chinello P, Petrosillo N, Pittalis S, Biava G, Ippolito G, Nicastri E, et al. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis. 2017;11:e0006034.
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad-spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449-463.
- Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85-94.
- Zhao Y, Harmatz J, Epstein C, Nakagawa Y, Kurosaki C, Nakamura T, et al. Favipiravir inhibits acetaminophen sulfate formation but minimally affects systemic pharmacokinetics of acetaminophen. Br J Clin Pharmacol. 2015;80:1076-1085.
- https://www.covid19-druginteractions.org/
Citation: Das AK (2020) Favipiravir: Promising Therapy for COVID-19. J Trop Dis 8:356. doi: 10.35248/2329-891X.20.8.356.
Copyright: © 2020 Das AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.