Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 11, Issue 5
Exploration of Temperature-Induced Quantum Proton Tunneling Probability in PCR-Mediated DNA Amplification
Joseph De Luna*Received: 20-Sep-2023, Manuscript No. BEG-23-23116; Editor assigned: 22-Sep-2023, Pre QC No. BEG-23-23116 (PQ); Reviewed: 06-Oct-2023, QC No. BEG-23-23116; Revised: 13-Oct-2023, Manuscript No. BEG-23-23116 (R); Published: 23-Oct-2023, DOI: 10.35248/2167-7662.23.11.228
Abstract
This study investigates the impact of temperature induced quantum proton tunneling probability on DNA amplification during Polymerase Chain Reactions (PCR). Using a simulation model based on a Gaussian wave function and finite-difference time-domain method, quantum tunneling of protons across square potential barriers is examined. The results unveil consistent probability distributions for quantum tunneling across various PCR temperatures, with distinct oscillation patterns emerging post-barrier crossing. Acknowledging limitations in initial conditions due to temperature-dependent proton energy, the study highlights the need for refined models and experimental validation. These findings accentuate the potential interplay between quantum mechanics and biological systems, prompting further research to understand quantum tunnelling’s comprehensive effect on genetic variations and molecular processes.
Keywords
Genetics; Molecular biology; Biophysics; Quantum mechanics
Introduction
In genetic research, Deoxyribose Nucleic Acid (DNA) amplification serves as a significant technique that empowers researchers to examine and manipulate genetic information effectively. This method involves generating multiple copies of a specific DNA sequence, enabling in-depth investigations into genetic material and the functions of particular genes. Its remarkable sensitivity to detect even minute amounts of target molecules makes it indispensable in molecular diagnostics [1].
Among the prominent techniques for DNA amplification, the Polymerase Chain Reaction (PCR) stands out as the high standard. PCR plays a pivotal role in duplicating individual DNA segments through various reactions under varying temperatures, which can then be sequenced to identify genetic variants or point mutations. Point mutations encompass alterations in a single DNA base, including additions, deletions, or changes. This is hypothesized to be caused by tautomeric mutations. Tautomers are isomers that can rapidly convert in a solution often differing in proton position [2]. Analyzing these mutations within the context of existing scientific knowledge provides insights into their implications across various disciplines related to personal health such as nutrition.
Quantum tunneling, a concept derived from quantum mechanics, holds fascinating potential for comprehending tautomeric mutations in DNA. This phenomenon allows particles to surpass energy barriers that classical physics considers insurmountable. Consequently, nucleotide bases can overcome energy obstacles and form unconventional base pairs during replication. The likelihood of quantum tunneling differs across various systems. However, in a biological context, this phenomenon leads to the creation of unconventional base pairs. These pairs play a role in genetic variation, vulnerability to illnesses, and responsiveness to therapies [3].
This paper aims to explore the effects of temperature fluctuations in PCR on the probability of quantum tunneling during DNA amplification. The significance of this research lies in its potential to inform the development of more sensitive and accurate molecular diagnostic techniques, provide insights into how point mutations occur at the molecular level, and expand knowledge of the interplay between quantum effects and biological systems, potentially opening up new avenues for scientific exploration and technological applications.
Materials and Methods
In the study conducted to examine the impact of temperature on the probability of quantum tunneling in DNA, a simulation model was employed. This model utilized a Gaussian wave function and the finite-difference time-domain method. The entire simulation was executed on Google Colab, utilizing a system configuration consisting of 12.7 GB of System RAM and 107.7 GB of Disk space.
The simulation methodology was rooted in the 1D time-dependent Schrödinger equation, as originally proposed by Nishantsule in 2019 [4]. However, certain adjustments were made to the model to align it with the specific research objectives of this study.
To initiate the simulations, initial parameters were chosen in accordance with the findings of these parameters encompassed the barrier height in eV, barrier width in Angstrom, proton energy in eV, and proton wave function spread in Angstrom [5].
A noteworthy constraint in the study was the computational limitations, which led to the decision to focus solely on the median temperature recorded during each PCR step. This pragmatic approach allowed for a meaningful exploration of the temperaturedependent aspects of quantum tunneling in DNA, effectively balancing the research goals with the available computational resources. However, these limitations have implications to the results and conclusion of this paper such as reduced statistical significance as the number of simulations, variations, and repetitions was restricted and can lead to limited generalizability of the findings. This also limits the exploration of parameter space and led to the oversimplification of the PCR system [6].
The integration of these methodologies and considerations constituted the foundation of the investigation into the intriguing phenomenon of quantum tunneling in DNA under varying temperature conditions [7].
Results
To establish uniform starting conditions, normalization was conducted through the implementation of the Boltzmann Distribution (Figure 1).
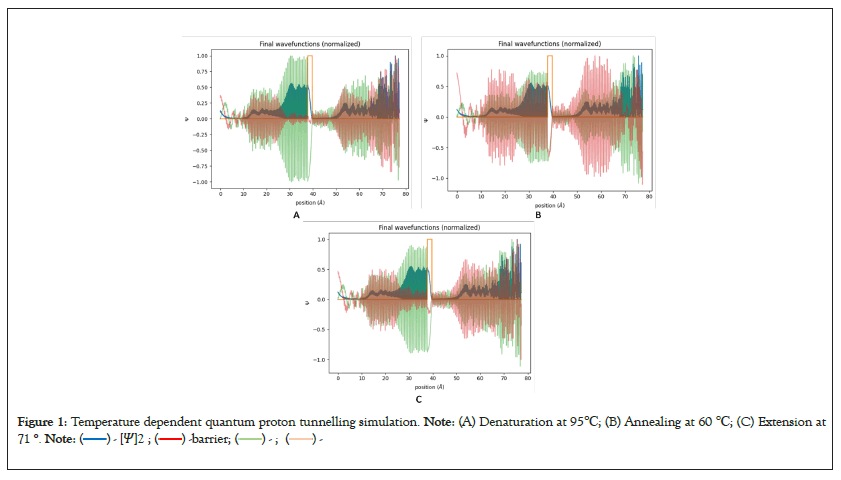
Figure 1: Temperature dependent quantum proton tunnelling simulation. Note: (A) Denaturation at 95℃; (B) Annealing at 60 ℃; (C) Extension at
71 °. 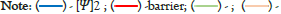
The simulation results demonstrated a consistent probability distribution for quantum tunneling, shown in blue, across a range of PCR temperatures, with probabilities spanning approximately 0.5 to 0. Interestingly, distinct oscillating patterns emerged after passing through the energy barrier [8]. These oscillations were particularly noticeable during the annealing and extension phases compared to the denaturation phase. The increased oscillations in the probability wave function after crossing the barrier suggest complex interactions involving multiple-path interference and quantum reflection. These observed oscillations suggest the potential for resonant tunneling [9]. These intricate oscillations arise from the interplay between energy level interactions, bounded states, and the particle's wavelength interacting with the energy barrier, resulting in complex interference phenomena [10]. Examining the oscillations' imaginary (green) and real (red) components provides valuable insights into the intrinsic nature of the tunneling process. Notably, the imaginary component dominates before breaching the barrier, while the real component becomes more prominent after passing the barrier. This difference suggests potential issues with the initial conditions imposed on the model [11]. Given the temperature-dependent changes in proton energy, temperature variations could significantly impact the overall energy distribution within the system [12]. As a result, the initial conditions for the denaturation simulation may not align with classical principles. On the contrary, a robust simulation model should accurately depict scenarios where real oscillations take precedence before surpassing the energy barrier. This behavior is expected when the particle's energy is lower than the barrier's height, indicating compliance with classically allowed realms [13]. Upon overcoming the barrier, the notable increase in imaginary oscillations marks the beginning of the quantum tunneling phenomenon. This is when the particle's wave function enters classically forbidden areas, and its amplitude experiences exponential decay while moving through the barrier [14]. The importance of the imaginary component increases as the amplitude decreases in the barrier zone.
Discussion
The observed constant tunneling probabilities across different PCR temperature dependent stages may indicates that the quantum tunneling probability in DNA is not heavily influenced by thermal fluctuations. This can also root to the nature of the DNA itself. In DNA, hydrogen bonds connect nucleotide base pairs (Figure 2) [15].
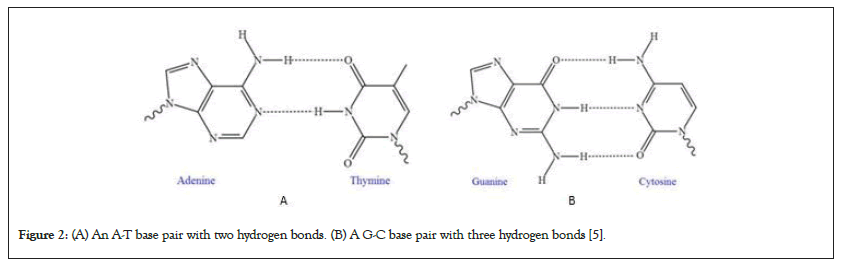
Figure 2: (A) An A-T base pair with two hydrogen bonds. (B) A G-C base pair with three hydrogen bonds [5].
The hydrogen bonds linking complementary base pairs play a crucial role as a molecular adhesive, thereby enhancing the overall stability of the helical structure of the DNA [16]. Within the DNA structure, two electron pairs compete for a single proton, establishing the foundation for complementary base pairing. This competitive interaction results in the hydrogen bond assuming two distinct equilibrium configurations.
The energy associated with these arrangements can be illustrated using a double well potential model (Figure 3). This theoretical model effectively captures the energy states available to protons [17].
Figure 3: Double well potential models (A): 2D - Model of symmetric hydrogen bonds double well potential [7]; (B): 1D - Model of asymmetric hydrogen bonds double well potential [8].
N : H - - - : N N : - - - H : N
In the model, the central elevation acts as a barrier. This barrier signifies the energy threshold essential for the proton's transition between equilibrium states. The inherent stability observed in natural DNA implies an asymmetry within the double well potential. Notably, one well exhibits lower potential energy compared to the other. Consequently, this results in a heightened potential energy barrier that effectively hinders the movement of protons toward the alternative equilibrium state [18].
However, it is important to take note the computational limitations stated in this study. Initial parameters of the simulation model used are constant across the PCR stages simulated that may affect the tunneling probabilities. Different DNA pairs have different barrier width, PCR processes have temperature ranges: Denaturation - 92°C to 98°C [9], Annealing - 55°C to 65°C, and Extension - 70°C and 72°C, and proton energy changes with temperature proven by Arrhenius equation. Exploration of these parameters can improve the model and reveal close to true value the quantum tunneling probability in DNA during PCR (Figure 4) [19].
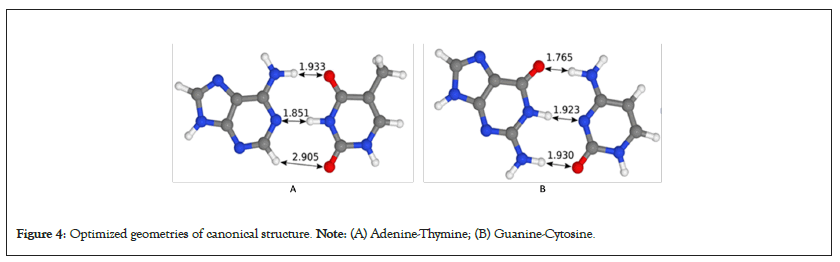
Figure 4: Optimized geometries of canonical structure. Note: (A) Adenine-Thymine; (B) Guanine-Cytosine.
Distinct oscillations observed are caused by the sine and cosine functions of the real (equation. 2) and imaginary (equation. 3) component of the proton wave function (equation. 1) simulated.
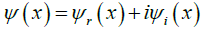
Where:
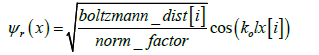
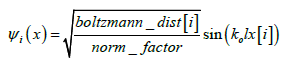
The wave vector, ko (équation. 4) is determined by the kinetic energy (ke) of the particle and the potential barrier. As the particle approches the barrier, ko becomes complex, contributing to oscillations in both real and imaginary parts.
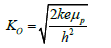
In the case of denaturation and extension, As the proton approches the barrier, ko becomes complex due to the presence of the barrier potential. The imaginary part of ko leads to an exponential decay of the wave function, meaning the wave function decreases rapidly as the proton approaches the barrier. The sine function, being an oscillatory function, con-tributes larger values as it oscillates rapidly due to the complex ko [20]. This dominant oscilla-tory behavior is captured by the imaginary component (Ψi), representing the particle's wave like nature and it’s tendency to interact with the barrier. After the proton passes through the barrier (tunneling), the real component (Ψr) becomes more prominent as ko transitions to have a significant real component. The cosine component, being oscillatory but with a different phase, starts to contribute significantly to the wave function. As the proton moves away from the barrier, the real part becomes more pronounced, representing the particle's behavior as it continues it’s motion beyond the barrier. The potential for resonant tunneling is implied by the observed oscillations [21]. In the simulation, as the particle's energy aligns with certain energy levels of the barrier, constructive interference occurs, leading to increased probability amplitudes. This quantum proton tunneling in DNA can lead on tautomeric mutations based on Lowdin’s Hypothesis (Figure 5) [22].
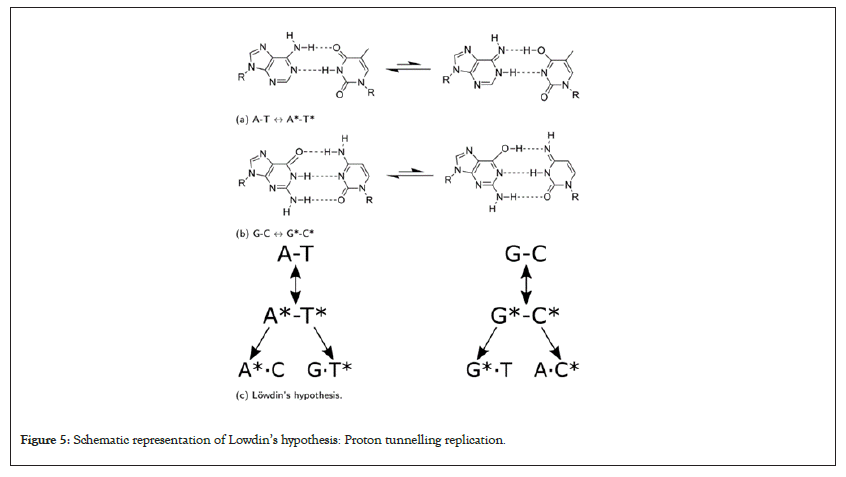
Figure 5: Schematic representation of Lowdin’s hypothesis: Proton tunnelling replication.
Lowdin’s hypothesis stated that these specific tautomeric forms result from a unique occurrence of double proton tunneling within the nucleotide base pairing. It's important to note that these mutations are short-lived [12]. In the context of DNA replication and transcription, the emergence of tautomeric forms through proton tunneling has the potential to introduce brief inaccuracies. When encountering these forms, the DNA replication ma-chinery could misinterpret their structure, leading to incorrect pairing with complementary bases. This mispairing, in turn, might lead to the insertion of incorrect nucleotides during DNA replication, ultimately causing mutations in the newly synthesized DNA strand [13]. Despite their intermittent and transient nature, determining the exact role of these tautomeric forms in replication and translation errors remains a challenging puzzle. Multiple studies have aimed to establish a definitive link between tautomeric mutations and these errors. However, due to the intricacies of the phenomenon and the challenges associated with experimental studies, ongoing research continues to shape our understanding in this area [23].
The findings presented in the discussion have significant potential implications. This can be used to better understood to design more efficient PCR processes. By optimizing temperature ranges and considering the quantum behavior of protons, faster and more precise DNA amplification can be achieved, which could improve the efficiency of molecular diagnostics. Insights into the quantum nature of proton behavior in DNA can impact drug design and development [24]. Understanding how protons tunnel through energy barriers within DNA could influence the design of drugs that specifically target DNA regions, potentially improving drug efficacy and reducing side effects. The quantum tunneling behavior observed in DNA could guide the development of tailored genetic therapies. Understanding how protons interact with DNA barriers at different temperatures might enable the precise targeting of specific DNA sequences for therapeutic interventions, advancing the field of personalized medicine. Knowledge of quantum tunneling in DNA may inspire advancements in nanotechnology. Designing nanoscale devices that harness the principles of quantum tunneling in DNA could lead to highly sensitive biosensors or innovative diagnostic tools, revolutionizing molecular diagnostics. Exploring the quantum nature of DNA could have implications for quantum computing in the field of genetic re-search. Understanding how protons interact with potential energy barriers at a quantum level might inspire the development of quantum algorithms for DNA analysis and genomic studies. The findings could spur advancements in theoretical models used in molecular dynamics simulations. Incorporating quantum tunneling behavior into these models could result in more accurate predictions of DNA behavior, aiding genetic re-search and drug discovery [25].
Conclusion
In conclusion, the study's exploration of quantum tunneling within the Polymerase Chain Reaction (PCR) process offers intriguing insights into the interplay between quantum phenomena and DNA amplification. However, it's important to acknowledge the limitations of the model in drawing definitive conclusions. The complex oscillations and behaviors observed during PCR, combined with the temperature-dependent nature of quantum effects, suggest intricate interactions that require further investigation. The study underscores the need for more comprehensive experimental validations and refined models to ascertain the true extent of quantum tunnelling’s influence on DNA amplification dynamics.
With these considerations in mind, several recommendations emerge:
Development of a more accurate and inclusive model
Future research should prioritize the creation of a model that can faithfully replicate biological samples from their natural environment. Such an improved model would better capture the complexities of quantum tunneling within DNA amplification.
Evaluation of alternative kinetic models
The exploration of other kinetic models that can provide a more comprehensive description of quantum proton tunneling is warranted. Diversifying the range of models may offer insights into different facets of quantum effects in biological processes.
Investigation of additional parameters
Beyond temperature, researchers should examine other parameters within PCR machines that may contribute to quantum tunneling probability. Identifying these factors could lead to a more complete understanding of the quantum mechanical aspects of DNA amplification.
Exploration of tautomeric mutations
Establishing a solid connection between tautomeric mutations and replication/translation errors is essential. Investigating this relationship can help clarify how quantum tunneling influences genetic variations and molecular processes more precisely.
These recommendations collectively represent crucial steps toward a deeper comprehension of the interplay between quantum mechanics and biology. While the study's results provide a promising glimpse into this potential connection, additional research is necessary to uncover the full scope of its impact on genetic variations and molecular processes.
Acknowledgement
The author would like to acknowledge Ms. Geraldine Pulido for having useful discussion. This work was supported by the Department of Science and Technology, Food and Nutrition Research Institute, Nutrigenomics Laboratory.
Conflicts of Interest
The author declare no conflict of interest
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
References
- Oliveira BB, Veigas B, Baptista PV. Isothermal amplification of nucleic acids: The race for the next “gold standard”. Front Sens. 2021;2:752600.
- Al-Sehemi AG, El-Gogary TM, Wolschann KP, Koehler G. Structure and stability of chemically modified DNA bases: Quantum chemical calculations on 16 isomers of diphosphocytosine. Int Sch Res Notices.2013;2013.
- Slocombe L, Al-Khalili JS, Sacchi M. Quantum and classical effects in DNA point mutations: Watson–Crick tautomerism in AT and GC base pairs. Phys Chem Chem Phys. 2021;23(7):4141-4150.
- Nishant Sule. Nishantsule/Quantum-Tunneling: A Wave Function Tunneling across a Barrier. 2017
- Wolfe M. Quantum Tunneling in DNA. 2013
- Nelson DL, Cox MM. Lehninger principles of biochemistry. New York, NY: Macmillan International Higher Education. 2021.
- Krasilnikov PM. Two-dimensional model of a double-well potential: Proton transfer upon hydrogen bond deformation. Biophys. 2014;59:189-198.
[Crossref] [Google Scholar] [PubMed]
- Isothermal amplification of nucleic acids: The race for the next “gold standard”.
- Wang X, Son A. Effects of pretreatment on the denaturation and fragmentation of genomic DNA for DNA hybridization. Environ Sci Process Impacts. 2013;15(12):2204-2212.
- Erjavec MS. Annealing temperature of 55˚C and specificity of primer binding in PCR reactions. InSynthetic Biology-New Interdisciplinary Science. IntechOpen. 2020.
- Burton E. Module 4.2: Denaturation, Annealing, and Primer Extension. 2021.
- Umesaki K, Odai K. A kinetic approach to double proton transfer in Watson–Crick DNA base pairs. J Phys Chem B 2020;124(9):1715-1722.
[Crossref] [Google Scholar] [PubMed]
- Ghasemi F, Tirandaz A. Environment assisted quantum model for studying RNA-DNA-error correlation created due to the base tautomery. Sci Rep. 2023;13(1):10788.
[Crossref] [Google Scholar] [PubMed]
- Biological Technology PCR and Molecular Biology Fundamental Principles. Boster. 2019
- Hood L, Rowen L. The human genome project: Big science transforms biology and medicine. Genome Med. 2013;5:1-8.
[Crossref] [Google Scholar] [PubMed]
- Acosta-Reyes FJ, Alechaga E, Subirana JA, Campos JL. Structure of the DNA duplex d (ATTAAT) 2 with Hoogsteen hydrogen bonds. PLoS one. 2015;10(3):e0120241.
[Crossref] [Google Scholar] [PubMed]
- Porta AR, Enners E. Determining annealing temperatures for polymerase chain reaction. Am Biol Teach 2012;74(4):256-260.
- Hoque ME, Rajaee N. Encoding information in DNA: From basic structure to nanoelectronics. Am J Nanomater. 2017;5(2):51-58.
- Gutzwiller MC. Chaos in classical and quantum mechanics. Springer Science and Business Media. 2013.
- Luo J. Sub-picosecond proton tunnelling in deformed DNA hydrogen bonds under an asymmetric double-oscillator model. Eur Phys J E. 2018;41:1-9.
[Crossref] [Google Scholar] [PubMed]
- Turaeva N, Brown-Kennerly V. Marcus model of spontaneous point mutation in DNA. Chem Phys. 2015;461:106-110.
- Parker BR, Van Everv J. Quantum tunnelling in DNA. Chem Phys Lett. 1971;8(1):94-99.
- Srivastava R. The role of proton transfer on mutations. Front Chem. 2019;7:536.
- Löwdin PO. Proton tunneling in DNA and its biological implications. Rev Mod Phys. 1963;35(3):724.
- Chaudhury R. Tautomeric mutation in DNA. A theoretical comparative study and synthesis between various proposed quantum models. 2015.
Citation: Luna JD (2023) Exploration of Temperature-Induced Quantum Proton Tunneling Probability in PCR-Mediated DNA Amplification. J Bio Energetics. 11:228.
Copyright: © 2023 Luna JD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
