Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 5, Issue 2
Evaluation of the Retinal Nerve Fiber Layer Thickness and the Choroidal Thickness in Generalized Vitiligo Patients
Muslum Toptan and Mustafa Aksoy*Received: 17-Apr-2020 Published: 09-May-2020, DOI: 10.35248/2684-1622.20.4.134
Abstract
Aim: The retinal nerve fiber layer (RNFL) thickness and the choroidal thickness are compared to healthy volunteers in vitiligo patients. Materials and Methods: The study included the right eyes of 60 healthy individuals and 60 generalized vitiligo patients. Following routine ophthalmological examination, the RNFL thickness and the choroidal thickness were analyzed with spectral domain optical coherence tomography (SD-OCT). Results: It was determined that the mean macular choroidal thickness decreased significantly in vitiligo patients, while the RNFL thickness did not change (p<0.05). Conclusion: As the fact that the RNFL thickness does not change despite the significantly reduced choroidal thickness in vitiligo patients may indicate that the melanin cells that are dense in the choroid are affected, the noninvasive, simple and rapid measurement of the choroidal thickness with OCT can help in determining the damage in vitiligo patients.
Keywords
Vitiligo; Choroid; Nerve fibers; Optical coherence tomography
Introduction
Vitiligo is a disease whose etiopathogenesis is unknown, characterized by the formation of depigmentation in different parts of the skin due to melanocyte destruction [1]. The loss of pigment cells may not be limited to the skin, and various ocular abnormalities can be seen as melanocytes in the eyes develop from neural crest cells [2-4]. These melanocytes are found in uveal tissues such as the eyelash, retinal pigment epithelium, choroid, ciliary body, and iris. Melanin in these tissues can disappear when destruction occurs in cutaneous melanocytes in vitiligo patients [5]. As a result, some studies reported hypopigmented spots on the iris and retina, atrophic changes in the peripapillary area, degeneration in the retinal pigment epithelium and chorioretinal areas [2,3,6].
The choroid is a highly vascularized and pigmented tissue that feeds the outer retina, and especially the stroma of the choroidal layer contains a high number of melanocytes. Melanin, produced in melanocytes in the choroidal layer and stored in melanosomes, has a crucial role in protecting and absorbing light from intraocular reflection [4]. There are a limited number of studies showing that the affected choroid is also affected in vitiligo in some systemic inflammatory diseases [5,7].
Rogosic found that there was an association between the duration of vitiligo and the development of glaucoma [8].
There are also studies indicating impaired retinal electrophysiological function in vitiligo patients [9,10]. Abnormal electro-oculographic findings of visual evoked potentials were observed, especially in patients with vitiligo with large skin involvement and longer disease duration [10].
We aimed to compare the choroid and the RNFL thicknesses of vitiligo patients using spectral domain optical coherence tomography (SD-OCT) because it is not known enough how and how much the sensitive retinal nerve fiber layer (RNFL) of specific importance is affected in the evaluation of glaucomatous damage with the highly pigmented and vascularized choroidal tissue.
Materials and Methods
This study was conducted in the Department of Eye Diseases and Skin and Venereal Diseases of Harran University in 2019-2020. Study protocol was approved by the Ethics Committee of Harran University (Document Number: E.35564) and informed consent was received from the participants. The right eyes of sixty generalized vitiligo patients and the right eyes of 60 healthy individuals paired with this patient group in terms of age and gender were included in the study as a control group.
A comprehensive medical history including the drugs used for each patient was taken and general dermatological physical examinations were performed by the same dermatologist. Both groups also underwent a full ophthalmological examination, which included the best corrected visual acuity, intraocular pressure measurement with the Goldmann Applanation Tonometer, refraction, biomicroscopy of the anterior and posterior segment of the eye. Axial length was measured using ultrasonic biometrics Pac-Scan 300p (Sonomed Escalon, NY) and visual field examination was performed using Humphrey perimetry (Carl Zeiss Meditec, Dublin, CA) with 30-2 program.
People under the age of 18, people with cataract or glaucoma, people with a BCVA of 20/40, people with a history of ocular surgery, people with optic nerve or retina disorders, people with systemic diseases that can affect the eye such as diabetes mellitus and hypertension were not included in the study and all patients between the ages of 18-40 with generalized vitiligo diagnosis were included in the study.
Choroidal thickness measurements were performed by the same experienced technician in increased depth imaging mode with the Heidelberg spectral domain optical coherence tomography device (SD-OCT, Heidelberg Engineering, Germany). The choroidal thickness was measured manually by marking the distance between the outer border of the retinal pigment epithelium and the choroid-sclera compound. In addition to the thickness of the subfoveal choroid, seven measuring points were determined, including 3 nasal and 3 temporal with 500 μm intervals in the temporal and nasal directions. N1, N2 and N3 abbreviations were given to the points identified in the nasal respectively, from the central to the periphery; and T1, T2 and T3 abbreviations were given to the points identified in the temporal respectively, from the central to the periphery (Figure 1).
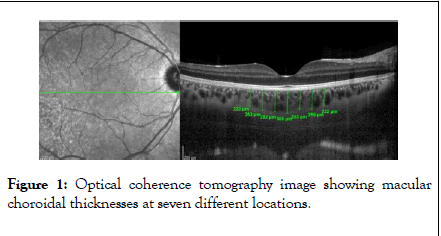
Figure 1: Optical coherence tomography image showing macular choroidal thicknesses at seven different locations.
Peripapillary RNFL thickness parameters were calculated automatically with fast RNFL mode. The software calculated average thickness values (μm) for each of the six sectors centred on the optical disk (temporal, temporal superior, temporal inferior, nasal, nasal inferior, and nasal superior) (Figure 2). Sections below signal strength index 6/10 were not evaluated. Measurements were made between 09.00-12.00 in the morning to prevent diurnal changes.
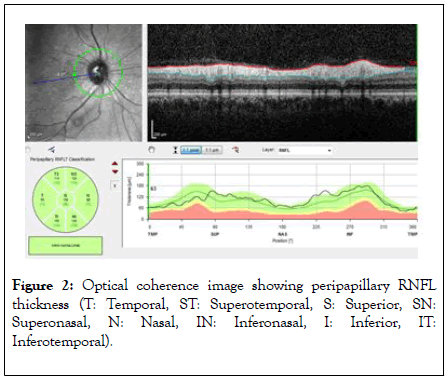
Figure 2: Optical coherence image showing peripapillary RNFL thickness (T: Temporal, ST: Superotemporal, S: Superior, SN: Superonasal, N: Nasal, IN: Inferonasal, I: Inferior, IT: Inferotemporal).
Statistical Analysis
Statistical evaluation was conducted with the IBM SPSS 15 package program (SPSS Inc., Chicago, IL, USA). Numerical variables with normal distribution are given as mean ± standard deviation, numerical variables without normal distribution are given as median (minimum-maximum), and categorical variables as frequency (percentages). In the case of two groups, the difference between the groups was determined with the Student t Test for the numerical variables with normal distribution, the Mann Whitney U Test for the numerical variables without normal distribution, the Pearson Chi-Square Test and the Fischer Exact Test. The relationship between numerical variables was determined with the Pearson correlation analysis. P value of 0.05 was considered statistically significant.
Results
The demographic characteristics and clinical findings of the individuals in the group are shown in Table 1. There is no statistically significant difference between the patient and the control group in terms of age, sex, IOP (intraocular pressure), axial length, MD (mean deviation), PSD (patent standard deviation) and visual acuity variables (p>0.05).
| Variable | Patient | Control | p |
|---|---|---|---|
| Age (Year) | 31(19-55) | 28(16-51) | 0,274* |
| Gender (1/2) | 28/32 | 28/32 | <0,001*** |
| IOP (mm Hg) | 16(13-18) | 16(11-20) | 0,585* |
| Axial length (mm) | 22,57(21,24-24,65) | 22,58(21,24-24,61) | 0,935* |
| Perimetric AD (dB) | -3,38 ± 1,22 | -3,21 ± 1,06 | 0,429** |
| Perimetric PSD (dB) | 3,7(1,19-5,63) | 4,11(1,19-5,63) | 0,960* |
| Visual acuity | 0,9 (0,7-1,0) | 0,9(0,8-1,0) | 0,715* |
*Mann Whitney U Test, ** Student t Test, *** Chi-Square Test
IOP: İntraocular pressure; AD: Average deviation; PSD: Pattern standard deviation
Table 1: Demographic and clinical features of the participants.
Ocular fundus findings such as peripapillary atrophy in three patients, focal hypopigmentation in two patients and widespread hypopigmentation in one patient were found.
The mean choroidal thickness was 275,42 (202,57-379,14) μm in the patient group and 297,50 (182,71-371,71) μm in the control group (p=0.006).
While there was a statistically inversely low association between subfoveal choroidal thickness and duration of disease in vitiligo (p=0,029, r=-0,283), there was no statistically significant association between age and the choroidal thickness (p>0.05) (Table 2).
| T3 | T2 | T1 | Subfoveal | N1 | N2 | N3 | Average | ||
|---|---|---|---|---|---|---|---|---|---|
| Duration of ilness | p | 0,121 | 0,059 | 0,054 | 0,029* | 0,606 | 0,629 | 0,572 | 0,152 |
| r | -0,202 | -0,245 | -0,250 | -0,283 | -0,068 | -0,064 | -0,074 | -0,187 | |
| Age | p | 0,181 | 0,364 | 0,582 | 0,759 | 0,793 | 0,789 | 0,449 | 0,492 |
| r | 0,175 | 0,119 | 0,072 | 0,040 | 0,035 | 0,035 | 0,100 | 0,090 |
*p<0,05
Table 2: Corelation between choroidal thickness with disease duration and age.
While there was statistically significant difference between the patient and the control group in terms of T3, T2, T1, subfoveal, N1 and mean values (p<0.05), there was no statistically significant difference between N2 and N3 values (p>0.05) (Table 3).
| Patient | Control | p | |
|---|---|---|---|
| T3 | 254,83 ± 48,76 | 279,80 ± 42,78 | 0,003* |
| T2 | 272,52 ± 49,05 | 298,25 ± 46,93 | 0,004* |
| T1 | 285,78 ± 50,66 | 317,5 ± 45,20 | <0,001* |
| Subfoveal | 318,77 ± 50,10 | 356,48 ± 41,05 | <0,001* |
| N1 | 297,22 ± 316,82 | 316,62 ± 46,03 | 0,029* |
| N2 | 279,92 ± 49,58 | 284,75 ± 48,95 | 0,592* |
| N3 | 252,43 ± 49,95 | 259,65 ± 50,39 | 0,432* |
| Average | 280,20 ± 45,06 | 301,86 ± 41,70 | 0,007* |
*Student t test. Average ± Standard deviation
Table 3: Comparison of choroidal thickness (µm) between groups.
While there was statistically significant difference between the patients and the control group in terms of superionasal and inferiotemporal values (p<0.05), there was no statistically significant difference between the groups in terms of global, temporal, superiotemporal, nasal, and inferionasal values (p>0.05) (Table 4).
| Patient | Control | p | |
|---|---|---|---|
| Global | 105(88-128) | 107,5(96-126) | 0,345* |
| Temporal | 75(55-108) | 78,8(46-99) | 0,235* |
| Superiotemporal | 131(100-167) | 137,5(103-169) | 0,055* |
| Superionasal | 104,5(72-161) | 112,5(86-141) | 0,012* |
| Nasal | 83,5(62-121) | 84,5(63-109) | 0,525* |
| Inferionasal | 116,5(89-165) | 118(95-149) | 0,282* |
| Inferiotemporal | 140,5(106-214) | 150(119-175) | 0,009* |
*Mann Whitney U Test. Median (Minimum-Maximum)
Table 4: Comparson of RNFL (µm) between groups.
While there was no statistically significant difference between periocular involvement and non-macular choroidal values in the patient group, macular choroidal thickness of periocular involvement patients was observed to be thinner (Table 5).
| Without periocular involvement | With periocular involvement | p | |
|---|---|---|---|
| T3 | 267,15 ± 48,46 | 251,43 ± 48,80 | 0,307* |
| T2 | 287,85 ± 47,29 | 268,28 ± 49,17 | 0,206* |
| T1 | 298,46 ± 49,63 | 282,28 ± 50,91 | 0,312* |
| Subfoveal | 323,54 ± 45,00 | 317,45 ± 51,80 | 0,702* |
| N1 | 300,15 ± 48,51 | 296,40 ± 51,15 | 0,814* |
| N2 | 284,15 ± 47,00 | 278,74 ± 50,69 | 0,731* |
| N3 | 261,46 ± 52,96 | 249,94 ± 49,39 | 0,466* |
| Average | 288,96 ± 45,15 | 277,78 ± 45,22 | 0,433* |
*Student t test. Average ± Standard deviation
Table 5: The effect of periocular involvement on choroidal thickness (µm) between groups.
There was no statistically significant difference in the RNFL thickness between those with periocular involvement and those without (Table 6).
| Without periocular involvement | With periocular involvement | p | |
|---|---|---|---|
| Global | 102(94-121) | 105(88-128) | 0,130* |
| Temporal | 74(60-98) | 75(55-108) | 0,829* |
| Superiotemporal | 128(100-141) | 134(100-167) | 0,080* |
| Superionasal | 111(83-161) | 104(72-161) | 0,584* |
| Nasal | 84(70-98) | 83(62-121) | 0,494* |
| Inferionasal | 120(102-164) | 116(89-165) | 0,364* |
| Inferiotemporal | 138(107-169) | 142(106-214) | 0,355* |
*Mann Whitney U Test. Median(Minimum-Maximum)
Table 6: The effect of periocular involvement on RNFL thickness (µm) between groups.
Discussion
Although many factors have been blamed for the pathogenesis of vitiligo, the etiology is not yet fully known. Antibodies against melanocytes are known to be destructive to melanocytes in the vast majority of patients [1].
Evidence of the destruction of uveal melanocytes and retinal pigment epithelium in vitiligo patients was first reported by Albert et al [11]. In some studies, hypopigmentation of the choroid was also detected in vitiligo patients [12,13]. Bulbul, in their study which examined ocular findings in 45 vitiligo patients, found peripapillary atrophy around the optic nerve in seven patients, atrophy of the retina pigment epithelium in two patients, diffuse in the retina in one patient, focal hypopigmented spots in one patient. Their study also found that ocular findings were significantly observed in patients with periorbital and to a lesser degree genital vitiligo [6]. Biswas found pigment change in iris and anterior chamber in 41 of 100 vitiligo patients, retinal pigment epithelial hypopigmentation in 9 of them, uveitis in 5 of them, chorioretinal degeneration in 11 of them, and reported no ocular findings in 34 of them, indicating a strong association between vitiligo and ocular disorder [3]. Karadağ et al. found abnormal retinal findings in 24 out of 122 eyes in 122 vitiligo patients; determined Tigroid retina in 11 eyes, oblique disc in 3 eyes, peripapillary atrophy in 3 eyes, drusen in 2 eyes, choroidal nevus in 1 eye, myelinated nerve fiber in 1 eye, cage degeneration in 1 eye [2]. In our study, we found ocular fundus findings such as peripapillary atrophy in three patients, focal hypopigmentation in two patients and widespread hypopigmentation in one patient.
Studies on the choroidal tissue in vitiligo patients have been limited, especially in-vivo study of the choroid with the EDI form of the SD-OCT device has helped to understand the diagnosis and pathogenesis of some diseases more clearly. The choroidal thickness is known to be affected by age, sex and ax [14]. In our study, there were no significant differences between age, gender, and ax among the groups.
The choroid is among the most vascularized tissues in the body, covering the outer retina. A pathology that can occur in the choroidal structure for any reason can result in decreased vision as a result of degenerative changes in photoreceptors. The stroma of the choroidal layer contains a high number of melanocytes [4]. A low choroidal thickness may be expected in vitiligo where melanocyte loss is observed. Foveal hypoplasia was found in several studies in patients with albinism [15,16]. In patients with oculocutaneous albinism with melanocyte deficiency, the choroidal thickness in the subfoveal region was significantly lower than in the control group and there was no difference in the peripapillary region compared to the control group [17]. Demirkan et al. determined that the choroidal thickness in vitiligo patients was significantly thin compared to the control group in all values except optic nerve area measurements, and it was indicated that choroidal thickness may be affected by melanocyte loss in vitiligo. The absence of differences between vitiligo patients and the control group in optic nerve regions was linked to less space coverage of melanocytes in histological structure in optic nerve regions. It was also stated that periorbital involvement had no effect on the choroidal thickness [18,19].
Vogt-Koyanagi-Harada (VKH) syndrome is a disease characterized by a T cell-mediated autoimmune process aimed at melanocytic antigens. Ocular symptoms of the disease include severe bilateral panuveitis associated with exudative retinal
detachment. Retinal edema, choroidal thickening, bilateral vitritis, papillitis and retinal detachment are seen. As exudation is not observed in vitiligo patients, increase in the choroidal thickness is not expected despite melanocyte destruction as in VKH [20]. We also determined that even though visual acuity of vitiligo patients was at the same level as the control group, the mean macular choroidal thickness was thinner. We found no significant changes in the choroidal thickness in patients with periocular attitude. We found a negative correlation between the choroidal thickness and the duration of the disease. Also, in our study, although there was significant decrease in the choroidal thickness of N1, T1, T2, T3 in vitiligo patients, there was no significant change in N2, N3, and this may indicate that the choroidal thickness changed slightly in this region due to the less dense melanin cells around the optic disc.
In the diagnosis of glaucoma, the determination of thickness thinning of OCT and RNFL is considered the most specific test for objective detection of damage [21]. In the studies of Rogosic, the age of the patient and the duration of vitiligo were significantly correlated with primary open-angle glaucoma. In their study, the RNFL thickness was measured to gauge neuroretinal damage [8].
Dertlioglu reported in their studies that normotensive glaucoma was seen in 18.4% of vitiligo group and that there was a statistically significant difference compared to control group. They found no correlation between periocular involvement and glaucoma [22]. Duplancic reported that the prognostic value of hemodynamic changes determined by color Doppler imaging in the ophthalmic artery and that there was a positive correlation between vitiligo and POAG [23]. In many sources, it was that glaucoma seen in vitiligo is a secondary complication, a direct result of corticosteroid therapy [24,25]. Studies of Khurrum demonstrated that patients with vitiligo and periorbital topical steroids did not have an increased risk of glaucoma or cataracts [26]. Ornek in their study, reported no significant differences between vitiligo patients and control groups in the upper, lower, nasal and temporal quadrants in the topographic distribution of RNFL, and concluded that the RNFL thickness was not affected in vitiligo patients [5].
Electroretinography (ERG) and visual evoked potentials (VEP), which are electrophysiological tests, are useful tests for determining retinal ganglion cell and RNFL dysfunction [27]. Shoeibi reported that overall retinal electrophysiological function in vitiligo patients decreased significantly compared to the normal population regardless of age and sex [9]. Perossini et al. identified abnormal VEP and electrooculography (EOG) in patients with severe vitiligo. They found more pronounced negative ocular electrophysiological results in patients with greater skin involvement and longer duration of disease [10]. In an animal study, abnormal flash ERG findings secondary to histopathological abnormalities were reported in mutant mice with vitiligo, and the apopitosis of photoreceptors in these mice was demonstrated in morphological and biochemical studies [28]. Aydın in their studies on vitiligo patients found that the photoreceptor segment was preserved in "SD-OCT", while in multifocal electroretinography (mfERG), there was a decrease in central retinal function, indicating a potential decrease. This study showed that even with normal fundus appearance and SDOCT findings, there may be a potential decline in central retinal function in vitiligo patients. Their study also noted that there was no statistically significant difference in the entire retinal layer thickness between the vitiligo and the control group [29].
Since the choroidal thickness is affected by many factors and diurnal choroidal thickness changes occur at 30-60 μm [14], we found it appropriate to measure the choroidal thickness values in a limited time period (between 09: 00 and 12:00 hours). Low case count, short follow-up time, the fact that factors which can affect the choroidal thickness measurements such as smoking and caffeine use are not studied, and manual measurement of the choroidal thickness are limited aspects of our study. Studies with a longer follow-up period and broader participation investigating all factors that may affect the choroidal and the RNFL thickness in vitiligo patients may provide greater clarity on this issue.
Conclusion
As far as we know, this study is the first to examine the choroidal thickness and the RNFL thickness with SD-OCT in vitiligo patients, analyzing its correlation with disease duration. In our study, it was determined that mean macular choroidal thickness decreased significantly in vitiligo patients, while the RNFL thickness did not change. Negative correlation was found between the choroidal thickness and the duration of the disease. There was no significant correlation between the choroidal and the RNFL thickness in vitiligo patients with periocular involvement. We are of the opinion that close monitoring of the vitiligo patients by the eye diseases department, the non-invasive OCT screening for posterior ocular segment disorders, and OCT-EDI examinations for the choroidal thickness containing dense melanin may be much more beneficial.
REFERENCES
- Schild M, Meurer M. Vitiligo: Clinical presentation and pathogenesis. Hautarzt. 2016;67(2):173-86.
- Karadag R, Esmer O, Karadag AS, Bilgili SG, Cakici O, Demircan YT, et al. Evaluation of ocular findings in patients with vitiligo. Int J Dermatol. 2016;55(3):351-355.
- Biswas G, Barbhuiya JN, Biswas MC, Islam MN, Dutta S. Clinical pattern of ocular manifestations in vitiligo. J Indian Med Assoc. 2003;101(8):478-480.
- 4. Cook CS, Ozanis V, Jakobiec FA. Prenatal development of the eye and its adnexa. In: Tasman W (ed) Duane's Ophthalmology. 1991;1–93.
- Ornek N, Onaran Z, Kocak M, Ornek K. Retinal nerve fiber layer thickness in vitiligo patients. J Res Med Sci. 2013;18(5):405-407.
- Bulbul Baskan E, Baykara M, Ercan I, Tunali S, Yucel A. Vitiligo and ocular findings: A study on possible associations. J Eur Acad Dermatol Venereol. 2006;20(7):829-833.
- Kurt A, Kurt EE, Kilic R, Oktem C, Tuncay F, Erdem HR. Is choroidal thickness related with disease activity and joint damage in patient with rheumatoid arthritis. Bratisl Lek Listy. 2017; 118(1):23-27.
- Rogosic V, Bojic L, Puizina-Ivic N, Vanjaka-Rogosic L, Titlic M, Kovacevic D, et al. Vitiligo and glaucoma: An association or a coincidence? A pilot study. Acta Dermatovenerol Croat. 2010;18(1):21-26.
- Shoeibi N, Taheri AR, Nikandish M, Omidtabrizi A, Khosravi N. Electrophysiologic evaluation of retinal function in patients with psoriasis and vitiligo. Doc Ophthalmol. 2014;128(2):131-136.
- Perossini M, Turio E, Perossini T, Cei G, Barachini P. Vitiligo: Ocular and electrophysiological findings. G Ital Dermatol Venereol. 2010;145(2):141-149.
- Albert DM, Nordlund JJ, Lerner AB. Ocular abnormalities occurring with vitiligo. Ophthalmology. 1979;86(6):1145-1160.
- Kim M, Kwon JW, Park YH. Atypical Pattern of Choroidal Hypopigmentation with Cutaneous Vitiligo. Korean J Ophthalmol. 2019;33(1):99-100.
- Ciardella AP, Horsley MB, Brown DM. Hypopigmentary fundus changes seen with cutaneous vitiligo. Arch Ophthalmol. 2007;125(4):576.
- Aksoy Y, Çolakoglu K, Kar T, Sevinc MK, Eyi YE. Choroidal thickness is affected by more factors than estimated. Invest Ophthalmol Vis Sci. 2014;55(3):1311.
- Thomas MG, Kumar A, Mohammad S, Proudlock FA, Engle EC, Andrews C, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity. Ophthalmology. 2011;118(8):1653-1660.
- Seo JH, Yu YS, Kim JH, Choung HK, Heo JW, Kim SJ. Correlation of visual acuity with foveal hypoplasia grading by optical coherence tomography in albinism. Ophthalmology. 2007;114(8):1547-1551.
- Karabas L, Esen F, Celiker H, Elcioglu N, Cerman E, Eraslan M, et al. Decreased subfoveal choroidal thickness and failure of emmetropisation in patients with oculocutaneous albinism. Br J Ophthalmol. 2014;98:1087–1090.
- Demirkan S, Onaran Z, Samav G, Ozkal F, Yumusak E, Gunduz O, et al. Decreased choroidal thickness in vitiligo patients. BMC Ophthalmol. 2018;29;18(1):126.
- Street D, Sivaguru A, Sreekantam S, Mollan SP. Vogt-Koyanagi-Harada disease. Pract Neurol. 2019;19(4):364-367.
- Perente I, Utine CA, Cakir H, Kaya V, Tutkun IT, Yilmaz OF. Management of ocular complications of Vogt-Koyanagi-Harada syndrome. Int Ophthalmol. 2009;29(1):33-37.
- Zangwill LM, Bowd C. Retinal nerve fiber layer analysis in the diagnosis of glaucoma. Curr Opin Ophthalmol. 2006;17(2):120-131.
- Dertlioglu SB, Oguz H, Cicek D, Yucel H. Prevalence of glaucoma in patients with vitiligo. Cutis 2016;97: 21–25.
- Duplancic D, Rogosic V, Puizina-Ivic N, Rogosic LV, Luksic B, Kovacic V, et al. Prognostic value of ophthalmic artery color Doppler sonography for progression to glaucoma in vitiligo patients. Acta Med Croatica. 2013;67(1):47-52.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56(3):164-169.
- David DS, Berkowitz JS. Ocular effects of topical and systemic corticosteroids. Lancet. 1969;2(7612):149-51.
- Khurrum H, Alhamdi KM, Osman E. Screening of glaucoma or cataract prevalence in Vitiligo patients and its relationship with periorbital steroid use. J Cutan Med Surg. 2016 Mar-Apr; 20 (2): 146-9.
- Kurysheva NI, Maslova EV, Zolnikova IV, Fomin AV, Lagutin MB. A comparative study of structural, functional and circulatory parameters in glaucoma diagnostics. PLoS One. 2018;13(8):201599.
- Tang M, Pawlyk BS, Kosaras B, Berson EL, Sidman RL. ERG abnormalities in relation to histopathologic findings in vitiligo mutant mice. Exp Eye Res. 1997;65(2):215-22.
- Aydin R, Ozsutcu M, Erdur SK, Dikkaya F, Balevi A, Ozbek M, et al. The assessment of macular electrophysiology and macular morphology in patients with vitiligo. Int Ophthalmol. 2018;38(1):233-239.
Citation: Aksoy M, Toptan M (2020) Evaluation of the Retinal Nerve Fiber Layer Thickness and the Choroidal Thickness in Generalized Vitiligo Patients. J Eye Dis Disord 5: 134 DOI: 10.35248/2684-1622.20.4.134
Copyright: © 2020 Toptan M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
