Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 13, Issue 3
Evaluation of the Effectiveness of Mud Acid in Well Stimulation
Udeagbara SG*Received: 07-Mar-2022, Manuscript No. JPEB-22-15742; Editor assigned: 11-Mar-2022, Pre QC No. JPEB-22-15742(PQ); Reviewed: 24-Mar-2022, QC No. JPEB-22-15742; Revised: 30-Mar-2022, Manuscript No. JPEB-22-15742(R); Published: 06-Apr-2022, DOI: 10.35248/2157-7463.13.2.452
Abstract
Production enhancement from oil and gas fields is of key importance to operators. In order to achieve enhanced production, well stimulation techniques are often deployed to maximize recovery from oil and gas wells. Matrix acidizing is the most prominent technique deployed among other well stimulation techniques; considering its relatively lower cost, compared to hydraulic fracturing. Also of importance is the suitability of matrix acidizing to generate extra production and restore original productivity in wells that are damaged. Matrix acidization involves injection of an acid solution into the reservoir formation, at a pressure below the fracture pressure to dissolve some of the minerals within the rock with the key objective of removing damage near the wellbore, subsequently restoring the natural permeability and improving the well productivity. The standard acid treatments used in this work were HCl-HF (mud acid) formulations to dissolve the plugging minerals, mainly silicates (clays and feldspars). Experiments were carried out on sandstone samples that had been immersed in drilling fluid to allow cake formation and mud cake samples to evaluate the effectiveness of the formulated acids in well stimulation. Three standard mud acid concentrations were prepared, (13%HCl-3%HF, 17%HCl-5%HF and 24%HCl-6%HF).Results derived from the experiments indicated that the highest concentration of acid used (24%HCl-6%HF), gave a good result (8.05% of the original mass was dissolved). For the experiments involving the mud cake, the highest concentration of acid used (24%HCl-6%HF) gave a good result (94.86% of the original mass was dissolved). This showed that the higher concentration mud acid was a good candidate for skin removal in sandstone reservoirs, and wellbores that have been damaged by drilling fluid invasion.
Keywords
Formation damage; Well stimulation; Acidization; Mud acid; Fluoroboric acid
Introduction
Background of the study
With the ever increasing demand for energy and a prediction of over 40% energy request in 2020 [1]; there is an urgent for more effective well production from oil wells. High-temperature reservoir acidizing is the main well stimulation technique deployed for new oil and gas reserve exploration [2]. This is because many reservoir conditions are at high temperature of 200 °F, whereas some even exist at ultra-high temperature of 500 °F. Hence, current technology is less suitable for reservoirs at such conditions. Hence, all aspects of acidization, from corrosion rates to treatment-fluid stability need to be improved. Reservoir stimulation has to do with improving well productivity. Hence, a successful well stimulation firstly requires that the parameters controlling well productivity are identified, as well as determining whether stimulation will positively influence the parameters that control production. This is hence the first step in any stimulation job design. Stimulation is performed to increase or restore well production, some wells may initially exhibit low porosity and permeability when this occurs; stimulation is employed to commence production. Other producing wells might have their pore spaces blocked by various particles and need stimulation to dissolve those particles and improve the rock’s porosity.
The term stimulation with respect to oil production refers to a range of activities used to increase the production of oil from petroleum reservoirs by increasing the permeability of the materials through which oil flows to the well. Typically, there are two distinct scenarios that often lead to the use of stimulation technologies. The first scenario is damage induced by well drilling and construction and the second scenario is via oil production [3]. Damage could also happen on the rock within the vicinity of the well as a result of the mechanical disturbances and chemical interaction with the fluids used during the drilling of the well bore. For instance formation pores may be plugged as a result of drilling mud plugging the rock pores, migration of fine particles in the rock, or swelling of clays in the rock. In this case, stimulation technologies may be applied that increases the permeability of reservoirs sufficiently to allow enhanced rates of oil production [3].
Well stimulation treatments used to increase well stimulation are of two main types; Matrix treatments, performed at pressures below the formation fracture pressure and are designed to remove nearwell bore damage. Hydraulic fracture treatments performed at high pressures above the formation fracture pressure and are designed to open highly porous flow paths between the reservoir and the well bore thereby by-passing near-wellbore damage and creating new flow patterns around the well.
Formation damage
Typically, any unintended resistance to the flow of fluids into or out of a wellbore is considered to be known as formation damage. This broad definition covers flow restrictions caused by a drop in permeability in the near-wellbore region, changes in relative permeability to the hydrocarbon phase, and unintended flow restrictions in the completion itself. Formation damage is the root cause of reduction in the production of many oil wells as a result of the reduction in the inflow from the reservoir to the well bore which is triggered by the reduction of the permeability in the well bore region.
Formation damage typically makes producing oil zones to be uneconomical, as a result of their low productivity. The reservoir rock and associated fluids are essentially in a state of physicochemical and thermodynamic equilibrium. Disruptions in this equilibrium as a result of changes in pressure, temperature and fluid chemistry around the wellbore region can create flow barriers, thereby yielding lower production rates. This formation damage problem which causes reduction in the overall oil recovery from well and consequently from the reservoir makes it necessary to diagnose and to treat the problem in the early life of a well.
Well stimulation
The concept of stimulation refers to a range of activities used to increase oil production from reservoirs by increasing the permeability of the materials through which oil flows to a well. Firstly, damage is induced via well drilling, construction and oil production [3]. Secondly, damage can also occur within the vicinity of the well because of mechanical disturbances and chemical interaction with the fluids (drilling mud) used during the drilling of the well bore.
This stimulation is also at some instances termed well stimulation but is perhaps more precisely called reservoir stimulation [3]. For a well to require any form of stimulation, damage to the formation must have occurred. Depending on the type of damage, an appropriate method of stimulation is then chosen to improve the formation properties once more.
Hydraulic fracturing: Hydraulic fracturing is a very old technique that industry has deployed in improving oil and gas field production rates. However, the technique has experienced a significant evolution till date. Hydraulic fracturing was first deployed in 1949; and has since grown substantially [4]. Originally, hydraulic fracturing was used mainly as a well stimulation method, applied in case-studies where the natural reservoir permeability was too low for economic petroleum recovery. Recent trends in the 1990s indicated that hydraulic fracturing started to be used for higher-permeability reservoirs as a method to remediate formation damage within wells [5].
Fracturing fluids: The main purposes of fracturing fluid are to extend fractures, add lubrication, change gel strength, and to carry proppant into the formation. There are two methods of transporting proppant in the fluid – high-rate and high-viscosity. High-viscosity fracturing tends to cause large dominant fractures, while high-rate (slick water) fracturing causes small spread-out micro-fractures. Water-soluble gelling agents (such as guar gum) increase viscosity and efficiently deliver proppant into the formation.
The fluid is basically slurry of water, proppant, and chemical additives. Furthermore; gels, foams, and compressed gases, including nitrogen, carbon dioxide and air can be injected. Typically, 90% of the fluid is water and 9.5% is sand with chemical additives accounting to about 0.5%. However, fracturing fluids have been developed using Liquefied Petroleum Gas (LPG) and propane in which water is unnecessary.
Matrix acidization: Matrix acidization is an old well stimulation method, with the first matrix acidization treatment performed on carbonate formations near Lima, Ohio in 1895. Matrix acidization may be differentiated from hydraulic fracturing discussed above in that the acid solution is injected below the parting pressure of the formation; therefore, hydraulic fractures are not created by matrix acidization [6].
Current application of matrix acidizing is classified into two key categories: carbonate acidizing and sandstone acidizing. Hydrochloric acid (HCl) is quite efficient at dissolving carbonate minerals. Hence, carbonate acidizing utilizes concentrated HCl injected into the formation to create wormholes that bypass formation damage around the well. However, considering that wormholes penetrate up to 6.1 m (20 ft) from the wellbore, carbonate acidizing may also be used to stimulate carbonate formations that do not have significant formation damage around the well [7].
Problem statement
This study is tailored towards improvement of well productivity by removing formation damage brought about by fine solids/ mud filtrate plugging the pore spaces of the formation around the wellbore. This study aimed at determining the effectiveness of mud acid (HF/HCl) in well stimulation.
Literature Review
Indicating the advantage of HBF4 in treating a sample oil well within Nigeria that faced severe fines migration related issues created by conventional mud acid. HBF4 had proven its compatibility in stabilizing fines migration [8]. After being acidized with mud acid, the production of the oil well was found to be 850 Barrels Liquid Per Day (BLPD). However, as a result of fines migration, the production declined to nearly zero. After successful HBF4 treatment, the production increased to 2500BLPD and maintained 220-Barrel Oil Per Day (BOPD) oil production even after 1 year. Figure 1 shows the production improvement in the Nigerian oil well.
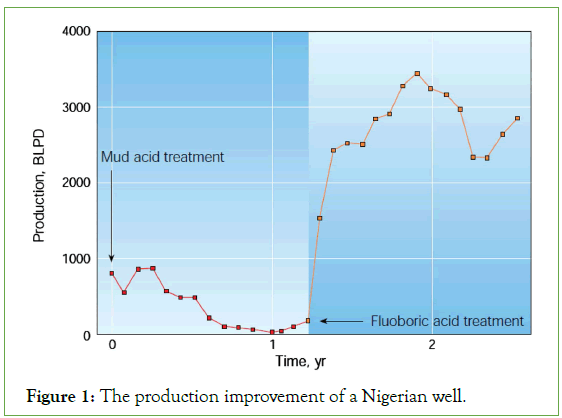
Figure 1: The production improvement of a Nigerian well.
Evidence of successful application of fluoroboric acid to stimulate sandstone in two injector wells in Brazilian off-shore [9]. The use of mixtures of fluoroboric acid, with a proper amount of Hydrochloric Acid (HCl) or an organic acid such as acetic acid, have been able to improve the performance of two Brazilian water-injection wells by removing clay damage. These two wells, which were injecting 11 and 15 m3/d, respectively, were sustaining injection rates at or above the desired quota of 30 m3/d 5 months after the treatments. The treatments were observed with a real-time monitoring program, which indicated that the skin factor had dropped from about 30/40 to zero. Their work highlighted the relevant role of secondary reaction of H2SiF6 with clays to remove formation damage, emphasizing the fact that comparing with conventional HF treatments, the less cost of fluoroboric acid as a by-product of sodium fluoride made it a better option to routine acidizing treatments.
The use of HBF4 acid in sandstone acidizing by mixing organic acid and HBF4 to form a new acid system named as Organic Clay Acid (OCA) [10]. Many wells had been stimulated using OCA and treated in low-temperature reservoirs at below 140 °F. The real field results proved the effectiveness of OCA in fines control and clay stabilization. In comparison with the initial production increase of the wells treated with an organic mud acid, it had been observed that higher initial production increase happened on the wells stimulated with OCA. This indicated that OCA had successfully mitigated the issue of fines migration caused by organic mud acid.
Developed chelant based on Hydroxyl Ethyl Amino Carboxylic Acid (HACA) and tested it on Berea sandstone. The results revealed that this HACA chelant can be used for high-temperature sandstone reservoir. The benefits of this chelant included reduced corrosion rate, reaction rate and close to neutral pH value. HACA acts as a corrosion inhibitor to form insoluble surface chelates. It also features a low reaction rate with dolomite. Also, the near-neutral pH value of HACA would eliminate the need for fluid treatment before disposal. Therefore, this chelant had advantages considering aspects of Health, Safety and Environment (HSE) due to lower HSE footprint [11,12].
An alternative approach to stimulate the production zone of Pinda formation that is found in West Africa [13]. The Pinda formation was having multilayers of carbonates. The Bottom Hole Static Temperature (BHST) of this formation was 300 °F. The six production wells from the formation zone were being stimulated with a pH of 4 HEDTA chelant during the main flush stage. Wells 1, 2, 3 and 5 were producing from a 7-in casing with a 4½ -in and 3½ -in tubing through a 3-in choke, after the stimulation well 1 was maintained at 208 BOPD from 114 BOPD. Well 2 were producing at 749 BOPD from an initial 197 BOPD. While Well 3 was producing at 1059 BOPD from an initial 830 BOPD. Well 5 as well were producing at 472 BOPD from an initial 217 BOPD. Well 4 and 6 specifically were completed using a 3½ -in monobore. Well 4 were producing at 1262 BOPD from an initial 762 BOPD. Well 6 were producing at 782 BOPD from an initial 761 BOPD. The results showed that all the six wells were then producing at an increased rate after the stimulation, indicating a high economical return resulted from the stimulation acid at a high temperature. Used a low 2.5 pH GLDA chelant to experimentally investigate its stimulation on high-quartz clean sandstone matrix and highclay heterogeneous sandstone matrix [14]. The results reflected a 20% permeability decrease for the clean sandstone, a regained permeability ratio of 0.8 indicating that some precipitation had occurred. No further investigation was carried out to determine the reason for the precipitation. There was however, a 30% permeability increase for the heterogeneous sandstone, a regained permeability ratio of 1.3 and no signs of core face damage was visually detected This indicated that this GLDA/HF chelant is more suitable for sandstone with clay content, but not clean sandstone [15].
Proposed a two-step injection process using chelating agents to treat high-temperature wells. First, the author suggested injection of low-volume but high concentration APC, and then followed by injection of high-volume but low-concentration APC such as GLDA [16].
In a research carried out by, Aluminium Chloride (AlCl3) was mixed with conventional mud acid to form retarded mud acid, (also known as fines control acid), which is comprised of 15% HCl, 1.5% HF and 5% AlCl3•6H2O. The experiment was done on Berea core samples at 75 and 200 °F respectively. Based on the solubility test result, no AlF3 precipitate was detected at both temperatures.
Discovered a new approach to retard the consumption rate of HF acid using methyl formate to generate formic acid, CH3COOH. Then, HF is generated at a controllable rate by adding ammonium fluoride; NH4F.They described laboratory work on the use of methyl format to generate formic acid in the presence of ammonium fluoride, thus dissolving clay suspended in the medium. In general, the experiment produced methyl format which slowly hydrolyzes to produce HF. The reaction equations to form HF were described as follows:
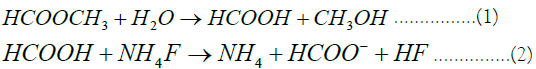
The experiment was found to be successful in producing HF which dissolved damage at up to 2 ft from the wellbore.
Use of two organic acids, acetic acid (CH3COOH) and formic acid (HCOOH), in stimulating HTHP wells. Both organic acids were good in sandstone acidizing, with weak ionization and slow reaction. These acids do not have much corrosion effect on the down whole tools and allow much reaction time. The acid blend had been used on Arun limestone formation in Indonesia with high temperature of 350 °F. The well and its corrosion response was good in technical and economic efficiencies of the acid blend used.
Concluded that the many advantages offered by nitrogen would suggest nitrogen fracturing as a very good technical solution. However, they also conclude that placing the proppant in high velocity gas stream is problematic, as well as resulting in erosion, and that the technology is limited to shallow wells or geologies that can fail the rock in a self-propping manner.
Materials and Methods
Table 1 shows list of materials and equipment used in this research work.
| Materials | Equipment |
|---|---|
| HCl acid | Weighing balance |
| HF acid | Hamilton beach stirrer |
| Bentonite | Mud balance |
| Barite | API filter press |
| Sodium Hydroxide | pH Meter |
| PAC-R | Brookfield programmable Rheometer |
| Water | Emulsion stability tester |
| Laboratory glass wares, etc. | Sand content kit beaker |
Table 1: List of materials and equipment.
Mud preparation: The API fresh water mud containing water and bentonite was prepared accordingly by adding 20 g of bentonite to 350 ml of water and some other additives in the right proportion. The resulting mixture was stirred with the aid of multi-beach mixer for (3-5) minutes to obtain homogeneous mixture. The mud was characterized and the results recorded. All rheological properties were measured at ambient conditions (at 75°F). Table 2 presents the proportion of each additive in the mud, while Table 3 shows the results of the characterization.
| Mud | Bentonite(g) | Barite(g) | PAC-R (g) | Sodium hydroxide(g) | Distilled water |
|---|---|---|---|---|---|
| Water-based drilling mud | 20 | 10 | 0.25 | 1 | 350 ml |
Table 2: Water-based drilling mud composition.
| Mud characterization | |
|---|---|
| Density (ppg) | 8.55 |
| Specific gravity | 0.95 |
| pH | 12.19 |
| Marsh funnel viscosity(sec/qt+) | 52 |
| Sand content (%) | 0.3 |
| Filtrate volume (ml) | 27.7 |
| Plastic viscosity (cp) | 8 |
| Apparent viscosity (cp) | 13.2 |
| Yield point (lb/100 ft2) | 10.8 |
Table 3: Mud characterization results.
Mud acid preparation: Mud acid of different concentration and strength would be tested in different reservoir rocks for matrix acidizing. This is to ascertain the concentration and strength of the acid that could dissolve the particles blocking the pore through which oil flows and improve productivity in return. The experiment was carried out using three mud acid concentrations; 13% HCl-3% HF, 17% HCl-5% HF, and 24% HCl-6% HF. To estimate the quantity of each substance to be added to get the acid mixture needed to displace the core particles, this relationship must be used based on preparing 100 ml of solution:
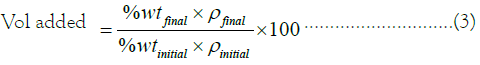
The acid mixture was prepared using the following steps.
1. Using the above equation (1), the volume of HCl and HF acid to
be used was prepared. Taken 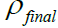 to be 1.064 g/cm3.
to be 1.064 g/cm3.
2. The calculated volumes of HCl and HF acids were added together and then deducted from 100. This is the volume of water used.
3. The volume of water calculated above was poured into a graduated beaker. The volumes of HCl and HF calculated were also added and the mixture was stirred. The resulting mixture was the mud acid mixture required.
4. It is important to always add acid to water as the reverse can cause an exothermic reaction that can splash or spray the acid at the operator.
Acidizing process
The acidizing process involved the use of sandstone samples and drilling mud cake obtained from a filter press. Both samples were acidized, and their rates of dissolution were measured and recorded.
Sandstone sample acidizing: The sandstone sample utilized in the experiment was prepared as follows
1. The sandstone sample was immersed in a drilling mud sample prepared above, it was brought out and allowed to dry which led to mud cake formation on it (Figure 2).

Figure 2: Mud Cake sample.
2. After it had dried, the sample was weighed and the result recorded, it was then immersed in the first mud acid concentrations for 30 minutes. After 30 minutes the sandstone sample was removed and weighed to determine the amount of mud cake that dissolved. The sample was immersed in the acid mixture again for additional 30 minutes after which it was removed and weighed again and the value was recorded (Figure 3).

Figure 3: Sandstone samples after being immersed in drilling mud.
3. Step 2 was repeated at 30 minutes interval until the total time became 2 hours (Figure 4).

Figure 4: Acidized sandstone sample.
4. The entire procedure was performed using the three different mud acid concentrations (Figure 5).

Figure 5: Mud cake sample during acidizing.
Mud cake sample acidizing: The mud cake sample utilized in the experiment was prepared as follows
1. The mud cake samples gotten from the filtration test was weighed, it was then placed in a petri dish.
2. The mud acid was poured into the petri dish and after 30 minutes the acid was drained off and the leftover mud cake was scrapped out and weighed.
3. The left-over mud cake in step 2 was placed into a cleaned petri dish again and the same mud acid in step 2 was poured into it. After 30 minutes the acid was drained, and the remaining mud cake was removed and weighed.
4. Steps 3 repeated until the total time was 2 hours.
5. The entire procedure was performed using the three different mud acid concentrations.
Results and Discussion
The acidizing experiments were performed using three similar sandstone rock samples with mud cake. Their initial masses and masses with mud cake were taken and recorded in Table 4. Three standard mud acid concentrations were prepared:
| Sample Name | Mass with mud cake(g) | Initial mass(g) |
|---|---|---|
| Sandstone 1 | 25.63 | 21.45 |
| Sandstone 2 | 108.4 | 103.87 |
| Sandstone 3 | 61.14 | 58.23 |
Table 4: Initial masses of sandstone sample.
1. 13% HCl-3% HF.
2. 17% HCl-5% HF.
3. 24% HCl-6% HF.
These different mud acid concentrations were prepared as outlined above and each sandstone sample was assigned to a particular mud acid concentration for the analysis. The results of the experiments were presented in Tables 5-7. Each sandstone sample was assigned to a mud acid concentration for the experiments. The results of the experiments are given in Tables 5-7.
| Time (mins) | Mass dissolved (g) | Mass Remaining |
|---|---|---|
| 30 | 1.65 | 23.98 |
| 60 | 1.72 | 23.91 |
| 90 | 1.9 | 23.73 |
| 120 | 1.91 | 23.72 |
Sandstone 1 (25.63 g), Treated with 13% HCl-3% HF Acid
Table 5: Results of first acidizing experiment.
| Time (mins) | Mass dissolved (g) | Mass Remaining |
|---|---|---|
| 30 | 1.87 | 106.43 |
| 60 | 2.74 | 105.66 |
| 90 | 3.37 | 105.03 |
| 120 | 3.78 | 104.62 |
Sandstone 2 (108.40 g), Treated with 17% HCl-5% HF Acid
Table 6: Results of second acidizing experiment.
| Time (mins) | Mass dissolved (g) | Mass Remaining |
|---|---|---|
| 30 | 2.28 | 58.86 |
| 60 | 3.59 | 57.55 |
| 90 | 4.17 | 56.97 |
| 120 | 4.92 | 56.22 |
Sandstone 3 (61.14 g), Treated with 24% HCl-6% HF Acid
Table 7: Results of third acidizing experiment.
From the results of the graph in Figure 6, it was found that the plots of all the experiments were linear with increasing mass of the sandstone sample dissolved for every 30-minute. The first concentration of acid used was able to dissolve 1.91 g of the sandstone sample from a total mass of 25.63 g. After physical observation of the sample it was noted that there was presence of the mud cake on it indicating that the acid was unable to completely remove the mud cake that formed on it.
Figure 6: Mud cake sample during acidizing. Note: 13% HCI-3% HF acid (Sandstone 1);
13% HCI-3% HF acid (Sandstone 1); 17% HCI-3% HF acid (Sandstone 1);
17% HCI-3% HF acid (Sandstone 1); 24% HCI-3% HF acid (Sandstone 1).
24% HCI-3% HF acid (Sandstone 1).
The second concentration of acid used was able to dissolve 3.78 g of the sandstone sample from a total mass of 108.40 g. After physical observation of the sample it was noted that there was still slight presence of the mud cake on it indicating that the acid was unable to completely remove the mud cake that had formed on it. The difference in the final mass and the initial mass of the sample was measured to be 1.25 g indicating that the acid was unable to dissolve the whole of the mud cake.
The third concentration of acid used was able to dissolve 4.92 g of the sandstone sample from a total mass of 61.14 g, and after physical observation, it was noted that there was none of the mud cake on it indicating that the acid was able to completely remove the mud cake that had formed on it. The difference in the final mass and the initial mass of the sample was measured to be -2.01 g indicating that the acid was able to dissolve the entire mud cake and part of the sandstone sample. From this analysis, it could be seen that the third acid concentration yielded a good result compared to others and therefore should be utilized for acidizing jobs involving formation damage caused by drilling fluids.
The acidizing experiment was also performed using mud cake samples against the three concentrations of acid. The results are given below (Tables 8-10).
| Time (mins) | Mass dissolved (g) | Mass Remaining(g) |
|---|---|---|
| 30 | 0.57 | 1.05 |
| 60 | 0.63 | 0.99 |
| 90 | 0.76 | 0.86 |
| 120 | 1.29 | 0.38 |
Mud cake (1.62 g), Treated with 13% HCl-3% HF Acid
Table 8: Results of fourth acidizing experiment.
| Time (mins) | Mass dissolved (g) | Mass Remaining(g) |
|---|---|---|
| 30 | 0.84 | 1.66 |
| 60 | 1.24 | 1.26 |
| 90 | 1.69 | 0.81 |
| 120 | 2.12 | 0.29 |
Mud cake (2.50 g), Treated with 17% HCl-5% HF Acid
Table 9: Results of fifth acidizing experiment.
| Time (mins) | Mass dissolved (g) | Mass Remaining(g) |
|---|---|---|
| 30 | 1.33 | 2.17 |
| 60 | 1.87 | 1.63 |
| 90 | 2.63 | 0.87 |
| 120 | 3.32 | 0.18 |
Mud cake (3.50 g), Treated with 24% HCl-6% HF Acid
Table 10: Results of sixth acidizing experiment.
From the results on the plot (Figure 7), it was found that the plots were linear with increasing mass of mud cake dissolved for every 30-minutes interval. The first concentration of acid used was able to dissolve 1.29 g of the mud cake sample from a total mass of 1.62 g. The second concentration of acid used was able to dissolve 2.12 g of the mud cake from a total mass of 2.50 g. The third concentration of acid used was able to dissolve 2.84 g of the mud cake from a total mass of 3.50 g. The results show increasing dissolution rates for the increasing acid concentration. From these observations, it could be seen that the third acid concentration gives a better result and should be used for well stimulation jobs involving formation damage caused by drilling fluid invasion and other sources of damage.
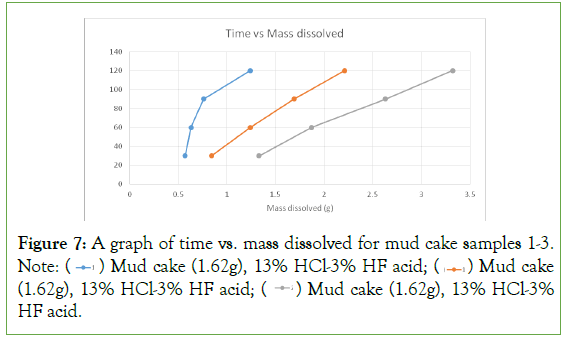
Figure 7: A graph of time vs. mass dissolved for mud cake samples 1-3. Note:  Mud cake (1.62g), 13% HCl-3% HF acid;
Mud cake (1.62g), 13% HCl-3% HF acid;  Mud cake (1.62g), 13% HCl-3% HF acid;
Mud cake (1.62g), 13% HCl-3% HF acid;  Mud cake (1.62g), 13% HCl-3% HF acid.
Mud cake (1.62g), 13% HCl-3% HF acid.
The results obtained from the experiments showed that the highest concentration of acid used (24%HCl-6% HF), gave a good result (8.05% of the original mass was dissolved). For the experiments involving the mud cake, the highest concentration of acid used (24%HCl-6%HF) gave a good result (94.86% of the original mass was dissolved). This showed that the highest concentration of mud acid was a good candidate for skin removal in sandstone reservoirs, and wellbores that have been damaged by drilling fluid invasion and other sources.
Conclusion
The experiments performed were based on laboratory analysis to determine the effectiveness of mud acid in well stimulation. Well stimulation involves a range of activities aimed at increasing the production of oil from reservoirs by increasing the permeability of the reservoir rock through which oil flows to the wellbore. An acidizing treatment restores permeability by removing damage around the wellbore, thus improving productivity in both sandstone and carbonate reservoirs. During acidizing, the acids dissolve the sediments and mud solids within the pores that are inhibiting the permeability of the rock. This process typically expands the natural pores of the reservoir, which stimulates hydrocarbon flow.
Three different acid concentrations were used to determine the dissolution rates of the samples while they were immersed in them. The detailed operational procedure of the apparatus and the acidizing process was described. Acidizing experiments were carried out using sandstone and mud cake samples. Three mud acid concentrations, 13% HCl-3% HF, 17% HCl-5% HF, and 24% HCl-6% HF acid mixtures were used. From these preliminary experimental results, some conclusions can be drawn.
The rate of dissolution of sandstone 1 and 2 is slow when compared to that of sandstone 3. This is because the acid concentration used is of a higher concentration and dissolves it at a faster rate.
The Mud acid prepared is capable of dissolving drilling mud cakes with thickness of 1/32 inches or less.
The rate of dissolution of mud cake 1 and 2 is slow when compared to that of mud cake 3. This is because the acid concentration used is of a higher concentration and dissolves it at a faster rate. At the end of the third experiment on the mud cake sample, there was less than 6% of the original mass that was used to start the experiment.
Recommendations
To further develop this research to be more suitable for commercial purposes, the following recommendations should be considered:
1. The research work should be extended to use of more acid concentrations to determine the rate of dissolution of sandstone reservoir rock.
2. The sandstone samples used should be tested for permeability increases before, during and after the experiments to ascertain the effectiveness of the mud acid.
3. Standard laboratory equipment should be made available for characterizing the drilling fluid.
4. An average of two or three readings should be used for each experiment carried out. This can help to eliminate some errors in the experiments.
5. Apart from mud acid, other research areas should be explored in terms of well stimulation. Nitro-shooting, hydraulic fracturing etc. should be investigated.
Acknowledgement
The support of the laboratory staff of the Department of Petroleum Engineering, Afe Babalola University Ado-Ekiti, Ekiti state is acknowledged.
REFERENCES
- Aboud RS, Smith KL, Forero Pachon L, Kalfayan LJ. Effective matrix acidizing in high-temperature environments. One Petro. 2007: 1-10.
- Al-Harthy S, Bustos OA, Samuel M, Still J, Fuller MJ, Hamzah NE, et al. Options for high-temperature well stimulation. Oilfield Rev. 2008; 20(4):52-62.
- Ehlig-Economides CA, Economides MJ. Pressure and temperature dependent properties of the rock-fluid systems in petroleum and geothermal formations. One Petro. 1981.
- Al-Nakhli AR, Abass H, Al-Ajwad HA, KwaK HT, Al-Harith A, Al-Otaibi A. Unconventional gas stimulation by creating synthetic sweetspot. One Petro. 2013.
- Al-Harbi BG, Al-Khaldi MH, Al-Dossary KA. Interactions of organic-HF systems with aluminosilicates: Lab testing and field recommendations. One Petro. 2011; (1):52-62.
- Al-Harbi BG, Al-Dahlan MN, Al-Khaldi MH. Aluminum and iron precipitation during sandstone acidizing using organic-HF acids. One Petro. 2012; 4(20):52-53.
- Ali SA, Ermel E, Clarke J, Fuller MJ, Xiao Z, Malone B. Stimulation of high-temperature sandstone formations from West Africa with chelating agent-based fluids. SPE Prod Oper. 2008; 23(01):32-38.
- Andotra G. Investigating the use of chelating agents for clay dissolution and sandstone acidizing purposes (Doctoral dissertation). 2014.
- Arthur JD, Bohm BK, Coughlin BJ, Layne MA, Cornue D. Evaluating the environmental implications of hydraulic fracturing in shale gas reservoirs. One Petro. 2019: 1-21.
- Ayorinde A, Granger C, Thomas RL. The application of fluoboric acid in sandstone matrix acidizing: a case study. 1992; 2:235-261.
- Crowe C, Masmonteil J, Thomas R. Trends in matrix acidizing. Oilfield Rev. 1992; 4(4):24-40.
- Da Motta EP, CM Dos Santos JA. New fluosilicic acid system removes deep clay damage. One Petro. 1999: 159-163.
- Van Domelen MS, Jennings AR. Alternate acid blends for HPHT applications. One Petro. 1995.
- Feng P, Wang D, Liu G, Wang H, Economides MJ. Sandstone reservoir stimulation using high-temperature deep-penetrating acid. One Petro. 2011: 1-11.
- Frenier W, Brady M, Al-Harthy S, Arangath R, Chan KS, Flamant N, et al. Hot oil and gas wells can be stimulated without acids. One Petro. 2004; 19(4):1-15.
- Garcia EA, LaBlanc A, Beuterbaugh A, Calabrese T. Developments in sandstone HF acidizing: HF fluid compatible with Na or K brines and carbonate-laden mineralogy for high temperatures (360 F). One Petro. 2016.
Citation: Udeagbara SG, Okereke NU, Oguamah IU, Kerunwa A, Nwanwe O (2022) Evaluation of the Effectiveness of Mud Acid in Well Stimulation. J Pet Environ Biotechnol. 13:452.
Copyright: © 2022 Udeagbara SG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.