Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Evaluation of the Antibacterial Potential of the Phytochemicals in Leaf Extracts of Newboudia laevis on Uropathogens
Felix O Alao1, Zacchaeus S Ololade2* and Yusuf A. Fagge12Department of Chemistry, University of Medical Sciences, Ondo, Nigeria
Received: 17-May-2021 Published: 07-Jun-2021, DOI: 10.35248/2155-9597.21.s10.003
Abstract
This study was conducted to determine the secondary metabolites and antibacterial potential of the leaf extracts Newbouldia laevis on some uropathogen strains and also to carry out phytochemical screening of the sample. The antibacterial activity of N. laevis leaves extracted with methanol, hot and cold water were evaluated against medically important bacteria strains of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus saprophyticus and Enterococcus faecalis. The results showed a remarkable inhibition of the bacteria growth against the tested organisms with Staphylococcus saprophyticus had highest activity (20 mm). In general, all the tested organisms showed a significant activity with the extracts. The conventional antibiotics used against these organisms showed that all the isolates were resistant to augmentin, ceftazidime, cefuroxime, and cloxacillin. The phytochemical analysis of the plants was carried out. The microbial activity of the Newbouldia laevis was due to the presence of various secondary metabolites. The GC-MS analysis showed that the most abundant phytochemical in the extract is linoleoyl chloride (76.3%). Hence, these plants can be used to discover bioactive natural products that may serve as leads in the development of new pharmaceuticals research activities.
Keywords
Newbouldia laevis; Leaf extract; Uropathogen; Antibacterial; Phytochemical
Introduction
Plants have been used in the prevention, treatment and cure of disorders and disease since ancient times. Natural products originated from plants constitute main resource base of almost all the traditional healthcare systems [1]. Phytochemicals contain complex mixtures of medicinally active organic compounds, such as polyphenols, terpenoids, fatty acids, sterols, alkaloids, flavonoids, glycosides, saponins, tannins, terpenes [2]. The mitigative potential of natural products originated from medicinal plants is used as a means of detoxification of poisonous agents in human and animals [3]. The importance of using medicinal plants can be attributed to some reasons, including affordability and limited availability of synthetic drugs in most developing nations. Moreover, the trust in drugs derived from natural products from plants is due to positive results when applying herbs [4]. In spite of their origin, natural drugs should not be viewed as simple tools for folk medicine since they are class of pharmaceutical products and should meet the requirements of quality, safety, and efficacy [5]. Excessive and uncontrolled use of antimicrobial agents among individuals had encouraged proliferation of antibiotic resistant pathogens in our society.
In view of this, there must be alternative means in curbing pathogens in our environment [6]. Newbouldia laevis is a genus of plant in the family of Bignoniaceae.
It is widely used in the African folk medicine for the treatment of several illnesses and diseases as an astringent in diarrhoea, dysentery and in the treatment of various diseases such as worms, malaria, sexually transmitted diseases, and in the reduction of dental caries [7].
This study was carried out to ascertain the antibacterial potential of secondary metabolites in the leaf extract of N. laevis leaves extract on uropathogens.
Materials and Methods
The sample was collected in the Bells University of Technology, Ota and was identified as Newbouldia laevis (P. Beauv.) Seem. Ex Bureau. The N. laevis leaves collected were carefully washed with clean water to remove all dirt, sand particles and insect droppings from the leaves. The leaves were air dried at room temperature and pulverized using an electric blender. 30 g each of blended leaves was extracted with 300 ml of methanol, hot water and cold water for 5 days, where it was allowed to settle and filtered with filter paper and the supernatant removed. The crude methanol, hot water and cold water extracts were poured into beakers and concentrated in water bath at 60°C [8].
Antimicrobial assay
Collection of isolates: Staphylococcus aureus, Staphylococcus saprophyticus, Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa isolates were obtained from Microbiology Laboratory of Bells University of Technology Ota, Nigeria.
In vitro antibacterial activity of the crude extracts was evaluated by the agar well diffusion method as per standard method. After getting the turbidity equal to 0.5 McFarland standards, inoculums were aseptically introduced on to the surface of sterile agar plates and sterilized cotton swabs were used for even distribution of the inoculums. Wells were prepared in the agar plates using a sterile cork borer of 8.0 mm diameter. The plant extracts were dissolved in tween twenty to get different concentration (4 mg/ml, 8 mg/ml, 16 mg/ml, 32 mg/ml, 64 mg/ml, 128 mg/ml, 256 mg/ml and 512 mg/ml). The wells were filled with plant extract. The plates are incubated at 37°C for 48 hr and then zone of inhibition was measured.
Antibiotic susceptibility test: Antibiotic susceptibility test was carried out on each of the pathogenic isolates as positive control (either drug resistant or sensitive). Multi-sensitivity discs bearing eight different antibiotics Augmentin, Ceftazidime, Cefuroxime, Cotrimoxazole, Cloxacillin, Erythromycin, Gentamicin, and Ofloxacin were aseptically placed with the aid of sterile forceps over the surface of Mueller Hinton agar which had been previously inoculated with the pathogenic test bacterial suspensions with prescribed turbidity (compared to that of 0.5 McFarland standard). The plates were incubated at 37°C for 24 hr. After incubation, the diameter of the zone of inhibition around each well was measured to the nearest millimeter.
Gas Chromatography–Mass Spectrometry (GC-MS) analysis: Determination of chemical composition of the N. laevis extract was performed on a GC-MS-Q P2010 plus Shimadzu model equipped with a Mass Spectrometer detector. The column used was RTX-1-Integral and contained (5%-diphenyl-95%- dimethylpolysiloxane). The column dimension was 30 m × 0.25 mm and the column oven was programmed at 70°C to hold at 0 min, ramped at 10°C/min up to 280°C at 5 min. The injection temperature was 250°C, the flow rate of the helium gas was 1.80 mL/min and the film thickness was 0.25 μm. Compounds were identified by means of the NIST library.
Results and Discussion
This study was carried out with a view to investigate the antimicrobial properties of N. laevis leaves extracts against uropathogens such as Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus saprophyticus. The leaf extract of this plant showed remarkable zones of inhibition against the five uropathogens isolates tested in this study. The highest zone of inhibition was observed with hot water extract used on Staphylococcus saprophyticus while the least was observed with cold water extract on Staphylococcus aureus. Different remarkable zones of inhibition were observed with all other uropathogens both Gram positive and Gram negative tested in this study as shown. The results from evaluation of antimicrobial properties of cold water, hot water and methanol extracts of the leaf of N. laevis on Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Staphylococcus saprophyticus and Pseudomonas aeruginosa showed that all extracts had measurable zones of inhibition against the selected human pathogens. The antimicrobial activities of the leaf extracts of N. laevis against Gram-positive bacteria and Gram-negative bacteria are shown (Figure 1). For the cold water, hot water and methanolic extracts. The extracts showed variable activities against tested bacteria. The extracts showed varying levels of effectiveness on all bacteria tested. The activities ranged as follows: Resistant (-), not sensitive (<8 mm), sensitive (9–14 mm), very sensitive, (15–19 mm) and ultrasensitive (>20 mm). The highest inhibitory effect of the cold water extract was observed against E. faecalis (20 mm) and P. aeruginosa (20 mm); followed by S. saprophyticus (19 mm), E. coli (18 mm) and S. aureus (15 mm), as depicted. This showed that E. faecalis was highly susceptible to the cold water extract of the leaf of N. laevis compared to the other tested bacteria.
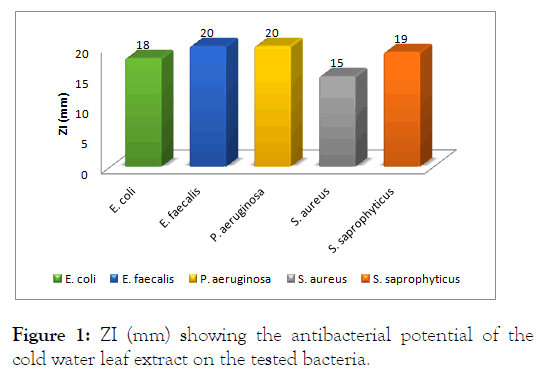
Figure 1: ZI (mm) showing the antibacterial potential of the cold water leaf extract on the tested bacteria.
For the hot water extract, the highest zone of inhibition was observed against S. saprophyticus (20 mm); followed by E. coli (19 mm), E. faecalis (19 mm), S. aureus (15 mm) and P. aeruginosa (20 mm) as showed (Figure 2).
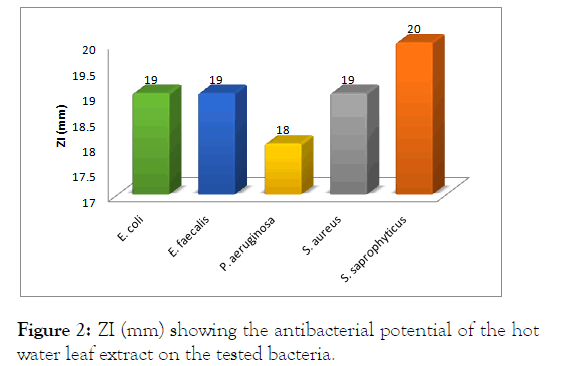
Figure 2: ZI (mm) showing the antibacterial potential of the hotwater leaf extract on the tested bacteria.
This showed that S. saprophyticus was highly susceptible to the hot water extract of the leaf of N. laevis unlike the cold extract.
For the methanolic extract, the highest zone of inhibition was observed against S. saprophyticus (20 mm); followed by P. aeruginosa (19 mm), E. coli (18 mm), E. faecalis (18 mm), S. aureus (16 mm) and as showed (Figure 3). This showed that S. saprophyticus was more susceptible to the methanolic extract of the leaf of N. laevis like the hot water extract.
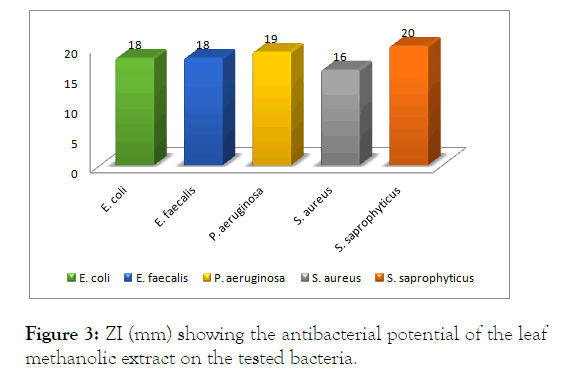
Figure 3: ZI (mm) showing the antibacterial potential of the leaf methanolic extract on the tested bacteria.
The antibacterial activities shown by the cold water, hot water and methanolic extracts were due the synergetic potential of both the major and minor constituent of the extracts.
Conclusion
The phytochemical screening of the crude methanolic leaf extracts of N. laevis revealed the presence of diverse secondary metabolites. These classes of compound are known to show curative activity against several pathogens and therefore could explain its use traditionally for the treatment of wide array of illnesses. The antimicrobial activity of N. laevis leaves extract against the tested isolates shows that this plant extract can be used to produce antimicrobial agents that can have broad spectrum of activity against uropathogens.
REFERENCES
- Othman L, Sleiman A, Abdel-Massih RM. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front Microbiol. 2019;10(1):911.
- Makhuvele R, Naidu K., Gbashi S, Thipe VC, Adebo OA, Njobeh PB. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon. 2020;6(10):e05291.
- Petrovska BB. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012;6(11):1-5.
- Onifade OF, Ayodele PF, Ololade ZS, Balogun DO. Mitigative effects of coconut oil and its water on the lungs of male albino rats exposed to petrol vapour. Biomed J Sci Tech Res. 2020;25(1):18865-18873.
- Yuan H, Ma Q, Ye L, Piao G. The Traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):1-18.
- Huang J, Tao G, Liu J, Cai J, Huang Z, Chen J-X. Current prevention of covid-19: natural products and herbal medicine. Front Pharmacol. 2020;11(1):588508.
- Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10(5):210-229.
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309-318.
Citation: Alao FO, Ololade ZS, Fagge YA (2021) Evaluation of the Antibacterial Potential of the Phytochemicals in Leaf Extracts of Newboudia laevis on Uropathogens. J Bacteriol Parasitol. S10: 003.
Copyright: © 2021 Alao FO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

