Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 8
Evaluation of Trichoderma Strains as Biocontrol of Fusarium oxysporum F. sp lycopersici in Tomato
Hope Takudzwa Mazungunye* and Elizabeth NgadzeReceived: 17-Mar-2021 Published: 20-Aug-2021, DOI: 10.35248/2157-7471.21.12.571
Abstract
Tomato (Lycopersicon esculentum) is an important crop which provides people with essential nutrients e.g. potassium and phosphorus as well as anti-oxidants important in fighting cancers. Its production is threatened by the wilts caused by a fungus called Fusarium oxysporum f. sp Lycopersici which can cause yield losses of 50-100%. The disease is mainly controlled by use of synthetic chemicals which can pose a threat to human health and the environment. This study evaluated the use of Trichoderma strains as potential biocontrol of the Fusarium oxysporum f. sp Lycopersici. In-vitro experiment was a dual culture experiment with six treatments arranged in a Completely Randomized Design. The results showed Trichoderma strains (T. harzianum, T. asperellum – CA, C9, NY) reduced mycelial growth of Fusarium significantly (p ≤ 0.05). The Trichoderma strains also showed a significantly high percentage inhibition of the pathogen (p ≤ 0.05). The in- vivo experiment of tomato variety Tengeru evaluating two factors (2 methods of application and 3 biocontrol spp. .) and was arranged in completely randomized block design with six treatment combinations and three blocks. The experiment showed that Trichoderma harzianum and Trichoderma asperellum CA significantly (p<0.05) reduced the effects of the pathogen when compared with the control and improved the growth parameters of the tomato plants as well as chlorophyll content. The Trichoderma strains did not influence chlorophyll florescence (p ≤ 0.05). Disease severity was significantly lower in Trichoderma inoculated treatments at (p<0.05) and lower in the control. Soil drenching proved to be a more effective method of application than seed treatment as shown in this study on many parameters.
Keywords
Biocontrol; Trichoderma; Fusarium rot; Soil drenching; Seed dressing
Introduction
Tomato (Solanum lycopersicum L.) is an important vegetable crop grown in Zimbabwe. Its popularity is due to its high nutritive value. Tomatoes are rich in many vital nutritional minerals (especially, phosphorus and potassium) and vitamins (B and C) [1]. Also, it helps in reducing the risk of common cancers like breast and prostate cancer. The crop provides an opportunity to generate cash income through sale of the fruit, offering many a livelihood. However, soil-borne pathogens cause a lot of economic yield losses through diseases [1]. Approximately 800 million people in the developing world face food insecurity and at least 10% of food is lost due to plant diseases [2]. Fungi have the biggest impact with regard to diseases and crop production losses compared to other plant parasites [3]. Tomato and other solanaceae crops like potatoes are threatened by various fungal diseases which include vascular wilts. Tomato yield are significantly reduced by Fusarium oxysporum f. sp. lycopersici which destroys roots and also blocks the xylem of tomato plants at various growth stages, causing vascular wilt [4]. The use of synthetic chemicals remains the most widely used disease control method in the ongoing struggle against plant pathogens, even though there is risk of plant pathogens becoming resistant [5]. There is growing need for safe and sustainable disease control methods, and when coupled with an increasing export market potential for organically produced agricultural crops, it creates a need to assess other alternatives to reduce the use of environmentally hazardous synthetic chemicals [5]. Several studies have shown the potential of biological agents in the control of Fusarium dry rots on solanaceae crops [6]. Trichoderma has been reported to show potential antagonistic effects on Fusarium in other continents or regions on the African continent [7]. The control of the disease by Trichoderma may be achieved by competition, production of antibiotics or by mycoparasitism. However, biological efficacy of the Trichoderma spp. may differ due to differences in ecosystems [7]. There is need to evaluate the effects of Fusarium spp. which have been causing losses in the Zimbabwean tomato production sector and also apply the possible biocontrol agent (Trichordema spp.) isolated from Zimbabwean soils.
Materials and Methods
The In-vitroand In-vivo experiment were conducted at the Department of Crop Science, University of Zimbabwe (17.78 °S, 31.05 °E and altitude of 1523 meters). This experiment was conducted to account for the antagonistic effect of Trichoderma spp. against Fusarium oxysporum f. sp Lycopersici.
In–vitro experiment
Fusarium oxysporum f. sp Lycopersici was regenerated from the University of Zimbabwe Plant Pathology lab culture collection. The Fusarium spp. was cultured on Potato Dextrose Agar (PDA) for one week before use in the dual cultures. Trichoderma harzianum strain T77, T. asperellum strain Nyehu, T. asperellum C9, T. CA was obtained from culture collection. They were cultured on PDA (Potato Dextrose Agar) at 27°C for one week and then used in the dual cultures against F. oxysporum f. sp Lycopersici. A completely randomized factorial design where treatments (Trichoderma spp. and controls as well as Fusarium spp. were the fixed factors. Means were separated using Fisher’s projected LSD at (P<0.05) (Table 1).
| Treatment | Pathogen | Trichoderma spp |
|---|---|---|
| 1 2 |
Fusarium oxysporum L | Trichoderma asperellum. (NY) |
| Fusarium oxysporum L | Trichoderma harzianum (T77) | |
| 3 | Fusarium oxysporum L | Trichoderma asperellum (TCA) |
| 4 5 6 |
Fusarium oxysporum L Fusarium oxysporum L Trichoderma asperellum(NY) |
Trichoderma asperellum (TC9) Fusarium oxysporum L Trichoderma asperellum (NY) |
Table 1: Treatment structure of the in-vitro experiment.
The dual culture technique was used in the evaluation of biocontrol potential of the Trichoderma spp. against Fusarium oxysporum f. sp L on PDA. The 90 mm diameter Petri dish was divided into two segments and the pathogen and antagonist were placed 20 mm equidistant from the line. A cork borer was used to obtain discs of 6 mm from the sub cultured pathogen and Trichoderma platesand placed on 20 mm equidistant points on opposite sides of the Petri dishes. Control Petri dishes had the same microorganism on either side so as to compare with pathogen antagonist treatments. A total of six treatments were used and replicated three times.
Radial growths as well as inhibition zones were measured at one, four, seven and ten days after culturing. Radial growth was measured using a ruler.
Pathogen growth inhibition was calculated using the following formula:
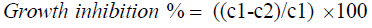
Where, C1: Mean diameter of pathogen colony in control plates and C2: Mean diameter of pathogen colony in presence of antagonist. Statistical analysis was performed using Genstat 14.
In-vivo experiment
The experiment was carried out at the Department of Crop Science greenhouses, University of Zimbabwe (17.78 ºS, 31.05 ºE and altitude of 1523 meters). The tomato seedling variety used is the local moderately susceptible variety Tengeru. Black plastic bags were dipped for 24 hours in 1% sodium hypochlorite solution at the crop science field laboratory and then rinsed with distilled water before planting commenced. The plastic pots were filled with red fersialitic soil. The soil was oven dried for 24 hours at 100ºC temperature to sterilize it. The Trichoderma species trial had one commercial strain T. harzianum (T77) and four Trichoderma isolates from culture collection namely, T. asperellum (NY), T. asperellum (C9) and T. asperellum (CA) which were tested for their antagonism in this experiment. The tomato variety which was used is Tengeru which is one of the popular local varieties on the market. The experimental design was a 2×3 factorial in Randomized Complete Block Design replicated 3 times i.e. (2 methods by 3 Trichoderma treatments. The two methods were (method 1- soil drenching and method 2 - seedling treatment). The control was with pathogen and without antagonist (Table 2).
| Treatment no | Treatment description 1 | Treatment description 2 |
|---|---|---|
| 1 2 |
T77 + method 1 | T77 + method 2 |
| T CA + method 1 | TCA + method 2 | |
| 3 | Control with pathogen + method 1 | Control with pathogen + method 2 |
Table 2: Treatment combinations of the in-vivo experiment.
The Trichoderma spp. T. harzianum (T77), T. asperellum (TCA and TC9) were sub cultured on PDA for 14 days and then the spores were scrapped off into 100 ml of sterile distilled water and shaken for 30 minutes in the magnetic stirrer. The number of spores was determined shortly after by the hemocytometer and the concentration adjusted to 104 spores ml-1 by using the following equation:
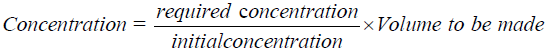
To apply the seedling treatment, the antagonists were applied before transplanting. The seedlings were soaked for 30 minutes in the antagonists’ suspension mixed adjusted to 104 spores ml-1 for Trichoderma. The treated seedlings were then transplanted.
Seedlings that were not treated were immersed in sterile distilled water for 30 minutes. These seedlings were later drenched with the antagonist which was the second method of application of the antagonists (soil drenching) by drenching with 100ml of the 104 spores ml-1 concentrated solutions of the antagonists (T77, TCA).
This method was conducted a week after the inoculation with the pathogen. Fusarium oxysporum f. sp lycopersici was cultured in PDA for 14 days and then scrapped off and mixed with sterile water and put on a magnetic shaker for 30 minutes. The final concentration was determined by the hemocytometer and the concentration was adjusted to 104 spores ml-1. The tomato plants in all treatments for the experiments were inoculated with the pathogen at the third and fourth leaf stage by punching each plant with sterilized needles at the base of the stem above the upper secondary roots [8]. A volume of 100 ml of the suspension were drenched in blocks 1, 2 and 3, respectively in all pots over the wounded area. After the inoculation process, all the pots were covered by polythene bags for 24 hours to maintain high humidity [8,9]. The tomato plants were monitored for the development of wilt symptoms at 14, 21 and 35 days after transplanting (DAT). The disease Appendix data were recorded using the scale developed by Mandal, et al. [10] which ranged from 0-100%. Fourteen days after the application of soil antagonist isolates, the effect of tested antagonists on plant growth were measured in terms of plant height, number of nodes per plant, number of flowers per plant 40 days after inoculation. Chlorophyll content was measured at two weeks after inoculation between 10 am and 3 pm by a portable chlorophyll meter. The readings were done on two fully expended leaves (two readings per leaf). The chlorophyll fluorescence was measured using a chlorophyll fluorescence meter. Disease severity, plant height, number of nodes per plant, number of flowers per plant and chlorophyll data were subjected to the repeated measures ANOVA using Genstat. Fisher’s LSD was used to separate the means at 5% significance level.
Results
Radial growth
Trichoderma spp. significantly reduced (P<0.05) radial growth of Fusarium oxysporum f. sp Lycopersici. The lowest radial growth of 23 mm was recorded in T. asperellum (NY), while the highest 37.75 mm was recorded in T. asperellum (NY) control. The second highest radial growth of 32.42 mm was recorded in the pathogen control (Table 3).
| Biocontrol | Radial Growth |
|---|---|
| T. asperellum NY | 23 a |
| T. harzianum | 27.67 b |
| T. asperellum CA | 26.58 ab |
| T. asperellum C9 | 25.08 ab |
| Fusarium(control) | 32.42 c |
| T. asperellum (NY) (control) | 37.75 d |
| F value | < 0.01 |
| S.E.D | 1.981 |
| LSD | 3.948 |
| CV% | 15.7 |
Table 3: A radial growth of Fusarium oxysporum f. sp Lycopersici in dual cultures with Trichoderma isolates in-vitro assays.
Percentage inhibition
Trichoderma harzianum and Trichoderma asperellum (CA) showed the lowest inhibition % of F. oxysporum f. sp Lycopersici (P<0.05).These two were not significantly different (p ≤ 0.05) from each other. Trichoderma asperellum (NY) had significantly (P<0.05) higher inhibition % of the pathogen at day four and day seven. Trichoderma asperellum (C9) had a significantly (P<0.05) higher percentage inhibition of the pathogen at day one and day ten (Figure 1).
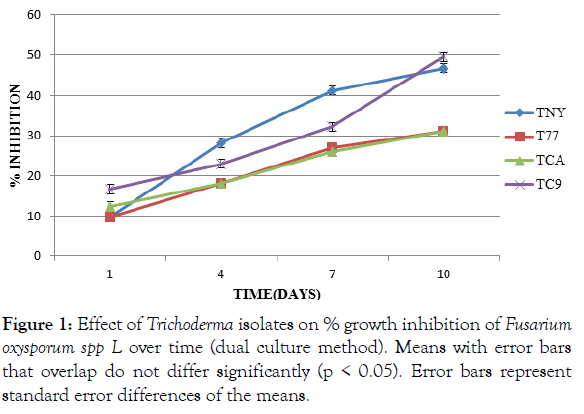
Figure 1: Effect of Trichoderma isolates on % growth inhibition of Fusarium oxysporum spp L over time (dual culture method). Means with error bars that overlap do not differ significantly (p < 0.05). Error bars represent standard error differences of the means.
Plant height
There was a significant (p<0.05) interaction between application method and biocontrol agent used. Trichoderma harzianum applied using the soil drenching methods recorded significantly (P<0.05) taller plants with an average height of 20.93 cm at 14 days after application. There was no significant difference between the Trichoderma treatments applied using the drenching method (Figure 2). Fusarium oxysporum f. sp Lycopersici applied using the soil drenching method recorded significantly (P<0.05) shorter plants with an average height of 9.43 cm at 14 days after application of biocontrol. Trichoderma asperellum applied using the drenching method recorded significantly (P<0.05) taller plants at 21 days after application. There was no significant difference between the Trichoderma treatments applied using the drenching methods (Figure 2).
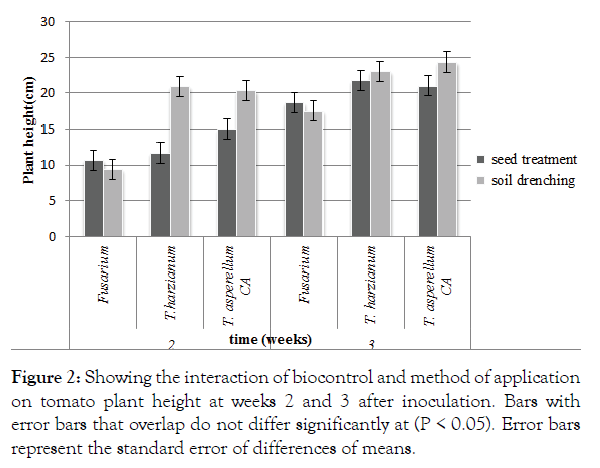
Figure 2: Showing the interaction of biocontrol and method of application on tomato plant height at weeks 2 and 3 after inoculation. Bars with error bars that overlap do not differ significantly at (P < 0.05). Error bars represent the standard error of differences of means.
Disease severity
There was a significant interaction (p<0.05) between biocontrol and method of application of the antagonist. The control plants which were soil drenched with Fusarium but not treated with the biocontrol recorded a significantly (P<0.05) high disease severity (1.498). Trichoderma harzianum soil drenched recorded a significantly (P<0.05) low disease severity. There was no significant difference between the Trichoderma treatments (Figure 3).
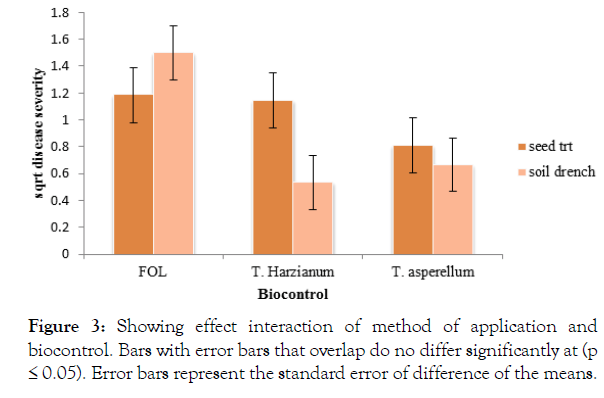
Figure 3: Showing effect interaction of method of application and biocontrol. Bars with error bars that overlap do no differ significantly at (p ≤ 0.05). Error bars represent the standard error of difference of the means.
Nodes per plant
There was a significant interaction (p<0.05) between method of application and biocontrol for the number of nodes count per plant. Trichoderma harzianum soil drenched treatments recorded significant (P<0.05) high numbers of nodes per plants (16.5). The Trichoderma treatments were not significantly different from each other (Figure 4). The soil drenched control plants recorded a significantly (P<0.05) low number of nodes 7.5 (Figure 4).
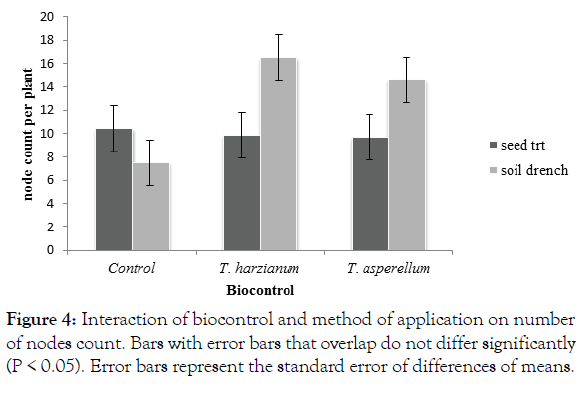
Figure 4: Interaction of biocontrol and method of application on number of nodes count. Bars with error bars that overlap do not differ significantly (P < 0.05). Error bars represent the standard error of differences of means.
Chlorophyll content and fluorescence
The control and biocontrol significantly (P ≤ 0.05) recorded different levels of chlorophyll (Figure 5). There was no significant difference (p<0.05) between the biocontrol treatments and T. harzianum (46.76) recorded the highest chlorophyll content at week 3 whilst the control with pathogen without antagonist recorded the least (Figure 5). There was an interaction between time and method of application.
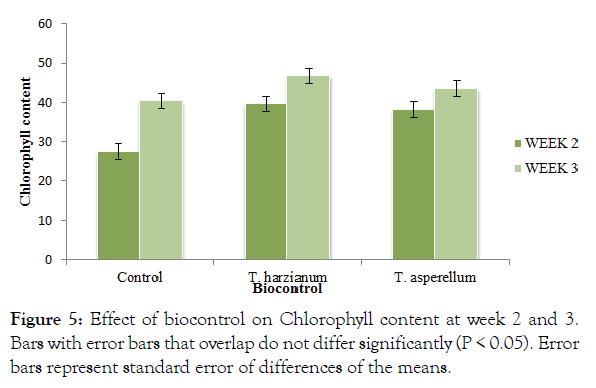
Figure 5: Effect of biocontrol on Chlorophyll content at week 2 and 3. Bars with error bars that overlap do not differ significantly (P < 0.05). Error bars represent standard error of differences of the means.
Soil drenched plants at week three had a significantly (P<0.05) high chlorophyll content 46.39 (Figure 6). Chlorophyll content of soil drenched treatments was significantly (P<0.05) high at week two than seed treated treatments at both week two and three (Figure 6).
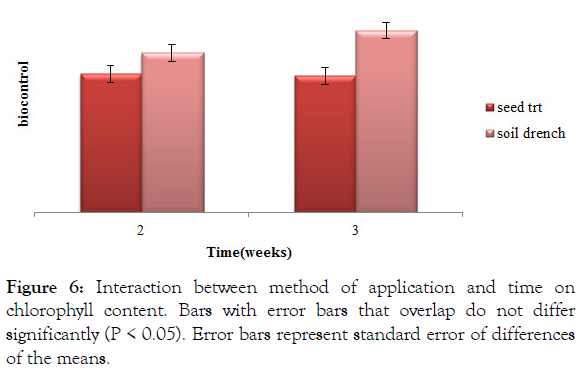
Figure 6: Interaction between method of application and time on chlorophyll content. Bars with error bars that overlap do not differ significantly (P < 0.05). Error bars represent standard error of differences of the means.
Chlorophyll fluorescence showed no significant difference in terms of biocontrol, method of application as well as the interaction of biocontrol and method of application and time.
Flower count
The Trichoderma strain, Trichoderma harzianum recorded a significantly (P<0.05) higher number of flowers per plant 8.5 flowers (Figure 7). The control treatment with pathogen without antagonist recorded the least number of flowers per plant (1.5 flowers) (Figure 7). Trichoderma asperellum recorded the second highest number of flowers (5 flowers) and was significantly (P<0.05) higher than the control treated plants and significantly lower than Trichoderma harzianum (Figure 7).
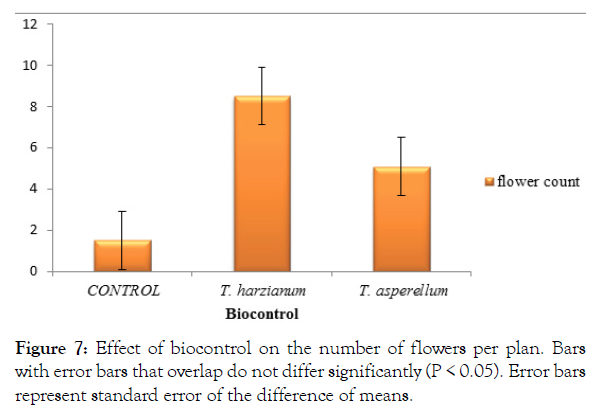
Figure 7: Effect of biocontrol on the number of flowers per plan. Bars with error bars that overlap do not differ significantly (P < 0.05). Error bars represent standard error of the difference of means.
Discussion
The Trichoderma isolates T. asperellum (NY, C9, CA and Nyehu) and T. harzianum (77) which were tested showed a higher mycelial radial growth and inhibitory effects on the growth of Fusarium oxysporum f. sp Lycopersici. The findings of this study findings are in agreement with Verma, et al. [11] study on T. viridae. The dual culture in this study displayed different levels of mycelium growth inhibition. Trichoderma asperellum (NY) showed a high radial growth rate over time and also the highest % inhibition. This isolate outperformed the commercialized T. harzianum in the in-vitro experiment. Isolate C9 also had a higher percentage of growth inhibition than T77. The advancing hyphae of isolates NY, CA, T77 and C9 covered the entire Petri dish suppressing the growth of F. oxysporum. The findings from the current study showed that Trichoderma strains have inhibitory effects on the growth of Fusarium oxysporum strain in all the treatments in the dual culture. The results also revealed that Trichoderma strains competed with F. oxysporum for nutrients and space, as well as mycoparasitism over the pathogen and probably secretion of antibiotics. This supports the findings from a study conducted by Theradimani, et al. [12] where activities of Trichoderma viridae and T. harzianum showed convincing results in terms of % inhibition zone shown against Fusarium species. The general mechanisms of antagonistic activity of Trichoderma spp. include antibiosis, lysis, competition and mycoparitism. Ramesh and Pandey [13] showed that T. Viridae produced diffusible substances toxic to the pathogens and these toxins could effectively act as ectoparasites by overcrowding the other organisms. Nisha, et al. and B Hunchoth,et al. [3,14] stated that Trichoderma spp. . secrete different compounds that have the potential to effectively suppress F. oxysporum f. sp Lycopersici although antibiotic substances from Trichoderma strains were not extracted and determined in this study. However, some antibiotics such as tubercidin, candicidin, phosphlactomycin, phenasin and 4-diacetylphloroglucinol, which have been produced by some antagonists, like Pseudomonas flourescens, Streptomyces spp. and Trichoderma spp. ., have been reported by Barari [15]. Another mechanism of mycoparasitism is described by Lu, et al. [16] and showed the branching of T. atroviride hyphae as an active, chemotactic response to the presence of the host. Papilla-like erections at the T. atroviride hyphal tips were observed and these occurred in the presence and in the absence of direct contact with the pathogen [16]. In that same study, there was an observation of adherence of T. atroviride spores to the hyphae of P. ultimum where the germination happened and parasitized the host pathogen. All these are possible reasons which can explain the inhibitory effect of Trichoderma on the radial growth of Fusarium oxysporum f. sp Lycopersicishown in this study.
The use of Trichoderma spp. . as biocontrol agents is promising in tomato production as it improves plant physiological and growth factors and thus improving crop yield. The general plant health is also improved and shows no adverse effect on the environment and consumers. Trichoderma isolates employs several mechanisms in influencing plant growth. Plant height and number of nodes are indicators of plant growth. This study showed that plant height and number of nodes were significantly affected by treatment of Trichoderma isolates. T. harzianum recorded significantly higher plant heights and number of nodes. The results are in affirmation with the study of Liu, et al. [17], where an increase in plant height and biomass was observed when plants were treated with Trichoderma viridae. Soil drenching application of the Trichoderma spp. recorded the highest plant heights as well as number of nodes. This supports the study conducted by Velmurugu, et al. [18] who stated that seeds inoculated with Trichoderma had no significant effect in seedling height of tomato plants. Further supporting results by Ramesh and Pandey [13] showed that in the Trichoderma seed inoculation treatments there was no significant effect observed in plant height. The plant heights in seed treated treatments were not significantly different from the control at week 3. Muhera S [8] stated that method of Trichoderma introduction is important in the success of Trichoderma action in seedling growth improvements. This is due to Trichoderma action, depending on the ability of Trichoderma spp. to survive and develop in the rhizosphere. Therefore seedling treatment may not offer effective inoculation compared to soil drenching into the root zone. The increase of plant growth due to Trichoderma inoculation is also associated with secretion of auxins, gibberellins and cytokinins which are important hormones that boost the development of roots and shoots. The results from the current study are in line with those reported by Verma, et al. [11] which showed the increase in plant growth factors related to yield by Trichoderma strains. The application of Trichoderma spp. on tomato plants under greenhouse conditions reduced disease severity of Fusarium wilts as evidenced by the significantly lower wilt severity on plants inoculated with the pathogen. T. harzianum (77) was the most effective agent in suppressing disease development followed by T. asperellum (CA). Disease severity was the highest in the control treatments were the plants were inoculated with Fusarium only. There was a significant reduction on disease severity by addition of the Trichoderma antagonists. Interestingly, Verma, et al. [11] stated that one of the mechanisms for disease reduction caused by Trichoderma spp. . is their ability to induce a potentiated state in the plant enabling it to become resistant to subsequent pathogen (Fusarium) infection. The same study also reported other mechanisms which include the induction of systemic resistance [19], the production of antibiotics to restrict the growth of the pathogen and competition with pathogens for nutrients or ecological niches [20]. T. harzianum showed the best efficacy compared to T. asperellum. This effect was also consistent as shown by results recorded at two, three and five weeks after inoculation with the Trichoderma strains.
Chlorophyll content was higher in the leaves of Trichoderma treated plants with Trichoderma harzianum 77 inoculated plants showing the highest significant (P<0.05) chlorophyll content compared to the controls. There was no difference in biocontrol between soil drenched treatments and seed treated treatments for chlorophyll content. In this study, the control recorded lower chlorophyll content as compared to treated plants and this can be attributed to the wilting caused by the pathogen in control plants. The results are in agreement with the findings by Madden and Dorrance [19], that indicated the occurrence of patchy stomatal closure in dehydrating leaves limiting the photosynthetic activity of these leaves. The control plants were stunted as well and this therefore reduced the leaf size, leaf area Appendix and subsequently the chlorophyll content (Verma, et al. 2017) [11]. Trichoderma treatments were superior in terms of growth parameters like plant height, number of nodes, and also vigour Appendix and all these are precursors to the amount of chlorophyll produced. This explains why the Trichoderma treatments produced more chlorophyll than the control treatment with pathogen only [21].
Trichoderma did not affect the chlorophyll florescence in this study as there was no significant difference between the control and the Trichoderma treatments. This could be attributed to the timings of the reading, and could potentially have an effect when readings are taken closer to physiological maturity. A study by Bora, et al. [22], observed that as the disease progressed, the maximum quantum efficiency of PS11 photochemistry decreased in the leaves of tomato plants with Fusarium wilt. The Fusarium wilt disease decreased the leaf area – there is a reduction in the ability of the tomato plants to capture photosynthetically active radiation, therefore determining the lower levels of chlorophyll content which is in agreement with this study. The experiment also expected to show lower chlorophyll fluorescence in pathogen inoculated treatments. However, this study showed no difference between the biocontrol treatments and the pathogen inoculated treatments.
The number of flowers produced per plant in this study was higher in T. harzianum followed by T. asperellum and the least number of flowers by the plants inoculated with the pathogen only. Clearly, the pathogen is affecting the number of flower produced or at least delaying the flowering process. Flowers produced are a proxy to the number of fruits to be produced and thus the yield of the tomato plants [13]. Therefore, Trichoderma is showing positive signs of biocontrol of the pathogen in terms of number of flowers produced
Conclusion
Trichoderma is effective in suppressing Fusarium oxysporum f. sp Lycopersici in-vitro as shown by the study. T. asperellum (NY) had the highest inhibition percentage of Fusarium. The other strains (T. harzianum, T. asperellum CA, C9) also significantly reduced the pathogen radial growth and had high percentage inhibition of the pathogen. These locally isolated Trichoderma strains are also effective in reducing the effects of Fusarium oxysporum Lycopersici in-vivo. Soil drenching in this study is a more effective method of application of the Trichoderma spp. than seed treatment.
Recommendations
The bio-efficacy evaluations of Trichoderma strains produced promising results and since this study was only done in the lab and in the greenhouse, there is need to further the study and do field evaluations. The experiment should include all strains and assess the residual efficacy under field conditions. The T. asperellum strains showed great potential in-vitro and in-vivo. Farmers can easily adopt biocontrol methods as the fungicides proliferate in the soil and will offer environmentally and health hazard free method of Fusarium wilt control in tomato production.
REFERENCES
- Barari H. Biocontrol of tomato Fusarium wilts by Trichoderma species under in vitro and in vivo conditions. 2016a;1(165):91-98.
- Aktaruzzaman M, Xu SJ, Kim JY, Woo JH, Hahm YI, Kim BS. First report of potato stem-end rot caused by Fusarium oxysporum in Korea. Mycobiology. 2014;42(2):206-209.
- Tapwal A, Garg S, Gautam N, Kumar R. In vitro antifungal potency of plant extracts against five phytopathogens. Braz Arch Biol Technol. 2011;54(6):1093-1098.
- Alwathnani HA, Perveen K. Biological control of fusarium wilt of tomato by antagonist fungi and cyanobacteria. Afr J Biotechnol. 2012;11(5):1100-1105.
- Mecteau M, Arul J, Tweddell R. Effect of different salts on the development of Fusarium solani var. coeruleum, a causal agent of potato dry rot. Phytoprotection. 2008;89(1):1-6.
- Mejdoub-Trabelsi B, Jabnoun-Khiareddine H, Daami-Remadi M. Interactions between four Fusarium species in potato tubers and consequences for fungal development and susceptibility assessment of five potato cultivars under different storage temperature. J Plant Pathol Microbiol. 2015;6(293):2.
- Zaker M. Evaluation of some Trichoderma isolates for biological control of potato Wilt disease (Fusarium oxysporum) under lab and green house conditions. J Plant Protection. 2012;1(4):279-286.
- Muhera S. Evaluation of Trichoderma in the biocontrol of Ralstonia solanaceum in Potatoes. University of Zimbabwe. 2017.
- Maji S, Chakrabartty PK. Biocontrol of bacterial wilt of tomato caused by 'Ralstonia solanacearum' by isolates of plant growth promoting rhizobacteria. Aust J Crop Sci. 2014;8(2):208-214.
- Mandal H, Chakraborty PS, Saha DA, Sarkar T, Saha D, Saha A. Biocontrol of virulent Ralstonia solanacearum isolates by an indigenous Bacillus cereus. International Journal of Agricultural Technology. 2017;13(1):19-30.
- Verma NP, Kaur I, Masih H, Singh AK, Singla A. Efficacy of Trichoderma in controlling Fusarium wilt in tomato (Solanum lycopersicum L.). Res in Environ Life Scienc. 2017;10(7):636-639.
- Theradimani M, Susitha S, Amudha C. Biocontrol of Fusarium Wilt in Tomato caused by Fusarium oxysporum f. sp. lycopersici. Int j curr microbial appl sci. 2018;7(9):420-429.
- Ramesh GC, Pandey BR. Evaluation of Trichoderma spp ., Pseudomonas fluorescens and Bacillus subtilis for biological control of Ralstonia wilt of tomato. F1000 Research. 2018:1-22.
- Bhunchoth A, Phironrit N, Leksomboon C, Chatchawankanphanich O, Kotera S, Narulita E, et al. Isolation of Ralstonia solanacearum‐infecting bacteriophages from tomato fields in C hiang M ai, T hailand, and their experimental use as biocontrol agents. J Appl Microbiol. 2015;118(4):1023-1033.
- Barari, H. Biocontrol of tomato Fusarium wilt by Trichoderma species under in vitro and in vivo conditions. 2016b;165(1):91-98.
- Lu Z, Tombolini R, Woo S, Zeilinger S, Lorito M, Jansson JK. In vivo study of Trichoderma-pathogen-plant interactions, using constitutive and inducible green fluorescent protein reporter systems. Appl Environ Microbiol. 2004;70(5):3073-3081.
- Liu L, Li Y, Li S, Hu N, He Y, Pong R, et al. Comparison of next-generation sequencing systems. J Biomed Biotechnol. 2012.
- Jegathambigai VR, Wijeratnam RW, Wijesundera RL. Trichoderma as a Seed Treatment to Control Helminthosporium Leaf Spot Disease of Chrysalidocarpus lutescens. World J Agric Res. 2009;5(6):720-728.
- Hoitink HA, Madden LV, Dorrance AE. Systemic resistance induced by Trichoderma spp.: interactions between the host, the pathogen, the biocontrol agent, and soil organic matter quality. Phytopathology. 2006;96(2):186-189.
- Tian Y, Tan Y, Yan Z, Liao Y, Chen J, De Boevre M, et al. Antagonistic and detoxification potentials of Trichoderma isolates for control of Zearalenone (ZEN) producing Fusarium graminearum. Front Microbiol. 2018;8:2710.
- Enespa DS, Dwivedi SK. Effectiveness of some antagonistic fungi and botanicals against Fusarium solani and Fusarium oxysporum f. sp. lycopersici infecting brinjal and tomato plants. Asian J Plant Pathol. 2014;8(1):18-25.
- Bora P, Bora LC, Deka PC, Bikram B, Sharma AK, Dutta HS, et al. Efficacy of Pseudomonas fluorescens and Trichoderma viride based bioformulation for management of bacterial wilt disease of ginger. Int J Plant Sci. 2016;11(2):180-186.
Citation: Mazungunye HT, Ngadze E (2021) Evaluation of Trichoderma Strains as Biocontrol of Fusarium oxysporum F. sp lycopersici in Tomato. J Plant Pathol Microbiol 12:571.
Copyright: © 2021 Mazungunye HT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

