Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2020) Volume 11, Issue 10
Evaluation of Common Bean (Phaseolous vulgaris L.) Varieties for the Reaction of Common Bean Anthracnose (Colletotrichum lindemuthianum) at Sirinka, Eastern Amhara, Ethiopia
Seid Hussien1, Negash Hailu2* and Eshetu Belete32Department of Plant Science, Debre Berhan University, Debre Berhan, Ethiopia
3Department of Plant Science, Wollo University, Dessie, Ethiopia
Received: 03-Sep-2020 Published: 14-Oct-2020, DOI: 10.35248/2157-7471.20.11.517
Abstract
Common bean (Phaseolous vulgaris L.) is the most important food legume consumed as source of protein and cash crop in Ethiopia. The production of the crops is constrained by biotic and abiotic factors. Common bean anthracnose caused by the fungus Colletotrichum lindemuthianum is a major production constraint in common bean growing regions of Ethiopia. Field experiment was conducted at Sirinka Agricultural research center during 2017/18 main cropping season to evaluate reactions of common bean varieties to the disease. The experiment consisted of twenty two common bean varieties evaluated for the reaction to anthracnose under natural infestation conditions. The highest disease severity (58%) was recorded from Awash-1 variety while the lowest disease severity (45%), was recorded from Awash Melka variety at final assessment day. The highest (3.03 t ha-1) yield was recorded from Awash Melka variety while the lowest (0.97 t ha-1) yield was recorded from KAT-B1 variety. From the present study, it is possible to conclude that, the advantage of screening resistant verities increases the opportunity to select for a broad range of anthracnose resistance and help to know the variability of the common bean anthracnose disease.
Keywords
Anthracnose; Common bean; Variety
Introduction
Common bean (Phaseolous vulgaris L.) is the most important food grain legume consumed worldwide [1]. It is grown and consumed principally in developing countries in Latin America, Africa, and Asia. Its production in sub-Saharan Africa is around 3.5 metric tons ha-1with 62% being produced in East African countries namely Burundi, DR Congo, Ethiopia, Kenya, Rwanda, Tanzania and Uganda [1]. The crop is grown worldwide for its edible, dry, fresh and green beans. Production is expanding slowly based on population growth with highest usage in poor developing countries, where beans provide an alternative to meat as a source of low-cost protein. The crop is well suited to low input systems as they can be stored for long periods without refrigeration and provide an excellent nutritional complement [2].
Common bean is an important legume crop in the daily diet of more than 300 million of the world’s population [3]. It has been rated as the second most important source of human diet and the third most important source of calories of all agricultural commodities produced in eastern Africa [4]. In Ethiopia, common bean is mainly cultivated in the Eastern, Southern, Southwestern and Rift Valley Regions [5,6]. The average white and red common bean productivity is 1.41 and 1.56 t ha-1 respectively. It is predominantly produced in Oromia region, SNNPR and Amhara region with their area coverage of 146,452.41 ha (41%), 117,969.97 ha (33%) and 81,235.07 (22.74%) ha respectively and the rest 3.25% is produced in other regions of Ethiopia [7]. The crop is a good source of income for small-scale farmers and fetches higher prices than cereals in the local market.
The low yield of the crop could be attributed largely to low adoption of improved agricultural technologies, drought, diseases and insect pests, lack of improved varieties, poor cultural practices and shortage of land and environmental degradation. From those constraint diseases are known to be the major factors affecting the production, productivity and the quality of the crop [8]. Common bean is attacked by a wide range of diseases that affect leaf, stem, root and seed. The major diseases that are affecting common bean production in Ethiopia include anthracnose caused by Colletotrichum lindemuthianum, rust caused by Uromyces appendiculatus, common bacterial blight caused by Xanthomonas axonopodis pv phaseoli, halo blight caused by Pseudomonas syringae pv. phaseolicola, angular leaf spot caused by Phaeoisariopsis griseola, Ascochyta blight caused by Ascochyta phaseolorum and common bean common mosaic virus [5,9]. Among them, Anthracnose caused by Colletotrichum lindemuthianum, is a destructive disease worldwide [10].
Before a decade common bean is not well distributed in eastern Amhara, although, the area is potential for common bean production. At present time, the production and area coverage area of common bean has been increasing from time to time because of the fact that the crop has immense potential for export and risk aversion in drought prone lowland areas of Wollo. However, intermittent drought, bean stem maggot, foliage beetle larvae, CBB, ALS and anthracnose have limited productivity of common bean in the lowland areas of Wollo [11]. Although management practices can improve the productivity of common bean in such marginal areas, more progress in improving yield will be realized through genetic improvement [12].
Anthracnose is one of the most serious diseases attacking common bean in cool weathers in Ethiopia. The infected seeds are the most important means of Colletotrichum lindemuthianum dissemination, which explains its worldwide distribution [13]. The crop is vulnerable to attack by the pathogen at all growth stage of the crop, from seedling to maturity, depending on the environmental conditions that are essential for the initiation and further development of the disease. Common bean anthracnose causes an estimated yield loss of up to 63% in Ethiopia [14-16]. Previously, Mohammed and Somsiri [17] reported that the intensity of anthracnose on white type common bean was higher in Ethiopia.
The first step for the management of seed-borne diseases is generally to eradicate or reduce the pathogen inoculum in the seed production field [18]. Seed treatment is an important measure for the control of anthracnose [19]. Moreover, utilization of resistant varieties has been the best way to manage the disease, it is one of the most economical and effective methods of anthracnose management [20].
The chemical control should form part of disease management practice and applications of contact or systemic foliar fungicide are the most important for the management of bean anthracnose [15]. Fitsum [21] reported that the possibility of using Flopan as foliar spray and Pseudomonas fluorescens as seed treatment, decreased anthracnose disease symptoms effectively in common bean plants and increased seed yield under field conditions. Recent studies showed that integrated management of crop diseases is getting increased attention as an environmentally sound approach.
Although the effect of common bean resistant and susceptible varieties to minimize the yield losses of anthracnose, it has received comparatively little attention in the Northeastern Ethiopia. Moreover, the reaction of commonly released common bean varieties to the disease is not well known in the area. Therefore, the objective of this study was to evaluate the effect of common bean varieties for the reactions of the disease.
Materials and Methods
Description of the study area
The experiment was conducted at Sirinka Agricultural Research Center experimental site located at (11°45’00’’ N latitude; 39°36’36’’E longitude; 1850 meters above sea level in northern Ethiopia during 2017main cropping season (July to October). The soil of the experimental fields is clay loam and clay with the pH of 6.9-7.23. The organic carbon is 1.37%, total N is 0.09%, available P 12.17 mg kg-1 soil and CEC 53.44 c molc kg-1 [22]. The total amount of rainfall is 750.4 mm with mean maximum and minimum temperatures of 28.6 and 14.7°C, respectively.
Experimental design and treatments
Experiments were conducted with natural infestation, during 2017 to evaluate the reaction of 22 bean varieties including resistant and susceptible check to bean anthracnose. Common bean varieties used in the experiment and their characteristics is indicated in Table 1. The treatment was arranged in a Randomized Complete Block Design (RCBD) with three replications. Spacing between plants and rows were 10 cm and 40 cm, respectively. There were 66 plots, each consisting of 2 rows. Spacing between blocks was 1 m and between adjacent plots 0.5 m. Each row had 20 plants. In general, there were 40 plants per plot in which ten of them were randomly pre-tagged from the two rows. Seeds was planted at the rate of two seeds per hole and thinned to one plant, 15 days after sowing (DAS) to ensure 40 plants per plot. All agronomic practices were performed uniform for all plots of each treatment.
| Varieties | Seed color | Seed shape | Amount of Rain fall | Days of maturity | Year of release | Altitude (m.a.s.l) | Suitable production area |
|---|---|---|---|---|---|---|---|
| Awash Melka | White | Flat | 350-700 | 85-100 | 1999 | 1400-1900 | All Ethiopia |
| ICB-0081 | Mottled | Kidney | 400-100 | 90-95 | 2013 | 1400-200 | Middle Rift Valley and south |
| Nazareth-2 | White | Elong | 350-1000 | 90-95 | 2005 | 1330-1850 | Middle Rift Valley |
| Tabor | Pinto | Elong | 1000-1300 | 98 | 1999 | 1300-1900 | Southern Ethiopia |
| Ser-125 | Red | Elong | 450-700 | 85-100 | 2014 | 1450-2000 | Middle Rift Valley |
| KAT B9 | Red | Round | 1400-200 | 85-90 | 2013 | 1400-200 | Middle Rift Valley |
| SAB-736 | White | Elong | 400-750 | 85-90 | 2015 | 1500-1800 | Middle Rift Valley, South and Harrga |
| Argene | White | Elong | 350-1000 | 90-95 | 2005 | 1300-1800 | Middle Rift Valley |
| ser-119 | Red | Elong | 450-700 | 85-100 | 2014 | 1450-2000 | Middle Rift Valley |
| KAT-B1 | Yellow | Round | 500-1500 | 75 | 2013 | 1500-1800 | Middle Rift Valley |
| Biofort (Large 5) | Mottled | Elong | 400-750 | 89 | 2016 | 1500-1800 | Middle Rift Valley |
| Awash-2 | White | Round | 400-750 | 85-90 | 2013 | 1300-1700 | Middle Rift Valley and the same place |
| Nasir | Red | Elong | 350-1000 | 86-88 | 2003 | 1200-1800 | All Ethiopia |
| Ibado, | Motteled | Elong | 350-500 | 90-120 | 2003 | 1400-2250 | Southern Ethiopia |
| Duristu | Red | Elong | 750 | 85 | 2008 | 1300-1800 | All Ethiopia |
| SAB632 | Speckled | Kidney | 400-750 | 85-90 | 2015 | 1500-1800 | Middle Rift Valley, South and Harrga |
| Gobe rash | Motteled | Kidney | 350-700 | 98 | 1998 | 1400-1900 | Jimma, South Western |
| Roba-1 | Cream | Elong | 350-700 | 75-95 | 1997 | 1400-1800 | All Ethiopia |
| GLP-2 | Motteled | Kidney | 550-100 | 91 | 2011 | 1500-200 | Middle Rift Valley |
| Crans cope | Speckled | Kidney | 400-1100 | 90-98 | 2007 | 1300-1950 | Middle Rift Valley and south |
| Gofta | Cream | Elong | 500-1200 | 110 | 1998 | 1500-200 | Eastern and Western Harereghe |
| Awash-1 | White | Round | 350-700 | 90 | 1990 | 1400-1800 | Middle Rift Valley |
Table 1: Common bean varieties and its characteristics.
Data collection
Disease assessment: Anthracnose severity was assessed every week after the onset of the disease. Ten plants were randomly selected and tagged from central rows of each plot and were used to determine the disease severity. The severity of anthracnose on the pre-tagged common bean plants was estimated six times as the area of plant tissue damaged using a 1-9 rating scale, as follows: 1= no visible disease symptoms; 3= presence of very small lesions, mostly on the primary vein of leaf’s lower side or on the pod, that covers approximately 1% of surface area; 5= presence of several small lesions on the petiole or on the primary and secondary veins of the leaf’s lower side or small round lesions on the pods, with or without reduced sporulation, that covers approximately 5% of the pods surface area, 7= presence of enlarged lesions on the lower side of the leaf. Necrotic lesions can also be observed on the upper leaf surface and on the petioles. On the pods, the presence of medium lesions are evident but also some small and large lesions generally with sporulation and that cover approximately 10% of the pod’s surface area may be found and 9= more than 25% of the leaf surface area covered with large coalescing and generally necrotic lesions resulting in defoliation [23]. The severity scores were then converted into Percentage Severity Index (PSI) according to the formula by Wheeler [24].
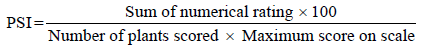
Area under disease progress curve and disease progress rate: The Area under Disease Progress Curve (AUDPC) was computes from the PSI data was record at each date of assessment as describing by Campbell and Madden [25].
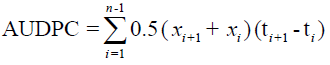
Where ‘n’ is the total number of assessments,’ ti’ is the time of the ith assessment in days from the first assessment date, ‘xi’ is percentage of disease severity at ith assessment. AUDPC is expressed in percent days because the severity (x) was expressed in percent and time (t) in days. The rates of disease progress in time were determine by recording the severity of anthracnose at 7 days interval right from the appearance of the first disease symptoms still the maturity of the crop in the different treatments.
Assessment of crop growth, seed yield and yield components: The Plant height, the number of pods per plant, infected pods per plant and seeds per pod were record from the 10 pre tagged plants. The harvested pods were sun dried and the respective seed yield of the different treatments was measured. Common bean yield data was adjusted at 10% moisture content after measuring with a moisture tester. Seed yield per plot was converted into yield in tons per hectare. The weight of 100 randomly select seeds was also measured.
Statistical analysis
Disease severity was assessed six times on weekly intervals starting from the first visible anthracnose symptoms in the experimental plots. Data on disease parameters such as (disease severity, PSI, AUDPC, disease progression rate and seed yield and yield components (pods per plant, infected pods per plant, seeds per pod, 100 seed weight) were subjected to analysis of variance (ANOVA) using General Linear Model (GLM) procedure of Statistical Analysis System (SAS) version 9.2 software [26]. The mean difference among the treatment were test with Fishers List Significant Difference (LSD) at (P ≤ 0.05) [27]. relationship among treatment yields, yield components, and disease assessment parameters, such as disease progress rate, percentage severity index, and area under disease progress curve (AUDPC). Logistic, ln ((Y/1-Y), [28] and Gompertz,-ln[-ln(Y)], [29] models were used for estimation of disease progression parameters from each treatment. The goodness of fit of the models was tested based on the magnitude of the coefficient of determination (R2).
Results
Disease severity on reaction of common bean variety
Differences in response to anthracnose severities and among 22 common bean varieties were clearly observed under field conditions at Sirinka. The analysis of variance for anthracnose severity and AUDPC were highly significant (P ≤ 0.001) during the whole date of disease assessment period. The severity of anthracnose measured in all 22 varieties had significantly higher values while showed significantly varying resistance to anthracnose (Table 2). During the final disease assessment comparatively, the lower severity level of anthracnose (45.7%, 46.7%, and 46.9%) were recorded from resistance varieties Awash Melka, Gofta and Roba-1 respectively (Table 2). The majority of them (68%) gave a susceptible reaction to the pathogen with more than 50% of disease severity, with heavy symptoms on leaves, stems and pods.
| Common bean Variety | Percentage Severity Index (PSI) % | AUDPC | |||||
|---|---|---|---|---|---|---|---|
| 51DAS | 58DAS | 65DAS | 72DAS | 79DAS | 86DAS | ||
| Nazareth-2 | 12bcdf | 13.5efgh | 18.1g | 37.6efg | 45.9fghij | 48.0hi | 1015.7ijk |
| Awash Melka | 10g | 11.3h | 16.4g | 33.1h | 43j | 45.7i | 921.2l |
| Tabor | 13.7ab | 16.8abcd | 24.1def | 45.9abc | 49.3bcdef | 53.3cdef | 1188.0cdefg |
| KAT B9 | 13.5abc | 16.7abcd | 23.7ef | 44.7bcd | 48.4cdefg | 53.2cdef | 1167.8defg |
| Dristu | 11.8cdefg | 13.3gh | 18. 0g | 36.2fgh | 45ghij | 47.8ghi | 996.4ijkl |
| Gofta | 10.5fg | 11.7h | 16.78g | 33.6gh | 43.7ij | 46.7hi | 932.0kl |
| SAB-736 | 13.2abcd | 16.6abcd | 23.6f | 42.9cd | 48.2cdefg | 52.3def | 1149.1efgh |
| Ibado | 11.1defg | 13gh | 17.7g | 36.1fgh | 45ghij | 47.5ghi | 988.0jkl |
| Ser-125 | 12.7abcd | 14.5defg | 22.0f | 38.3ef | 47.3efghi | 50.3efgh | 1074.3hi |
| Argene | 12.4abcdf | 14.7defg | 21.8f | 38.2ef | 47efghi | 49.3fghi | 1068.2hij |
| Awash-1 | 14.3a | 18.9a | 29a | 49.2a | 53.3a | 58a | 1305.8a |
| Nasir | 13.7ab | 16.97abcd | 24.7bcdef | 46.9abc | 49.4bcdef | 53.5bcdef | 1201.2bcdef |
| Crans cope | 14.1a | 18.1ab | 27.1abcd | 48.4ab | 51.1abcd | 56.6abcd | 1260.3abc |
| Gobe rash | 14.0a | 18ab | 26.7abcde | 48.3ab | 50.7abcde | 55.6abcd | 1249.0abcd |
| Awash-2 | 13.8ab | 17.2abcd | 24.5cdef | 47.3ab | 50.2abcde | 53.4cdef | 1209.4bcdef |
| Roba-1 | 10.83efg | 11.97gh | 17.05g | 33.88gh | 44hij | 46.9ghi | 950.3kl |
| Ser-119 | 12.9abcd | 16.0bcdef | 23f | 40.7de | 47.5defgh | 51efg | 1114.3gh |
| GLP-2 | 14.2a | 18.3ab | 27.5abc | 48.5ab | 51.7abc | 56.8abc | 1270.5abc |
| SAB-632 | 13.0abcd | 16.3abcde | 23.4f | 41.2de | 47.8defg | 52.5def | 1130.8fgh |
| KAT-B1 | 13.8ab | 17.3abc | 24.7bcdef | 47.7ab | 50.2abcde | 54.1abcde | 1218.1bcde |
| ICB-0081 | 13.9ab | 17.7ab | 24.8bcdef | 47.9ab | 50.6abcde | 55.3abcd | 1228.6abcd |
| Biofort | 14.3a | 18.4ab | 27.6ab | 48.7ab | 52.3ab | 57.7ab | 1280.8ab |
| LSD | 1.96 | 2.81 | 3.03 | 4.33 | 3.77 | 4.26 | 85.21 |
| CV (%) | 9.19 | 10.81 | 8.06 | 6.18 | 4.75 | 4.96 | 4.56 |
Table 2: Percentage severity index and area under progress curve (AUDPC) of the response of common bean varieties to anthracnose at Serinka during 2017 main cropping season.
The maximum disease severity levels of anthracnose; 58%, 57.7% and 56.8%, were recorded from susceptible varieties of Awash-1, Biofort and GLP-2, respectively (Table 2). The main reason of less resistant varieties is the possible breakdown of resistance due to the race change of the pathogen to the host resistance [30]. The current finding is consistent with the investigations of Sharma et al. [31] who found highly significant differences in common bean anthracnose severities were recorded from the resistant and susceptible varieties. Varieties mixtures containing at least 60% of a resistant variety have been reported to offer a good control of anthracnose [19].
Area under disease progress curve
The use of the area under disease progress curve (AUDPC) as a disease severity measure and as a tool for plant resistance evaluation helps to reflect disease progress throughout the whole growing season [25]. Analysis of variance for AUDPC values showed a very highly significant difference (P ≤ 0.001) between varieties.
There were differences in the AUDPC values among common bean varieties. The differences observed in AUDPC values of varieties indicated differences in resistance level of individual varieties. The lower AUDPC values of bean anthracnose 921.2, 932.0 and 950.3 were recorded from resistance varieties Awash Melka, Gofta and Roba-1, respectively (Table 2). While the higher AUDPC values of 1305.8, 1280.8,1270.5,1260.3,1249.0,1228.6 and 1218.1 were recorded from susceptible variety such as Awash-1, Biofort, GLP-2, Crans cope, Gobe rash ICB-0081 and KAT-B1, respectively, (Table 2). In this study, the highest AUDPC values represented bean varieties with the highest disease infection.
Reaction of common bean variety on yield and yield components
Analysis of variance indicated that, very high significant differences (P ≤ 0.001) were observed among varieties in plant height. Higher plant height, 100.3, 97.8, 87.7 and 87.3 cm were recorded from Nasir, Tabor, Nazareth-2 and Gofta varieties, respectively, while lower plant height, 30.2,30.8,38.3,42.3and 46.1 were recorded from variety KAT-B1, SAB-736, SAB632, KAT-B9 and Biofort, respectively (Table 3).
| Treatment | Yield and yield components | |||||
|---|---|---|---|---|---|---|
| PH | NPP(cm) | IPP (%) | SPP | 100SW(g) | tha-1 | |
| Nazareth-2 | 87.73ab | 20bc | 4.7f | 5.47d | 21.59hi | 2.52cd |
| Awash Melka | 55.83defg | 13.63e | 3.67g | 6.33a | 23.67ghi | 3.03a |
| Tabor | 97.83a | 20.8ab | 5.8bcd | 4.2hi | 29.63ef | 1.58ghi |
| KAT B9 | 42.27ij | 12.4efg | 5.77bcd | 4.37gh | 41.14d | 1.66gh |
| Dristu | 76.2acb | 18.67cd | 4.07g | 5.6d | 25.99fgh | 2.64bcd |
| Gofta | 87.3b | 18.83cd | 3.7g | 6.1ab | 32.50e | 2.93ab |
| SAB-736 | 30.87j | 11.2fgh | 5.73bcd | 4.47fgh | 40.10d | 1.82fg |
| Ibado | 47.7hij | 13.07e | 4.03g | 5.7bc | 55.15ab | 2.65abcd |
| Ser-125 | 44fghi | 13.4e | 5.33de | 4.95e | 33.51e | 2.36de |
| Argene | 66.27cde | 18.03d | 4.87ef | 5.44d | 19.105 | 2.38de |
| Awash-1 | 68.27cd | 7.8i | 6.733a | 3.67kl | 22.76hi | 1.32hij |
| Nasir | 100.27a | 21.73a | 5.83bcd | 4ij | 26.26fgh | 1.47ghij |
| Crans cope | 61.2de | 11.13gh | 6.13abc | 3.47lm | 48.77c | 1.18jk |
| Gobe rash | 55.47efgh | 11gh | 6.07bc | 3.72kl | 58.31a | 1.27ijk |
| Awash-2 | 68.23cde | 12.57efg | 5.87bcd | 3.93ijk | 23.99ghi | 1.48ghij |
| Roba-1 | 67.47cde | 18.07d | 3.93g | 5.93bc | 29.49ef | 2.81abc |
| Ser-119 | 65.27cde | 12.73ef | 5.67dc | 4.73ef | 28.46efg | 2.28de |
| GLP-2 | 58.33def | 10.73h | 6.13abc | 3.43lm | 55.45ab | 1.41hij |
| SAB-632 | 38.27ij | 11.33fgh | 5.7cd | 4.61fg | 50.32bc | 2.04ef |
| KAT-B1 | 30.2j | 7.83i | 5.93bcd | 3.07n | 41.047d | 0.972k |
| ICB-0081 | 65.13cde | 10.47h | 6.03bc | 3.87jk | 59.87a | 1.32ihj |
| Biofort (Large5) | 46.13fghi | 10.33h | 6.33ab | 3.3mn | 51.29bc | 1.34hij |
| Lsd (5%) | 12.78 | 1.598 | 0.61 | 0.32 | 5.44 | 0.38 |
| CV (%) | 12.65 | 6.98 | 6.91 | 4.32 | 8.88 | 12.07 |
The mean values in the column with different letters are significantly different whereas the mean values with the same letters are not significant different.
Table 3: Yield and yield component of different common bean varieties in the presence anthracnose pathogen.
Moreover data on yield parameters showed very highly significant differences (P ≤ 0.001) among varieties in the number of pods per plant, infected per pod, hundred seed weight, seeds per pod and seed yield. The higher numbers of pod per plant; 21.7, 20.8, 20, 18.8 18.7, and 18.0 were recorded from Nasir, Tabor, Nazareth-2, Gofta, Dursitu and Aregne varieties, respectively. However, the lowest number of pod per plant; 7.8, 7.8, 10.3, 10.5 and 10.7 were recorded from KAT-B1, Awash-1, Biofort, ICB-0081, and GLP-2, varieties, respectively (Table 3).
Considering infected pods per plant, the reaction of anthracnose showed significant difference among varieties. The lower number of infection of pods per plant; 3.67, 3.70 and 3.9 were recorded from Awash-Melka, Gofta and Roba-1 varieties, respectively while higher numbers of infection pods per plant; 6.7, 6.13, 6.07, 6.13, 6.03 and 6.33 were recorded from Awash-1, Corans cope, Gobe rash, GLP-2, ICB-0081 and Biofort, respectively (Table 2).
Analysis of variance indicated that, there was very highly significant (P ≤ 0.001) difference among varieties on grain yield of bean. The mean yield varied widely among varieties from 0.972 – 1.47 t ha-1 for susceptible varieties and from 2.04 – 3.03 t ha-1 for relatively resistant bean varieties. The highest yield (3.03 t ha-1) was recorded from Awash Melka while the lowest yield (0.972 t ha-1) hectare was recorded from Awash-1 variety (Table 3) [31].
It can thus be noted that the measurement of disease may not give a direct relationship to yield, while gives an indication of the amount of yield that may be lost if the plant is susceptible to the pathogen. Nkalubo [32] reported that, differences in yield varied significantly between different accession and not between resistant classifications. There were accessions with an intermediate resistant reaction that yielded significantly higher than accessions with a resistant reaction. This might be due to the apparent nature of resistant for some common bean genotypes against the disease.
Association of common bean anthracnose and yield parameters
Correlation analysis revealed that, significant negative relationship between anthracnose severity and area under disease progress curve (AUDPC) on plant height, number of pods per plant, number of seeds per pod and grain yield per hectare (Table 4). While highly significant (P ≤ 0.001) positive correlations were observed between area under disease progress curve (AUDPC), PSI, and percentage pod infection. Disease parameters such as, AUDPC and disease severity (PSI) showed highly significant (P ≤ 0.01) negative correlations with the seed yield and seed per pod (Table 4). As reported by Sharma et al. [31], highly significant negative correlations between anthracnose severity and percentage reductions in the number of seeds per pod and seed weight [32]. Marcinkowska and Borucka [33] found significant positive correlation between the incidences of C. lindemuthianum in P. vulgaris seeds and leaf, pod and stem infection by the pathogen under natural field conditions.
| Variables | PH | PPP(cm) | IPP (%) | SPP | 100SW (g) | tha-1 | PSI | AUDPC |
|---|---|---|---|---|---|---|---|---|
| PH | 1.0 | |||||||
| PP | 0.7*** | 1.0 | ||||||
| IPP | -0.12ns | -0.52*** | 1.0 | |||||
| SP | 0.18ns | 0.55*** | -0.89*** | 1.0 | ||||
| SW | 0.17ns | 0.32** | -0.5*** | 0.6*** | 1.0 | |||
| tha-1 | 0.14ns | 0.5*** | -0.84*** | 0.9*** | 0.57*** | 1.0 | ||
| PSI | -0.1ns | -0.52*** | 0.8*** | -0.81*** | -0.56*** | -0.79*** | 1.0 | |
| AUDPC | -0.12ns | -0.52*** | 0.9*** | -0.91*** | -0.56*** | -0.86*** | 0.9*** | 1.0 |
Table 4: Correlation coefficient (r) among disease parameters and yield and yield components in different varieties.
Conclusion and Recommendations
The primary goal of this study was to evaluate the reactions of common bean varieties on epidemics of the disease. The anthracnose severity, infected pods per plant and area under disease progress curve (AUDPC) were recorded highest in susceptible varieties Awash-1 followed by Mexican-142 and Awash Melka. The highest anthracnose disease severity was observed in susceptible varieties Awash-1 could be the reason for highest yield loss in the varieties. Anthracnose attacked plant leaves, stems and pods and not only interrupts the plant's ability to take in photosynthetic materials but also utilizes the plant's substrates and damages the host's functions thus reducing its ability to yield effectively.
From twenty two common bean varieties evaluated for the reaction to bean anthracnose under natural infestation conditions, varieties showed significantly (P ≤ 0.001) different levels of bean anthracnose severity and AUDPC during the disease assessment period. The highest disease severity (58%) was recorded from Awash-1 varieties while the lowest disease severity (45%), was recorded from Awash Melka varieties at final assessment of the day. In the present study confirmed that the efficiency of the reaction of bean verities benefit in terms of the genetic ability resistant varieties to anthracnose.
From the present study, it is possible to conclude that, the advantage of screening resistant verities increases the opportunity to select for a broad range of anthracnose resistance and help to know the variability of the common bean anthracnose disease. Moreover, more extensive screening resistant varieties with different seasons and location studies should be planned for a full assessment of the disease distribution and identifying germplasm materials, as a source of resistance could be important for common bean breeding in Ethiopia.
REFERENCES
- Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J. Beans (Phaseolus spp.)–model food legumes. Plant and soil. 2003;252:55-128.
- Ferris S, Kaganzi E. Evaluating marketing opportunities for haricot beans in Ethiopia. IPMS (Improving Productivity and Market Success) of Ethiopian Farmers Project Working Paper 7. ILRI (International Livestock Research Institute), Nairobi, Kenya. 2008.
- Hadi H, Kazem G, Rahimzadeh K, Mostafa V, Reza S. Response of common bean to different levels of shade of agronomy. J Agronomy. 2006;5:595-599.
- Pachico D. The Demand for Bean Technology. pp. 60-73. In: Henry, G. (edn). Trends in CIAT commodities 1993. CIAT Working Document, Cali-Columbia. 1993.
- Fininsa C. Relationship between common bacterial blight severity and bean yield loss in pure stand and bean-maize intercropping systems. Int J Pest Manag. 2003;49:177-185.
- Hailu N, Fininsa C, Tana T, Mamo G. Effect of Climate Change Resilience Strategies on Common Bacterial Blight of Common Bean (Phaseolus vulgaris L.) in Semi-arid Agro-ecology of Eastern Ethiopia. J Plant Pathol Microbiol. 2015;6:1-10.
- CSA (Central Statistics Agency of Ethiopia). Report on area and crop production of major crops for 2016 Meher season. 2016;1:125.
- Kutangi E, Farrow A, Mutuoki T, Gebeyehu S, Karanja D. Improving common bean productivity: An Analysis of socioeconomic factors in Ethiopia and Eastern Kenya. Baseline Report Tropical Legumes II. Centro Internacional de Agricultura Tropical-CIAT. Cali, Colombia. 2010.
- BARC (Bako Agricultural Research Center). Bako Agricultural Research Center progress report of pulse crop team. 2011.
- Barcelos Q, Souza E, Damasceno E, Silva K. Vegetative compatibility and genetic analysis of Colletotrichum lindemuthianum isolates from Brazil. Genet Mol Res. 2011;10:230-242
- Fikru M, Asresie H. Evaluation of Improved White Pea Bean Varieties in Eastern Amhara. Proceeding of the First Annual Conference of Amahara Agricultural Research Rreview, Bahirdar, Ethiopia August, 2006;25-29.
- White JWR, Ochoa MF, Ibarra P, Singh S. Inheritance of seed weight of common bean (Phaseolus vulgaris) under semiarid rain fed conditions. J Agric Sci. 1994;122:265-273.
- Allen D. Pests, diseases, and nutritional disorders of the common bean in Africa. Centro International De Agricultural Tropical (CIAT), Cali, Colombia.1996.
- Tesfaye B. Loss assessment study on haricot bean due to bean anthracnose. Pest Management Journal Ethiopia. 1997;1:69-72.
- Amin M, Amare A, Nigussie D. Effect of integrated management of bean anthracnose (Colletotrichum lindemuthianum Sacc. & Magn.) through soil solarization and fungicide applications on epidemics of the disease and seed health in Hararghe highlands, Ethiopia. J Plant Pathol Microbiol. 2013;4:1-7.
- Amin M, Teshale J, Tesfay A. Evaluation of bioagents seed treatment against Colletotrichum lindemuthianum, in haricot bean anthracnose under field condition. Res Plant Sci. 2014;2:2-26.
- Mohammed Yusuf and Somsiri, S. 2005. Seed transmission and epidemics of Colletotrichum lindemuthianum in the major common bean growing areas of Ethiopia. Kasetsart Journal (Natural Science) 39: 34 -45.
- McGee D. Epidemiological approach to disease management through seed technology. Ann Rev Phytopathol. 1995;33:445-466.
- Tesfaye B. Biology and control of bean anthracnose in Ethiopia. Doctoral Dissertation, University of Free State, Bloemfontein, South Africa. 2003.
- Daniel R, De-Vos D, Desair J, Raedschelders G, Luyten E, Rosemeyer V, et al. The cinquorum-sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J Biol Chem. 277:426-430.
- Fitsum S. Evaluation of some fungicides and bio-agents for the management of Common Bean Anthracnose (Colletotrichum lindemuthianum (Sacc. & Magnus) in Ambo, West Showa, Ethiopia, M.Sc., thesis, Ambo University, Ethiopia. 2014;65.
- Mekonnen G, Sharma J, Lisanework N, Tamado T. Effect of Integrated Weed Management Practices on Weeds Infestation, Yield Components and Yield of Cowpea [Vigna unguiculata (L.) Walp.] in Eastern Wollo, Northern Ethiopia Am J Exp Agri. 2015;7(5):326-346.
- Sharma P, Pathania K. Evaluation of bio-agents and bio-pesticides against Colletotrichum lindemuthianum and its integrated management in common bean. CSK HP Agricultural University, Molecular Plant Pathology Laboratory, Department of Plant Pathology, India. 2010.
- Wheeler B. An Introduction to Plant Diseases. John Wiley & sons, London. 1969.
- Campbell C, Madden L. Introduction to plant disease epidemiology. John Wiley and Sons, New York. USA. 1990.
- SAS (Statistical Analysis System) software. Version 9.2. Inc. Carry, North California, USA. 2009.
- Gomez K, Gomez A. Statistical procedures for agricultural research (2nd edn), John Willey and Sons, Inc. New York, USA. 1984;680.
- Vander PJ. Analysis of epidemics. In: Horsfall, J.G. and Cowling, E.B. (eds) Plant Pathology: An Advance Treatise, Academic Press, New York, USA. 1960; 229–289.
- Berger R. Comparison of the Gompertz and Logistic equations to describe plant disease progress. Phytopathol. 1981;71:716-719.
- McDermott J. Gene flow in plant patho systems. Annu Rev Phytopathol. 1993;31:353-357.
- Sharma PN, Sharma O, Padder BA. Kapil R. Yield loss assessment in common bean due to anthracnose (Colletotrichum lindemuthianum) under sub-temperate conditions of Northwestern Himalayas. Ind Phytopathol. 2008;6:323-330.
- Nkalubo S. Study of anthracnose (Colletotrichum lindemuthianum) resistance and its inheritance in Ugandan dry bean germplasm: M.Sc. Thesis University of Kwazulu-Natal Pietermantz burgh, South Africa. 2006.
- Marcinkowska J, Borucka K. Colletotrichum lindemuthianum in Phaseolus vulgaris seeds. Plant Breed Seed Sci. 2001;45:59-64.
Citation: Hussien S, Hailu N, Belete E (2020) Evaluation of Common Bean (Phaseolous vulgaris L.) Varieties for the Reaction of Common Bean Anthracnose (Colletotrichum lindemuthianum) at Sirinka, Eastern Amhara, Ethiopia. J Plant Pathol Microbiol 11:517. doi: 10.35248/2157-7471.20.11.517.
Copyright: © 2020 Hussien S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

