Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 9
Evaluation of Antioxidant, Antibiofilm, Cytotoxic and Antimicrobial Activities of Calligonum Comosum
Nallusamy Sivakumar*, Neelam Sherwani and Mohamed Abdullah Al MahrouqiReceived: 21-Jan-2021 Published: 30-Sep-2021, DOI: 10.35248/2157-7471.21.12.575
Abstract
Objective: Calligonum Comosum is a native omani medicinal plants used traditionally by the locals to treat inflammation, toothache, gum sores and ulcer. As antimicrobial resistance poses a serious threat on global scale by rendering many currently available antibiotics ineffective. This demands the need to look for novel therapeutic agents and there has been an expanded interest in natural products as sources of pharmacologically active principles. The present study was thus initiated to corroborate the therapeutic uses of this medicinal species by evaluating, the phyto-constituents, antibacterial, anti-biofilm and antioxidant activities of C. comosum.
Methods: Quantitative and qualitative phytochemical analysis was done, using standard protocols. The antioxidant activity was assessed applying DPPH free radical scavenging assay, hydrogen peroxide radical scavenging assay and the total antioxidant capacity. Biofilm inhibition activity was evaluated using micro titer plate assay and well diffusion method was employed to determine the antibacterial activity. Cytotoxity was assessed in terms of LC50 value using brine shrimp lethality assay.
Results: The methanolic extracts of C. comosum showed significant antibacterial activity against all the five tested bacterial strains, with MIC values for all observed to be 1.25 mg/ml except for E. coli. Substantial reduction of the biofilm formation was observed for all bacterial species treated with C. comosum leaf and stem extracts and the extracts also displayed significant cytotoxicity against brine shrimp nauplii, exhibiting LC50 value of 56.797 μg/ml. The total phenolic content and flavonoid content was observed to be 56.6 ± 1.66 mg GAE/g and 49.33 ± 1.34 mg of QE/g of dry extract respectively. C. comosum exhibited potent DPPH scavenging activity, with IC50 value of 44.90 μg/ml and the total antioxidant capacity was 130 ± 2.04 mg AAE/g.
Conclusion: The result validates its ethno-medicinal use and suggests that the C. comosum can be exploited for its antibiofilm, antioxidant and antibacterial properties.
Keywords
Calligonum comosum; Antimicrobial resistance; Antibiofilm; Antibacterial; Antioxidant; Cytotoxic
Introduction
Microbes are becoming resistant to available antimicrobial medicines at an alarming rate. Antimicrobial Resistance (AMR) has rendered many currently available antibiotics ineffective. For instance, the methicillin resistant Staphylococcus aureus has shown resistance against different antibiotics such as tertracycline, chloramphenicol and aminoglycosides in addition to methicillin [1]. Multi-drug resistant Escherichia coli and Klebsiella pneumoniae strains are also common [2]. According to the World Health Organization WHO Research and Development blueprint, pathogens like Pseudomonas aeruginosa, S.aureus and E.coli, poses greatest threat to human health, if the AMR continues at the current rate [3]. The quest for new antimicrobials sources in order to overcome the antibiotic resistance has been a top research priority, as currently 700,000 deaths occur each year due to drug-resistant diseases [4].
Traditionally, plants have been the sources of novel chemical entities which are capable of suppressing the bacterial activities. Nowadays, traditional herbal medicines are widely accepted in the conventional healthcare systems due to the therapeutic potential of several secondary compounds of plant origin [5]. In light of the above, the traditional medicine could be the best resource to counteract antibiotic resistance. Out of the 28,000 herbal species currently being categorized for medicinal use, less than 16% of the species are actually cited in the medicinal regulatory publications [6]. Thus, these untapped resources have a substantial potential for the discovery of novel therapeutic medicines.
Calligonum comosum L-Her commonly called ‘Arta’ belonging to family Polygonaceae is a nearly leafless perennial woody shrub growing up to 2.5 m tall. It is distributed widely across sandy deserts of Oman, Saudi Arabia, United Arab Emirates, deserts of North Africa, Pakistan and Thar Desert in India. C. comosum is an arid zone plant that can tolerate extreme drought conditions. It is widely distributed among the sand dunes and sandy soils in Oman. In traditional Omani folklore, many medicinal uses are enlisted to C. comosum species. For instance, chewing of stems and leaves to cure toothache, decoction from roots to treat gum sores, antiinflammatory and anti-ulcer properties.
The pharmacological nature of medicinal plants is due to the presence of natural antioxidants such as flavonoids and phenols which exhibits a strong antioxidative activity being capable of scavenging free radicals, inhibiting oxidative reactions [7], easing lipid peroxidation and preventing cellular damages during oxidative stress [8]. Due to the toxicity of synthetic antioxidants at higher concentrations, the focus of scientific attention has sharply shifted towards plants as a source of antioxidants [9] and there has been an expanded interest in plant phenolic compounds as natural novel antioxidants negating the Reactive Oxygen Species (ROS) induced oxidative stress without any side effects of synthetic antioxidants. Other important phytochemicals in medicinal plants responsible for therapeutic potential and bioactivity includes alkaloids, tannins, terpenes, steroids, saponins, and quinones. Tannins are a diverse group of high molecular weight polyphenolic compounds and have shown effective anti-inflammatory antimicrobial antitumor, antioxidant and anticancer activities [10]. Saponins, the glycosylated steroids have shown numerous pharmacological activities such as anti-inflammatory [11] and antibacterial [12]. Quinones, the oxidized phenols, are highly electrophilic compounds, well known for their antibacterial and antitumor properties [13].
Hence, the present study was carried out to evaluate the antibacterial potential of C. comosum due to the growing resistance against the currently used antibacterial agents and to evaluate the antioxidant activity, antibiofilm ability, cytotoxity and phytochemical constituents present in the stem and leaf extract.
Materials and Methods
Sample collection and extraction
C. comosum plant growing wild in sand plains of Bowsher area (23.5821° N, 58.0271°E) were collected, this area is in Governorate of Muscat, which is the north-eastern part of Oman. Healthy plants were selected and collected between March 15th and 15th April 2018. Voucher specimens were collated with the herbarium center at the Biology department of Sultan Qaboos University, Oman. Young stem and leaves of C. comosum were washed with water to clear the debris, cut into small pieces and air dried under shade (25°C) until completely dry. Dried pieces powdered to fineness (100 g) were extracted with 300 ml 99.9% concentrated methanol in a shaker for 24. Post first extraction residues were subjected to 2 more extractions. Afterwards using a rotary evaporator the methanol filtrate was evaporated to complete dryness. The residue was dissolved in dimethyl sulfoxide to yield 100 mg/ml of extract. Stored inside brown bottles the extract was refrigerated until its further use.
Antibacterial activity
Microorganisms: The following ATCC cultures of Escherichia coli (ATCC 9637), Pseudomonas aeruginosa (ATCC 10231), Bacillus subtilis (ATCC 6633) Streptococcus mitis (ATCC 49456) and Staphylococcus aureus (ATCC 29213), were used for the antimicrobial activity.
Agar well diffusion method: The effectiveness of the C. comosum leaf extract against the above specified bacterial strains was estimated following agar well diffusion method [14]. Petri dishes filled with 25 ml of nutrient agar were left to solidify. Subsequently, the agar plate surface was inoculated by evenly swabbing an overnight broth culture of bacteria by using sterile cotton swabs. Using a sterile cork borer, wells with a diameter of 8 mm were made on these plates aseptically. The methanolic plant extracts were dissolved in DMSO at a concentration of 100 mg/ml, then 100 μl of plant extract was added into these wells and allowed to diffuse for 2 h at room temperature. DMSO (100 μl) and gentamicin (10 μg ml−1) were used as negative and positive controls. Post diffusion plates were incubated at 37°C for 24 h and the diameter of the inhibition zone (mm) was measured. Experimental analysis was done in triplicate, mean, standard deviation and standard error were calculated.
Minimum Inhibitory Concentration (MIC): The MIC of the extracts of C. comosum was determined by the microtiter test [15]. Based on the results of preliminary screening in agar well diffusion method, the methanolic extracts that demonstrated potent antibacterial activity were tested for MIC. A serial dilution of the plant extract, ranging from 10 mg/ml to 0.078 mg/ml, was done and 100μl of different concentrations (10 mg/ml, 5 mg/ml, 2.5 mg/ ml, 1.25 mg/ml, 0.625 mg/ml, 0.3125 mg/ml, 0.15655 mg/ml, and 0.078 mg/ml) were loaded in each wells of 96-well microtiter plate. The plates were incubated at 37°C for 24 h. The lowest concentrations of the plant extract (μg/ml) that inhibited bacterial growth, demonstrating no turbidity were defined as MICs. Experimental analysis was done in triplicate, mean, standard deviation and standard error were calculated.
Minimum Bactericidal Concentration (MBC): The minimum bactericidal concentration was determined using [16] assay. 100 μL of the test culture from the well of the microtiter plate with no visible turbidity was taken, inoculated on nutrient agar plates and incubated for 24 h at 37°C. The highest dilution of the methanolic plant extract which represents the lowest concentration of plant extract with no bacterial growth on the new set of nutrient agar plate was taken as MBC. Experiment was repeated thrice.
Antibiofilm activity
The biofilm inhibition activity of C. comosum extracts by preventing initial cell attachment was evaluated using microtiter plate assay [17]. Tryptone Soy Broth (TSB) (180 μL) and 20 μL of bacterial suspension of each E. coli, B. subtilis, S. aureus, P. aeruginosa and S. mitis was added into each of the 96-well of the polystyrene flat bottom plate and incubated without shaking at 37°C for 24 h. After incubation 200 μl (1 mg/ml) aliquot of different concentrations (75%, 50%, and 25%) of plant extract was added in three replicates into each well of 96-well microtitre plates and then incubated further at 37°C for 24 h, TSB broth alone was used as a negative control. After incubation, the broths were removed from the microtiter plate wells and washed with sterile distilled water twice.
In order to evaluate the biomass formed in adhered biofilm the wells were strained with 200 μL of 0.4% crystal violet solution and incubated for 30 min. Following incubation, the dye was removed, plates were washed thrice in order to remove any free floating bacteria and plates were dried at 40°C for 15 min. After drying, 200 μL of 95% of ethanol was added to each well, the biofilm was quantified and the OD was measured at 570 nm.
Biofilms were measured using the formula SBF = AB-CW/G, where SBF stands for the specific biofilm formation, AB is the Optical Density (OD) at 570 nm of the attached stained bacteria, CW is the Optical Density (OD) at 570 nm of the stained control wells which contain bacteria-free medium and G is the Optical Density (OD) at 570 nm of cell growth in the suspended culture. The values for SBF were grouped into three categories as following; the strong biofilm producers with an SBF index of ≥ 2.00, intermediate biofilm producers with SBF index between 1 and 2 and the weak biofilm producers with SBF index ≤ 1.00.
Brine Shrimp Lethality (BSL) assay
Brine shrimp lethality assay was determined using [18] assay. To conduct the BSL assay, 250 mg of Artemia salina (brine shrimp) eggs were incubated with 400 ml of synthetic sea water medium (40 g sea salt/l and supplemented with yeast extract 6 mg/l) in a hatching chamber with dark and light areas partitioned. Eggs were added to the dark side, the hatched nauplii (larva) will be attracted towards the light, after 48 h the 10 mature nauplii were collected in each vial containing 5 ml of the artificial seawater and different concentrations of plant extract. Concentrations of 1 mg/ ml, 100 μg/ ml, 50 μg/ ml, 10 μg/ ml, 5μg/ ml and 1 μg/ ml were prepared by serial dilution from the stock solution. After 24 h of incubation, the nauplii were counted.
The percentage mortality was determined as follows;
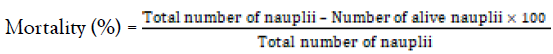
The median lethal concentration (LC50), was calculated from the best fit line derived from the linear regression analysis of the % mortality versus the concentration of plant extract.
The lethality of the C. comosum extracts on the brine shrimp was categorized as
LC50 ≥ 1000 μg ml–1- Non Active – nontoxic, LC50 = 500–1000 μg ml–1 -Active - weakly cytotoxic,
LC50 = 100–500 μg ml–1 -Active -moderately cytotoxic and LC50 ≤ 100 μg ml–1 - Active - strongly toxic.
The LC50 value was calculated using AAT Bioquest LC50 calculator, lower values of LC50 indicates greater antioxidant capacity.
Phytochemical screening
Presence or absence of certain phytochemicals like saponins (Frothing Test), terpinoids (Salkowski test), flavonoids (Shinoda’s test), steroids (Liebermann-Burchard’s or Acetic anhydride test), tannins (Ferric chloride test), Baeyer’s test for unsaturation, quinones and carbohydrates (Molisch’s test) in C. comosum was evaluated using the standard methods [19,20]. All the tests were performed in triplicate.
Determination of total phenolics (Folin-Ciocalteu reagent): Folin- Ciocalteu reagent assay was applied to ascertain the total phenolic content spectrophotometrically [21]. 0.5 ml of methanolic extract (100 μg/mL) was mixed with 2.5 mL of Folin-Ciocalteu reagent and 7.5% Na2CO3 (2.5 mL), the test tube was covered with paraffin and then kept for 30 min under dark conditions. Absorbance was read at 765 nm. Solution of Folin-ciocalteu, Na2CO3 and methanol acted as blank and reference standard was gallic acid. Assessment of total phenolic content was made using the linear equation of the calibration curve, expressed as milligrams of gallic acid equivalents per gram (mg GAE/gG1 extract). Analysis was done 3 times, mean, standard deviation and standard error were calculated and applied.
Determination of total Flavonoids (Aluminium chloride colorimetric method): Total flavonoid content was estimated quantitatively using aluminium chloride colorimetric assay [22]. To 1 ml extract was added 2.8 ml of distilled H20, followed by 0.1 ml solution of potassium acetate (prepared as 1 mg/ml) and 0.1 ml solution of 10% aluminium chloride (AlCl3). Absorbance of this mixture was noted at 415 nm, after keeping the mixture standstill for 30 min. The flavonoid content was estimated as Quercetin equivalent, 10 to 100 μg/ml quercetin was taken as a standard and a calibration curve was plotted. Analysis was performed thrice.
Antioxidant activity
DPPH Radical Scavenging Activity: The DPPH radical scavenging effect was estimated by using [23] method. A solution of DPPH (0.135 Mm solution) was prepared in methanol, 1 ml of which was mixed with 1.0 ml aqueous extract under different concentrations (varying from 0.2 to 1 mg/ml). After mixing, the reaction mixture was kept for half an hour under dark conditions. Absorbance was recorded spectrophotometrically at 517 nm. DPPH scavenging capacity was estimated using the equation:
(%) DPPH scavenging activity = {(Abs control – Abs sample)/(Abs control)}x 100
Abs control stands for - DPPH and methanol, absorbance
Abs sample stands for - DPPH radical + sample extract/standard, absorbance
The IC50 value was calculated using AAT Bioquest IC50 calculator, lower values of IC50 indicates greater antioxidant capacity.
Determination of hydrogen peroxide radical scavenging capacity: The capacity of C. comosum extracts to scavenge H2O2 was evaluated [24]. Hydrogen peroxide solution (40 mM ) was made in phosphate buffer (with pH 7.4). One ml methanolic leaf extract (100 μ g/ml) was mixed with H2O2 solution (0.6 ml), after waiting for 10 min the absorbance was noted at 230 nm. Phosphate buffer alone acted as blank. The % scavenging by C. comosum extract was estimated by the formula. The analysis was performed thrice and values were averaged
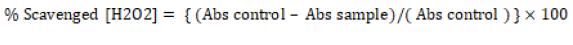
Abs control stands for the absorbance of the control which is without extract
Abs sample stands for absorbance in presence of extract. Varied concentrations of methanolic extracts of C. comosum ranging from 10-100 μ g/ml were taken and scavenging activity evaluated.
Different concentrations (10-100 μ g/ml) of methanolic extracts of C. comosum were taken and scavenging activity evaluated and IC50 value which shows 50% inhibition was determined using regression analysis.
Determination of antioxidant activity
Determination of total antioxidant activity (Phosphomolybdenum method): The antioxidant capacity was assessed according to [25] assay. H2SO4 (0.6 M), sodium phosphate (28 mM), ammonium molybdate (4 mM) were mixed together to form a 3 mL solution, to this 0.3 ml extract was added. Solution was incubated in a water bath for 90 min, at a temperature of 95°C. Later solution cooled off and absorbance noted at 695. Methanol (0.3 ml) without extract and reagent solution (3 ml) was used as blank. Using ascorbic acid as a standard reference calibration curve was derived from varied concentrations of ascorbic acid, ranging from 1000, 500, 250, 125, 62.5 to 31.25 μg/ml. Analysis was done 3 times.
Results and Discussion
Phytochemical screening of the plant extract
The therapeutic properties of medicinal plants are associated with the physiological effects of bioactive compounds such as alkaloids, phenols, flavonoids, saponins, tannins and caumarins. Phytochemical screening results of aerial parts of C. comosum extracts are summarized in Table 1, the results revealed the presence of flavonoids, phenols and tannins in strong intensity while quinones in medium intensity and steroids, flavonoids and saponins in low intensity. Similar results were recorded by earlier study in C. comosum [26].
| Compounds | Levels |
|---|---|
| Flavonoids | +++ |
| Triterpenes/steroids | + |
| Quinones | ++ |
| Alkaloids | ++ |
| Tannins | +++ |
| Phenols | +++ |
| Saponin | + |
| Carboxyl group | ++ |
| Carbohydrates | ++ |
Table 1: Phytochemicals screening of methanolic extract of C. comosum.
Antibacterial effect of plant extract: The methanolic extracts of C. comosum showed significant antibacterial activity against all the five tested bacterial strains (Table 2). The results depicted that the extracts of C. comosum demonstrated the highest activity against S. mitis with an inhibition zone of 26.5 mm, followed by S. aureus, B. subtilus, P. aeruginosa and least inhibition was observed for E. coli (18.7 ± 0.274).
| Bacteria | Inhibition zone (mm) |
|---|---|
| E. coli | 18.7 ± 0.274 |
| S. aureus | 23.4 ± 0.150 |
| B. subtilis | 21.7 ± 0.325 |
| S. mitis | 26.5 ± 0.150 |
| P. aeruginosa | 21.2 ± 0.057 |
Table 2: Diameter of inhibition zones of methanolic extract against five different bacteria.
Similar results were obtained earlier in C. comosum [27] where least inhibition was shown against E. coli. The outer membrane of Gram-negative bacteria acts as a permeability barrier and confers are more resistant to antibacterial agents [28]. The high antibacterial activity in the methanolic extract may be attributable to the presence of tannins, saponins, flavonoids, alkaloids and terpenoids [29]. Tannins inhibit bacterial growth by inducing complexation with enzymes and precipitation of proline rich proteins in membranes [30], saponins antibacterial activity stems from their ability to cause leakage of certain enzymes and proteins from the cell [31]. Terpenoids work by weakening the membranes of the microorganisms [32], steroids inhibit bacterial growth by inducing leakage of contents from the phosphatidylethanolaminerich liposomes [33] and flavonoids by inhibiting nucleic acid synthesis, by damaging cytoplasmic membranes and altering the membrane permeability [34].
MIC and MBC: MIC is the minimum concentration of a chemical which inhibits the visible growth of the test organism. All the strains showed visible inhibition. MIC values of for S. aureus, B. subtilis, S. mitis and P. aeruginosa, were 1.25 mg/ml of the extract (Table 3). The extract was least effective against E. coli with MIC value of 2.5 mg/ml. MBC of the methanolic extracts of C. comosum ranged between 2.5 and 5 mg/ml. Similar results were recorded in C. comosum in an earlier study. According to the proposed classification [35], the antibacterial activity of the plant extracts can be classified as following: noteworthy or significant if MIC <1 mg/ml, moderate if MIC ranged between 1–8 mg/ml and weak if MIC from 8–12.5 mg/ml where the extracts were considered weak inhibitors. Moderate (MIC ranging from 1.25 to 2.5 mg/ml) activity of the methanolic extracts against all the five bacterial strains was observed in the results. Our findings support the traditional use of C. comosum by local people as antibacterial plant. High MIC value for E. coli is due to the presence of cell wall lipopolysaccharides which provide more resistant to antibiotics [36].
| Bacteria | MIC (mg/ml) |
MBC (mg/ml) |
|---|---|---|
| E. coli | 2.5 | 5.0 |
| S. aureus | 1.25 | 2.5 |
| B. subtilis | 1.25 | 5.0 |
| S. mitis | 1.25 | 2.5 |
| P. aeruginosa | 1.25 | 2.5 |
Table 3: Minimal inhibitory concentration and minimal bacterial concentration in mg/ml.
Anti-biofilm activity of C. comosum extracts: One of the adaptive resistance strategies implied by many pathogenic bacteria is formation of biofilms, the multispecies surface-adherent bacterial communities embedded in an extracellular matrix [37]. The antibiofilm activity of methanolic extracts of C. comosum against five different strains of bacteria was evaluated under different subinhibitory concentrations (75, 50, and 25% of MBC) and a substantial reduction of the Specific Biofilm Formation (SBF) was observed for all. Maximum inhibition of biofilm was observed in gram negative E. coli where biofilm was reduced from 2.8 (strong biofilm) to levels of 0.3 (weak biofilm) under 75% MBC concentration. In gram positive S. mitis biofilms were reduced from approximately 2.5 (strong biofilm) to 0.7 (weak biofilm) under 75% MBC and to 1 (moderated biofilm) under 50% MBC (Figure 1).
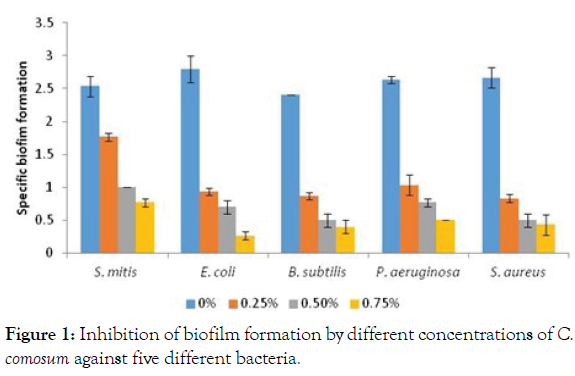
Figure 1: Inhibition of biofilm formation by different concentrations of C. comosum against five different bacteria.
Numerous studies till date, have demonstrated that the bioactive compounds screened from the plant extracts are capable of inhibiting the initiation of biofilm formation by targeting quorum sensing system [38,39]. The antimicrobial potency of the plant extracts is due to inhibition of quorum sensing. The phytochemicals act as Quorum Sensing Inhibitors (QUIs) due to their structural similarities to the quorum sensing signals like acylated homoserine lactone-AHL in gram negative bacteria where they mimic the AHLs and the LuxR-type receptors in the absence of AHLs, are not able to fold and thus degrade [40]. In gram positive bacteria quorum sensing is regulated by oligopeptide pheromones [41]. The inhibition in biofilm formation may have been due to presence of flavonoids, like quercetin, kaempferol, astragalin, taxifol, which have the ability to inhibit biofilm synthesis by suppressing the activity of autoinducer-2 which is responsible for cell-tocell communication. Polyphenolic acid like gallic acid reduces biofilm formation by blocking the AHL -acylated homoserine lactone mediated communication between the bacteria species by degrading LuxR/LasR signal receptors [42]. More inhibition in Gram-negative E. coli and P. aeruginosa species can be due to the fact that the autoinducers in gram negative bacteria are mainly AHLs which are inhibited more by the phenolic compounds [43].
Cytotoxicity associated with C. comosum extracts: The bioactive compounds of many medicinal plants at higher concentrations are found to be toxic, in order to ensure safe treatment from their decoctions, the in vivo lethality bioassay in brine shrimp is a useful tool to evaluate the cytotoxic potential of the herbal extracts. In this bioassay LC50 values below 1000 ppm are considered cytotoxic. The methanolic extracts of C. comosum displayed significant lethality against brine shrimp nauplii, (Figure 2) with the percentage mortality of Artemisia salina nauplii significantly increased with increasing concentrations of the C. comosum extracts, exhibiting LC50 value of 56.797 μg/ml (Figure 3) (Table 4), indicating potent cytotoxic compounds in this species. The results are in line with [44] and [45] who documented significant cytotoxic activity of C. comosum against HepG2 and MCF-7 cell lines. As the preliminary screening for phytochemicals exhibited the presence of alkaloids, phenolic compounds and steroids, thus the displayed cytotoxicity may be attributed to the existence of such bioactive compounds. Our results are in agreement with previous studies which have demonstrated the role of alkaloids, steroids and phenolic compounds [46] in cytotoxic ability of plant extracts.

Figure 2: Cytotoxicity of C. comosum on brine shrimp nauplii.
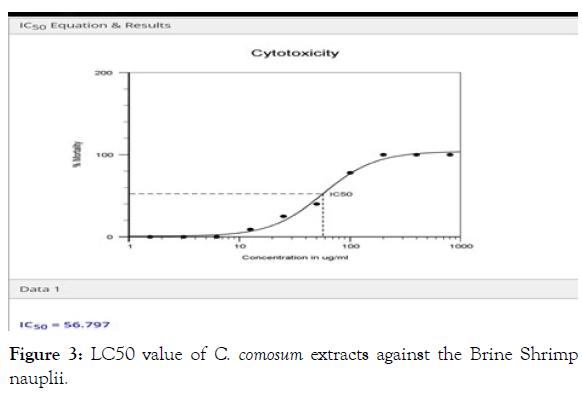
Figure 3: LC50 value of C. comosum extracts against the Brine Shrimpnauplii.
| Sample | LC50 (µg/ml) | Toxicity status |
|---|---|---|
| Methanolic extract of C. comosum | 56.797 µg/ml | Toxic |
Table 4: LC50 values for methanolic extracts of C. comosum against the brine shrimp.
Alkaloids exert their cytotoxic effect by inhibiting the NF-κB pathway activation and by inducing cell cycle arrest [47], phenolic compounds like phenolic acid and flavonoids exert by inducing cell cycle arrest and apoptosis in various human cancer. Thus it is necessary to screen a given medicinal plant for the phytochemicals and their cytotoxic potential for a safe administration of herbal remedies. According to NCI -The United States National Cancer Institute a plant extract is usually safe after incubation between 48 - 72 h if its LC50 value is more than 20 μ g/ml( LC50 >20 g/ ml), for LC50 value is less than 20 μg/ml are cytotoxic [48]. As the plant extract exhibited a value of LC50 >20 g/ml thus this plant is regarded as safe for use as local remedies against toothache and inflammation.
Quantifications of polyphenols, flavonoids and total antioxidant
activity: Total phenolic concentration was determined as gallic acid equivalent from the standard gallic acid calibration curve and expressed as gallic acid equivalents/g dry extract weight of the extract. The total phenolic content was 56.6 mg GAE/g of extract. Previous studies have reported the presence of phenolic acids such as gallic acid, chlorogenic acid, quinic acid, vanillic acid, and protocatechuic acid as the abundant compounds in the methanolic extracts of C. comosum [49].
The total flavonoid content was derived from the calibration curve of quercetin and determined as Quercetin Equivalents (QE)/g dry extract weight of extract. The total flavonoid content was 49.33 mg of QE/g of dry extract. Earlier the flavonoids like, quercetin, kaempferol, astragalin, taxifol, kaempferol glycoside, (+)-catechin, dehydrodicatechin, were identified and isolated from C. comosum. The phenols and flavonoids are the free radical scavenging molecules due to their ability to donate hydrogen to free radicals and the total antioxidant capacity depicts a combined effect of total phenolics, total flavonoids and other reducing compounds in the plant extracts. The Total Antioxidant Capacity (TAC) is expressed as Ascorbic Acid Equivalents (AAE). The TAC of methanolic extracts of C. comosum was estimated to be 130 ± 0.21 mg AAE/g (Table 5).
| Total Phenolics (mg GAE/g DW) |
Total Flavonoids Antioxidant Activity (mg QE/g DW) |
Total (mg AAE/g DW) |
|---|---|---|
| 56.68 ± 1.66 | 49.33 ± 1.34 | 130.68 ± 2.04 |
Table 5: The total phenolics, total flavonoids and total antioxidant activity of methanolic extracts of C. comosum.
An oxidative stress in a biological system derives from an imbalance created among the generation of free radicals and the biological system’s ability to eradicate these reactive oxygen species through scavenging by antioxidants. Free radicals work by attacking the unsaturated fatty acid chains in phospholipids in the membranes, inducing lipid peroxidation a key process causing oxidative stress. The ROS reacts with the basic biomolecules [50] initiating a chain reaction and damaging DNA, RNA and many enzymes. As some synthetic antioxidants were found to demonstrate toxic and carcinogenic effects, the focus has shifted to natural plant phytochemicals. The plants endogenous defense system is mainly the phenolic compounds, like phenolic acids, flavonoids, tannins, stilbenes. The –OH group of phenolic compounds mainly determines their antioxidant ability by donating H –atom to the free radicals and to scavenge the reactive oxygen species like OH•, N2O3, NO2•, and ONOOH, chelate transition metal ions, inhibit lipid peroxidation [51]. Gallic acid demonstrates its effect by elevating the levels of antioxidant enzymes like GPx and CAT and by protecting peroxidation of lipids [52]. Flavonoids antioxidant abilities are ascribed to their ability to activate antioxidant enzymes, transfer the stable free radicals, inhibit oxidases such as xanthine, peroxidase and nitric oxide, chelate metal catalyst and reduce α-tocopherol radicals [53]. The antioxidant properties of quercetin are manifested through regulating the levels of glutathione, showing inhibitory effect against enzymes associated with oxidative properties like acetylcholinesterase and butyrylcholinesterase and modulating the enzymes of various signal transduction pathways [54].
Antioxidant capacity of C. comosum methanolic extract
DPPH radical scavenging activity of C. comosum: C. comosum displayed strong DPPH scavenging activity in a concentrationdependent manner. This assay works on the principle that plant extract with antioxidant capacity by donating a hydrogen atom, reduces the odd electron of nitrogen atom in α, α diphenyl-β- picrylhydrazyl (DPPH) to α, α-diphenyl β-picrylhydrazine, with a marked discoloration from deep violet colour to light yellow. The scavenging activity was recorded to vary from 53.45% to 81.81% with the concentrations, ranging from 100 μg/ml to 500 μg/m (Figure 4), whereas DPPH scavenging activity of α-tocopherol at 500 μg/m was observed to be 83.4% respectively. The IC50 value of methanol extract was 44.90 (Figure 4). IC50 values and individual dose-response curves were calculated using the AAT Bioquest IC50 calculator. Similar results were observed by an earlier study in C. comosum [55]. A number of previous studies have suggested a correlation between the antioxidant activity of plant extracts and the content of total phenols and flavonoids [56,57]. Thus a strong DPPH scavenging activity in this study may be linked with high total phenol and flavonoid concentration.
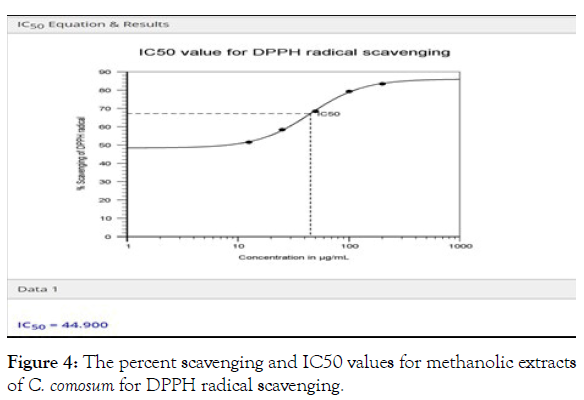
Figure 4: The percent scavenging and IC50 values for methanolic extracts of C. comosum for DPPH radical scavenging.
Hydrogen peroxide scavenging activity of the C. comosum extracts: Hydrogen peroxide scavenging activity of the plant extracts is based on the plant phenolics electron-donating ability to H2O2 and thus neutralizing it to water. The C. comosum extracts exhibited strong hydrogen scavenging activity of 50.25%. Among the free radicals, hydroxyl radical (OH•) causes the maximum cellular damage, the results in this study demonstrate a strong quenching activity of phenolic compounds.
The present study was a validation approach to verify the local traditional use of C. comosum used for toothaches and gum sores, as an anti-inflammatory, as an astringent and a stimulant. Inflammation is a vital biological response of the immune system to injury and infection through releasing pro-inflammatory cytokines like interleukin (IL)-1b and TNF- tumor necrosis factor. Tannins the polymeric polyphenols are astringent and exhibit an effective anti-inflammatory effect [58] through inhibition of expression of the inflammatory mediators like cytokines, COX- 2 and iNOS- inducible nitric-oxide synthase. Saponins antiinflamatory effect is also exhibited by down regulation of COX-2 and lipopolysaccharide (LPS)-induced iNOS expression leading to nitric oxide (NO) inhibition [59]. Phenolic acids like gallic acid reduces inflammation by inhibiting TNF-ɑ [60]. Flavinoids like kaempferol down regulates pro-inflammatory cytokines by suppressing the translocation of cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) proteins [61], while flavonoids like quercetin exhibited their anti-infammatory mechanism by blocking cyclooxygenase and the lipooxygenase pathways, similar mechanism is followed by coumarins which also inhibit the lipoxygenase and the cyclooxygenase pathways of the arachidonic acid metabolism. Alkaloids potential anti-inflammatory potential is based on their ability to inhibit the synthesis of some proinflammatory cytokines. Previous work has demonstrated the antiinflammotory effect of C. comosum extracts [62].
Conclusion
The phytochemicals present in C. comosum with strong radical scavenging, antibiofilm, cytotoxic and antibacterial capacity proves that this species has a pharmaceutical potential. This study validates the traditional use of C. comosum for toothaches and gum sores.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
Authors are grateful to Herbarium Staff at Life science unit, Sultan Qaboos University for access to herbarium plant specimens for comparison.
REFERENCES
- Li B, Webster TJ. Bacteria antibiotic resistance: New challenges and opportunities for implant associated orthopedic infections. J Orthop Res. 2018;36:22–32.
- Stanley IJ, Kajumbula H, Bazira J, Kansiime C, Rwego IB. Multidrug resistance among Escherichia coli and Klebsiella pneumoniae carried in the gut of out-patients from pastoralist communities of Kasese district, Uganda. PLoS One.2018;13:e0200093.
- WHO Media Centre News Release WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017.
- WHO Media Centre News Release News report cause for urgent action to avert antimicrobial resistance crises. 2019.
- Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258.
- Allkin B. Useful Plants – Medicines: At Least 28,187 plant species are currently recorded as being of medicinal use. State of the World's Plants. London: Royal Botanic Gardens, Kew; 2017.
- Rahman K. Studies on free radicals, antioxidants, and cofactors. Clin Interv Aging. 2007;2:219-236.
- Huyut Z, Beydemir Ş, Gülçin I (2017) Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem Res Int. 2017:1-10.
- Gholamreza D, Abbas S, Ghahremani M, Ardestani SK, Abdollahi M. Antioxidant potential of various extracts from Ferula szovitsiana in relation to their phenolic content. Pharm Biol. 2007;45:691-699.
- Maisetta G, Batoni G, Caboni P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement Altern Med. 2019;19:82.
- Hassan HS, Sule MI, Musa AM, Musa KY, Abubakar MS. Anti-inflammatory activity of crude saponin extracts from five Nigerian medicinal plants. Afr J Tradit Complement Altern Med. 2011;9:250–255.
- Tagousop CN, Tamokou J, Kengne IC. Antimicrobial activities of saponins from Melanthera elliptica and their synergistic effects with antibiotics against pathogenic phenotypes. Chem Cent J. 2018;12:97.
- 13. Kemegne GA, Mkounga P, Essia Ngang JJ. Antimicrobial structure activity relationship of five anthraquinones of emodine type isolated from Vismia laurentii. BMC Microbiol. 2017;17:41.
- NCCLS (1993) Performance standards for antimicrobial disc suspectibility tests. Approved Standard NCCLS publication M2-A5, Villanova, PA, USA.
- Eloff J. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713.
- Ozturk S, Ercisli S. Chemical composition and in vitro antibacterial activity of Seseli libanotis. World J Microbiol Biotechnol. 2006;22:261–265.
- Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996-1006.
- Meyer BN, Ferrign RN, Putnam JE, Jacobson JE, Nicholas DE. Brine Shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31-34.
- Harborne JB. Textbook of phytochemical methods. A guide to modern techniques of plant analysis. (5th Edn), Chapman and Hall Ltd, London. 1998.
- Trease GE, Evans WC. Pharmacognosy. Saunders Publishers, London. 2002.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1999;299:152–178.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555-559.
- Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433-2440.
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003-1008.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341.
- Cheruth AJ, Al Naqbi KMA, El-Kaabi AAS, Odeh O, Kandhan K. In vitro antioxidant activities and screening of phytochemicals from methanolic and ethyl acetate extracts of Calligonum comosum L’Her. Orient Pharm Exp Med. 2016;16:209–215.
- Riadh H, Imen F, Abdelmajid Z, Fedhila S. Detection and extraction of anti-listerial compounds from Calligonum comosum, a medicinal plant from arid regions of Tunisia. Afr J Tradit Complement Altern Med. 2011;8:322-327.
- Nalubega R, Kabasa J, Olila D, Katerega J. Evaluation of antibacterial activity of selected ethnomedicinal plants for poultry in Masaka district, Uganda. Res J Pharm. 2011;5:18-21.
- Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int. 2014;2014:497606.
- Othman L, Sleiman A, Abdel-Massih RM. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front Microbiol. 2019;10:911.
- Suurbaar J, Mosobil R, Donkor AM. Antibacterial and antifungal activities and phytochemical profile of leaf extract from different extractants of Ricinus communis against selected pathogens. BMC Res Notes. 2017;10:660.
- Ja'afreh M, Khleifat K, Qaralleh H, Allimoun M. Antibacterial and antioxidant activities of Centeurea damascena methanolic extract. J Basic App Res Biomed. 2019;5:55-63.
- Epand RF, Savage PB, Epand RM. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds. Biochem Biophys Acta. 2007;1768:2500–2509.
- Xie Y, Yang W, Chen X, Ren L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Cur Med Chem. 2015;22:132-149.
- Aligiannis N, Kalpotzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem. 2001;40:4168–4170.
- Zgurskaya HI, Löpez CA, Gnanakaran S. Permeability Barrier of Gram-Negative cell envelopes and approaches to bypass it. ACS Infect Dis. 2015;1:512–522.
- Berger D, Rakhamimova A, Pollack A, Loewy Z. Oral Biofilms: Development, control, and analysis. High Throughput. 2018;7:24.
- Bouyahya A, Dakka N, Et-Touys A, Abrini J, Bakri Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac J Trop Med. 2017;10:729-743.
- Asfour HZ. Anti-quorum sensing natural compounds. J Microsc Ultrastruct. 2018;6:1–10.
- Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016 14: 576–588.
- Ziemichód A, Skotarczak B. QS – systems communication of gram-positive bacterial cells. Acta Biol. 2017;24:51–56.
- Slobodnikova L, Fialova SB, Rendeková K, Kováč J, Mučaji P. Antibiofilm activity of plant polyphenols. Molecules. 2016;21:1717.
- Rutherford ST, Bassler BL. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427.
- Badria F, Ameen M, Akl M. Evaluation of Cytotoxic Compounds from Calligonum comosum L. Growing in Egypt. Z Naturforsch C J Biosc. 2007;62:656-660.
- Ahmed H, Moawad A, Owis A, Abouzid S, Abdel-Rahman R. Phytochemical screening and evaluation of biological activity of Calligonum polygonoides L. subsp. comosum. J App Pharm Sci. 2015;5:022-026.
- Tonisi S, Okaiyeto K, Hoppe H, Mabinya L, Nwodo U. Chemical constituents, antioxidant and cytotoxicity properties of Leonotis leonurus used in the folklore management of neurological disorders in the Eastern Cape, South Africa. 3 Biotech. 2020;10:141.
- Habli Z, Toumieh G, Fatfat M, Rahal ON, Gali-Muhtasib H. Emerging cytotoxic alkaloids in the battle against cancer: Overview of Molecular Mechanisms. Molecules. 2017;22:250.
- John M. Natural compounds in cancer therapy. John Boik, Oregon Medical Press, Princeton, MN. Pharm Biol. 2001;40:1-79.
- Khelef Y, Chouikh A, Rebiai A, Neffar S, Chefrour A. Biochemical, quantitative and qualitative phenolic compounds, anti-free radical's activities of Calligonum comosum collected from different sites in the Algerian Desert. Biharean Biol. 2019;13:71-76.
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11–26.
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:217037.
- Badhani B, Sharma N, Kakkar R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5:27540-27557.
- Sarian M, Uddin Q, Mat So'Ad S, Alhassan A, Murugesu S. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. Antioxidant and antidiabetic effects of flavonoids a structure-activity relationship based study. Biomed Res Int. 2017;2017:8386065.
- Anand DAV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn Rev. 2016;10:84–89.
- Cheruth AJ, Naqbi KMA, El-Kaabi AAS, Odeh O, Kandhan K. In vitro antioxidant activities and screening of phytochemicals from methanolic and ethyl acetate extracts of Calligonum comosum L’Her. Orient Pharm Exp Med. 2016;16:209–215.
- Aryal S, Baniya M K, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants. 2019;8:96.
- Bisognin DA, Luz LV, Lencina KH, Santos CO, Sautter CK. Contents of total phenolics and flavonoids and antioxidant activity in leaves of Ilex paraguariensis. Pesqui Agropec Bras. 2019;54:e00856.
- Park M, Cho H, Jung H, Lee H, Hwang K. Antioxidant and anti-inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J Food Biochem. 2013;38: 259-270.
- Jang KJ, Kim HK, Han MH, Oh YN, Yoon HM, Chung YH. Anti-inflammatory effects of saponins derived from the roots of Platycodon grandiflorus in lipopolysaccharide‑stimulated BV2 microglial cells. Int J Mol Med. 2013;31:1357-66.
- Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A Review. Int J Mol Sci. 2018;19:1573.
- Yeon M, Lee M, Kim H, Yang Y, Woo H, Kwon H. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci Biotech Biochem. 2019;83:166-173.
- Kiani K, Rudzitis-Auth J, Scheuer C, Movahedin M, Sadati N, Ardakani H. Calligonum comosum (Escanbil) extract exerts anti-angiogenic, anti-proliferative and anti-inflammatory effects on endometriotic lesions. J. Ethnopharmacol. 2019;239:111918.
Citation: Sivakumar N, Sherwani N, Mahrouqi MAA (2021) Evaluation of Antioxidant, Antibiofilm, Cytotoxic and Antimicrobial Activities of Calligonum Comosum. J Plant Pathol Microbiol 12:573.
Copyright: © 2021 Sivakumar N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

