Indexed In
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 11, Issue 2
Evaluating Croton Macrostachyus: Honey, Nectar, and Pollen Antimicrobial Activities against Escherichia coli, Shigella boydii,Staphylococcus aureus, and Bacillus subtilis
Kasim Roba1* and Zufan Bedewi22Department of Biology, College of Natural and Computational Science, Hawassa University, Hawassa, Ethiopia
Received: 16-Feb-2022, Manuscript No. CMO-22-15630; Editor assigned: 21-Feb-2022, Pre QC No. CMO-22-15630(PQ); Reviewed: 07-Mar-2022, QC No. CMO-22-15630; Revised: 11-Mar-2022, Manuscript No. CMO-22-15630(R); Published: 18-Mar-2022, DOI: 10.35841/2327-5073-22.11.270
Abstract
Background: Ethiopia is one of the plant species-rich countries in the world and the centre of origin of many medicinal plants. Studying antimicrobial activities is vital to investigate plants resources for medicinal values and the study was conducted to identify and evaluate Croton macrostachyus honey’s antimicrobial sources from its nectar and pollen against mentioned bacteria.
Methods: Completely Randomised Design was used for the laboratory. After adjusting turbidity, consistent growth of bacterial culture was made using sterilized cotton. The extract of C. macrostachyus’ pollen 3.6 gm. was added to 12 ml of distilled water to prepare stock solutions as ppm stock solution and antimicrobial activities of pollen, nectar, and honey were tested against mentioned bacteria above. Data were inserted into Microsoft excel 2010 and imported to R software version 3.44. Multilevel analysis was used to see the interaction between bacteria species and each concentration of honey, nectar, and pollen of Croton and Anova was used to see the significance of these concentrations on bacteria species. A p-value of <0.05 was considered statistically significant.
Results: Results indicated that bacteria were more inhibited at 72 hours than 48 and 24 hours and the result identified honey’s antimicrobial source was due to pollen composition found in honey that only pollen had antimicrobial activities while nectar had no antimicrobial activities against both Gram-negative and Gram-positive bacteria mentioned above. Time has significant effects on tested bacteria (p=0.000) and treatments have significant effects on tested organisms (p=0.000). Honey inhibited the growth of more Gram-negative bacteria: Escherichia coli and Shigella boydii. Bacillus was mostly inhibited by crotons’ pollen than others.
Conclusion: Water extract of Crotons ‘pollen had antibacterial activities against all tested bacterial strains, but inhibited more Gram-positive bacteria; while Crotons’honey inhibited more Gram-negative bacteria than Gram-positive bacteria. Negative controls (sterilized water) and nectar did not show an inhibitory effect on tested bacteria, while positive control (Chloramphenicol) had antimicrobial activities. Further isolation and characterization of bioactive compounds from Croton macrostachyus pollen are useful to develop a novel botanical formulation for further applications.
Keywords
Antimicrobial sources; Crotons honey; Crotons nectar; Croton’s pollen; Evaluation; Identification
Introduction
C. macrostachyus is a species of the genus Croton. Euphorbiaceae family, commonly known as the spurge family and croton is regarded as a multipurpose tree playing an important role in primary healthcare [1]. Ethnobotanical studies on traditional medicinal plants are the means to increase the capacity of the pharmaceutical industries [2]. Alternative medicine is considered as a primary health care modality in resource-constrained health care settings [3]. Healing with medicinal plants is as old as mankind itself [4]. Pollens are a rich source of essential amino acids and are often considered as a complete food for human beings healthy [5]. According to my knowledge in this field, no data related to the biological activity of Crotons’ pollen grain, nectar, and honey has been in literature, and to fill this gap the researcher is interested to do this research. Each flower species has a unique pollen grain [6].
Pollen-based microcapsules such as hollow sporopollenin exine capsules have emerged as excellent drug delivery and microencapsulation vehicles [7]. Comparative study on the antibacterial activities of (pollen, and honey) has antibacterial efficacy against pathogenic Gram-positive and Gram-negative bacteria [8]. Honey contains appropriate antioxidants which are responsible for biological activity, defense, and increase red blood cells functions and pharmaceutical and biological scientists need to identify the floral species which give anti-microbial characteristics [9]. The use of honey as a traditional remedy for microbial infections dates back to ancient times [10]. The reason to study the antibacterial property of honey and pollen is to find safe and natural antibiotics; since several microorganisms have developed resistance to commonly prescribed antibiotics, there is a need to find alternatives [11].
Intoxications and infections caused by food-borne pathogens like Escherichia coli represent increasing public health problems; S. aureus is a Gram-positive, non-spore-forming spherical bacterium that belongs to the Staphylococcus genus that subdivided into 32 species and subspecies [12]. Shigella species are members of the family Enterobacteriaceae, classified based on biochemical and serological characteristics [13]. The Bacillus species constitute an interesting group of probiotic bacteria that received limited attention [14].
Methodology
Selected trees were caged by insects proved to avoid visiting insects to get more pollen for the experiment [15]. A pollen grain of C.macrostachyus was collected when it was warm, windy, and particularly when humidity was low and preferably during the middle of the day [16]. Four species of bacteria were selected for the experiment; two Gram-positive: Staphylococcus aureus and Bacillus subtilis and two-Gram negative: Escherichia coli and Shigella boydii and they were cultured. With the help of a sterile wire loop, the test bacteria were transferred into test tubes having a sterile nutrient broth and incubated at 37°C for 24 hours until the noticeable turbidity and density was equal to that of 0.5 McFarland standards by adding 0.5 ml of BaCl2 solution into 99.5 ml of H2SO4. After adjusting turbidity, a sterile cotton swab was dipped into suspension and streaked over the whole surface of the plate to make sure the consistent growth of bacterial culture [17].
For the extraction 10, gm. of collected pollen of croton was added to 250 ml of beakers that contains 100 ml of distilled water and shaken for 24 hours and filtered by whatman filter paper and then dried in the water bath. The extracts of Crotons’ pollen were measured by sensitive balance and then stock solutions and working concentrations were prepared for its honey and pollen. It was pure honey that was used for the experiment and no need of extracting honey and only working concentrations were prepared by three different concentrations based on the amount of the extract obtained from extractions of pollen. As well, nectar was used directly in all concentrations since no need for extraction nectar. To prepare a stock solution, 3.6 gm. of the extract of C. macrostachyus’ pollen was added to 12 ml of distilled water. It was calculated as ppm stock solution. Working concentrations of 100%, 70%, and 30% were prepared from stock.
Antimicrobial activities of C. macrostachyus’ nectar, pollen, and honey were tested against mentioned bacteria after they incubated. Disks were mixed into working concentrations of 100%, 70%, and 30% and then added to labeled bacteria species inoculated into Petri dish respectively and stayed for 10-15 minutes until introduced with media, inverted and then brought to incubator incubated at 37⁰C. The inhibition zone of bacteria was checked continuously for 24, 48, and 72 hours and measured at 48 and 72 hrs. The number of experiments was replicated thrice for all bacteria species and the results were inserted to computer Microsoft excel 2010 and imported to R software version 3.44. Multilevel analysis was used to see the interaction between bacteria species and each concentration of treatments and Anova was used to see the significance of these concentrations on bacteria species. A p-value of <0.05 was considered statistically significant.
Results
Positive control Chloramphenicol had the highest antimicrobial activities against; Bacillus subtilis and Staphylococcus aureus than Escherichia coli and Shigella boydii (Figure 1). Chloramphenicol shows more significant effects than Croton pollen 30% concentrations against mentioned bacteria.
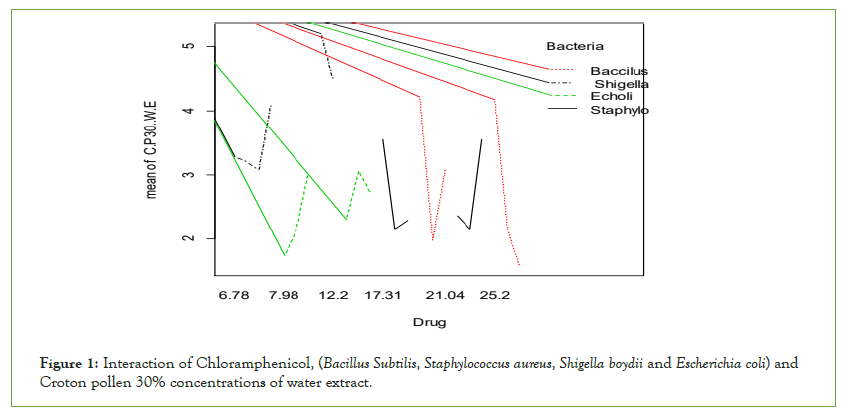
Figure 1: Interaction of Chloramphenicol, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and Croton pollen 30% concentrations of water extract.
Bacillus subtilis and Staphylococcus aureus were more inhibited by Chloramphenicol than Gram-negative bacteria: Escherichia coli and Shigella boydii (Figure 2). Chloramphenicol inhibited more Grampositive bacteria than Gram-negative bacteria. Chloramphenicol shows more significant effects than Croton pollen 70% concentrations against Gram-positive bacteria but Croton pollen 70% concentrations have more significant effects than standard drug against Gram-negative bacteria.
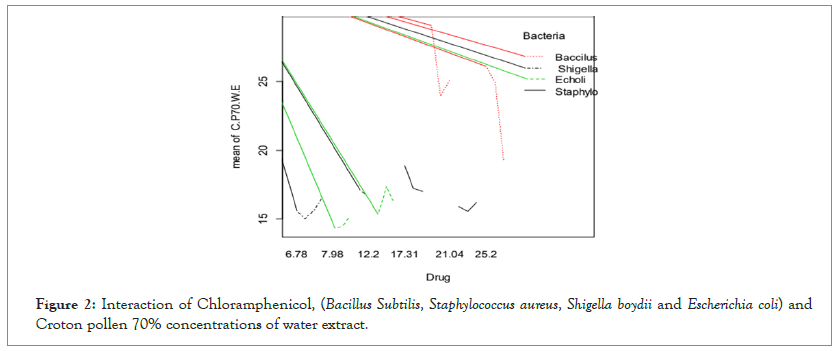
Figure 2: Interaction of Chloramphenicol, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and Croton pollen 70% concentrations of water extract.
Bacillus subtilis and Staphylococcus aureus were more inhibited by Chloramphenicol than Gram-negative bacteria: Escherichia coli and Shigella boydii (Figure 3). Chloramphenicol shows more significant effects than Croton pollen 100% concentrations against Grampositive bacteria but Croton pollen 100% concentrations inhibited more Gram-negative bacteria than standard drug.
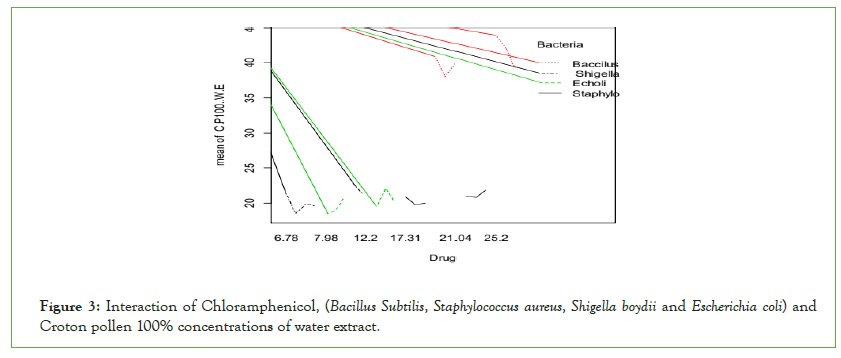
Figure 3: Interaction of Chloramphenicol, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and Croton pollen 100% concentrations of water extract.
A 30% concentration of Crotons’ honey shows more significant effects than Crotons’ pollen 30% water extracts (Figure 4). 30% concentrations of honey inhibited Gram-negative bacteria: Escherichia coli and Shigella boydii more than Gram-positive Bacillus: subtilis and Staphylococcus aureus.
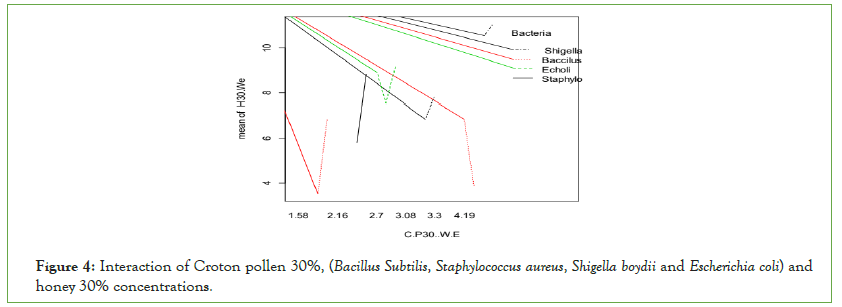
Figure 4: Interaction of Croton pollen 30%, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and honey 30% concentrations.
70% concentrations of honey show more significant effects than Croton pollen 70% water extracts against Gram-negative bacteria (Figure 5). Honey 70% concentrations inhibited more Gramnegative bacteria (Escherichia coli and Shigella boydii) more than Gram-positive (Bacillus subtilis and Staphylococcus aureus), but Crotons’ pollen inhibited more Gram-positive bacteria (Bacillus subtilis).
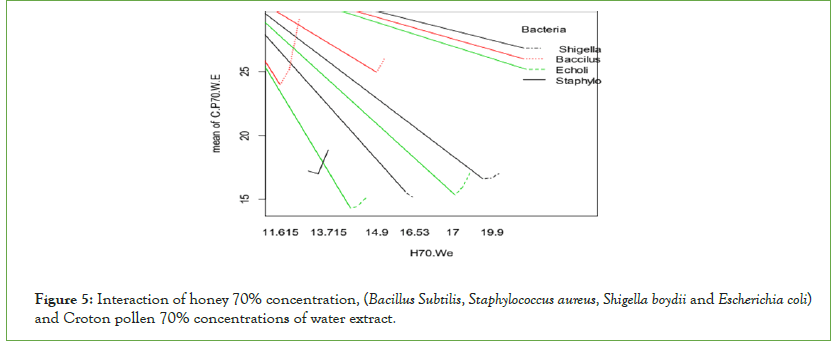
Figure 5: Interaction of honey 70% concentration, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and Croton pollen 70% concentrations of water extract.
Escherichia coli were more inhibited by honey 100% concentrations than Croton’s pollen 100% water extracts (Figure 6). Crotons’ pollen 100% water extract inhibited more Gram-positive bacteria (Bacillus subtilis) than all the others. Croton’s pollen shows more significant effects than honey against mentioned Gram-positive bacteria. Honey 100% concentrations of water extract inhibited more Gram-negative bacteria (Escherichia coli) than Gram-positive (Bacillus subtilis and Staphylococcus aureus).
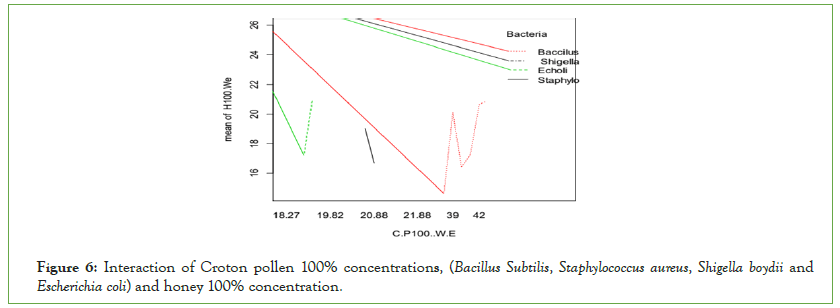
Figure 6: Interaction of Croton pollen 100% concentrations, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and honey 100% concentration.
Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) were inhibited more than Gram-negative (Escherichia coli and Shigella boydii) by crotons pollen water extract (Figure 7). Grampositive bacteria were more inhibited than Gram-negative bacteria by 100% concentration of croton pollen and highly inhibited at t2 than t1.
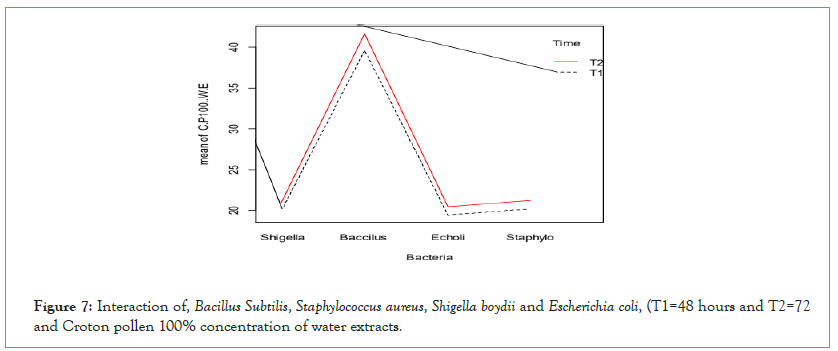
Figure 7: Interaction of, Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli, (T1=48 hours and T2=72 and Croton pollen 100% concentration of water extracts.
Bacillus subtilis and Staphylococcus aureus bacteria were inhibited more than Gram-negative (Escherichia coli and Shigella boydii) by crotons pollen water extract (Figure 8). Nectar did not inhibit either Gram-negative bacteria or Gram-positive bacteria as shown by the figure below.
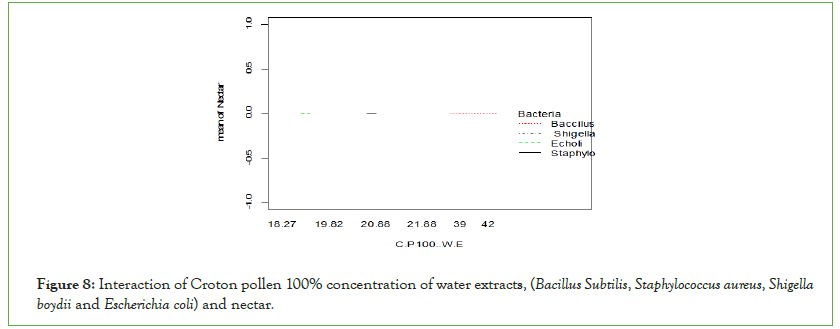
Figure 8: Interaction of Croton pollen 100% concentration of water extracts, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and nectar.
Bacillus subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) were inhibited by honey and it has highly significant effects on all species of bacteria on both Gram-negative and Gram-positive mentioned bacteria (Figure 9). Nectar inhibited neither Gramnegative nor Gram-positive bacteria.
Figure 9: Interaction of honey 100% concentration, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) and nectar.
Time of the experiment has highly significant effects on all species of bacteria on both Gram-negative and Gram-positive bacteria (Figure 10). Escherichia coli and Shigella boydii were mostly inhibited than Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) by honey 100% concentration as shown in the figure below.
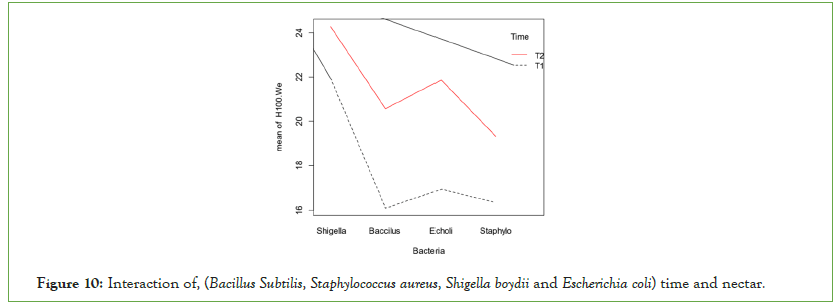
Figure 10: Interaction of, (Bacillus Subtilis, Staphylococcus aureus, Shigella boydii and Escherichia coli) time and nectar.
Discussion
The present study summarizes that Crotons macrostachyus’ pollen has high antimicrobial effects against both Gram-negative and Gram-positive bacteria however; highly inhibited Gram-positive bacteria (Bacillus Subtilis, Staphylococcus aureus) than Shigella boydii and Escherichia coli) at 30, 70, and 100 working concentrations. Honey inhibited more Gram-negative bacteria than Gram-positive bacteria and his result is in line with the reports of [18]. Gramnegative bacteria were more inhibited by 100% concentrations of honey than Gram-positive bacteria. This result is contrasted with the reports of [19]. Pollen 100% concentrations inhibited more Gram-positive bacteria at 72 hours. The antimicrobial activity of the extract is dependent on concentration and exposure periods.
Nectar did not inhibit either Gram-negative bacteria or Grampositive bacteria that might be due to its composition nature that it is carbohydrate bees used as energy sources [20-29]. This study confirmed that honey’ medicinal value is due to pollen composition found in it that this study tested antimicrobial effects of honey, nectar, and pollen of croton; honey and pollen has antimicrobial effects while nectar did not show any antimicrobial effects and the study confirmed that it is because of pollen that honey enable to have medicinal uses for many diseases. This study was limited to study antimicrobial activities of croton against these mentioned bacteria and studying many indigenous plants’ antimicrobial activities at against more test organisms by different concentration is important to find alternative solutions for resistant developing bacteria against prescribed drugs [30-38].
Conclusion
In this research program antimicrobial activities of crotons pollen, honey and nectar were tested against mentioned test organisms at concentrations of 30, 70, 100 ppm. Crotons’ pollen extract showed antibacterial activities against all tested bacterial strains at the concentrations of 30%, 70%, and 100% and the highest inhibition zone observed on 100% concentration, but in the case of the 48 hours and 70% concentrations of C. pollen solution, a lower inhibition zone is observed at the time of 72 hours than 48 hours against B. subtilis that might be due resistance of bacteria. Our study confirmed that this Gram-negative and Gram-positive bacterium inhibition zone differs based on treatment type, working concentration prepared from a stock solution of the experiment, and exposure period of the experiment. We conclude that crotons pollen inhibited more Gram-positive bacteria: B. subtilis and S. aureus than E. coli and S. boydii. Honey inhibited more Gramnegative bacteria than Gram-positive bacteria. Honey’ antimicrobial sources are from its pollen that no inhibitions were seen by the nectar that might be due to its chemical compositions since it contains carbohydrates which help bees as an energy source; while pollen inhibited both Gram-negative and Gram-positive bacteria. Pollens and honey might be an alternative natural food resource due to their preventative properties. This study is one of the first where an antimicrobial property of Crotons’ pollen, nectar, and honey against Gram-negative and Gram-positive bacteria species is quantified. A future study is necessary to pinpoint the functional components in a wide variety of local honey and honeybee forages and test their biological activities to find alternatives for diseases developing resistance against prescribed drugs these days that may be important solutions.
Acknowledgments
First and foremost, I am grateful to acknowledge my advisor Dr. Zufan Bedewi, I am very much thankful to Dr. Beyene Dobo, Mr. Mosisa Daba, and Tigist for their support during laboratory experimental work.; I need to acknowledge Dr. Abiot Deddefo who was encouraging me on everything for my success and Dr. Feto Esmo Beriso general director of Oromia research institute for his help in teaching me R software that helped me to analyze my thesis data. I need to concede my wife Dr. Zemzem Ahmed tendered her inspiration while I was writing the paper. I need to confess researcher Osho Tibesso from the USA for his fatherly advice when I was joining Hawassa University to learn my master of science, Dr. Musa Jarso director of Holeta agricultural research institute and finally I need to acknowledge Oromia agricultural research institute for financial support for thesis work in all aspects.
Ethics Approval and Consent Participate
The purpose of the study was explained to Dr. Zufan Bedewi the head of the department of natural and computational science at Hawassa University, Ethiopia and she agreed to test antimicrobial activities and identification of honey reasons for medicinal value from its pollen and nectar against mentioned Gram-negative and Gram-positive bacteria.
Funding
The authors would like to express their deepest gratitude to the Oromia agricultural research institute as financial support with which to conduct this research.
Availability of Data and Materials
The author declares that all other data supporting the findings of this study are available within the article and its supplementary information files.
Authors’ Contributions
Mr. Kasim Roba did all laboratory work and inserted data to Microsoft Excel and imported it to R software and analyzed and interpreted and wrote this paper for publication and did all necessary things. All authors have read and approved the final manuscript.
REFERENCES
- Obey JK, Von Wright A, Orjala J, Kauhanen J, Tikkanen-Kaukanen C. Antimicrobial activity of Croton macrostachyus stem bark extracts against several human pathogenic bacteria. J Pathogens. 2016.
[Crossref] [Google Scholar] [Indexed]
- Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomedicine. 2017;13(1):1-29.
[Crossref] [Google Scholar] [Indexed]
- Karunamoorthi K, Jegajeevanram K, Vijayalakshmi J, Mengistie E. Traditional medicinal plants: a source of phytotherapeutic modality in resource-constrained health care settings. J Evid Based Complementary Altern Med. 2013;18(1):67-74. [Crossref] [Google Scholar] [Indexed]
- Petrovska BB. Historical review of medicinal plants’ usage. Pharmacognosy reviews. 2012;6(11):1.
[Crossref] [Google Scholar] [Indexed]
- Al-Yousef HM, Amina M, Alqahtani AS, Alqahtani MS, Malik A, Hatshan MR, et al. Pollen bee aqueous extract-based synthesis of silver nanoparticles and evaluation of their anti-cancer and anti-bacterial activities. Processes. 2020;8(5):524.
- Petersen S, Bryant V. The study of pollen and its role in the honey market. American Bee Journal. 2011;151(6):591.
- Fan T, Park JH, Pham QA, Tan EL, Mundargi R, Potroz MG, et al. Extraction of cage-like sporopollenin exine capsules from dandelion pollen grains. Scientific reports. 2018; 8(1):1-1.
[Crossref] [Google Scholar] [Indexed]
- Comparative Study on the Antibacterial Activities of Bee Product (Propolis, Pollen, Bee Wax and Honey) Int J Pharm Boil Sci.2018.
- Meo SA, Al-Asiri SA, Mahesar AL, Ansari MJ. Role of honey in modern medicine. Saudi J Bio Sci. 2017;24(5):975-978.
[Crossref] [Google Scholar] [Indexed]
- Boukraâ L, Abdellah F, Ait-Abderrahim L. Antimicrobial properties of bee broducts and medicinal plants antimicrobial properties of bee products and medicinal plants. J Sci Educ Technol. 2013:960-970.
[Crossref] [Google Scholar] [Indexed]
- Johnston M, McBride M, Dahiya D, Owusu-Apenten R, Nigam PS. Antibacterial activity of Manuka honey and its components: An overview. AIMS microbiology. 2018;4(4):655.
[Crossref] [Google Scholar] [Indexed]
- Loir L. Staphylococcus aureus. 2012;1-9.
- Temu MM, Kaatano GM, Miyaye ND, Buhalata SN, Shushu ML, Kishamawe C, et al. Dysenteriae isolated from stool specimens of patients with bloody diarrhoea in Mwansa, Tanzania. Tanzan J Health Res. 2007;9(3):186-189.
[Crossref] [Google Scholar] [Indexed]
- Ritter AC, Paula A, Correa F, Veras FF, Brandelli A. Characterization of Bacillus subtilis available as probiotics. J Microbiol Res. 2018;8(2):23-32.
- Roba K, Bedewi Z. Evaluating Croton macrostachyus: honey, nectar, and pollen antimicrobial activities against Escherichia coli, Shigella boydii, Staphylococcus aureus, and Bacillus subtilis. 2022.
- Roba K, Bedewi Z. Evaluating Croton macrostachyus: honey, nectar, and pollen antimicrobial activities against Escherichia coli, Shigella boydii, Staphylococcus aureus, and Bacillus subtilis.
- Gebreyohannes G, Nyerere A, Bii C, Berhe Sbhatu D. Determination of antimicrobial activity of extracts of indigenous wild mushrooms against pathogenic organisms. Evid Based Complementary Altern.2019.
[Crossref] [Google Scholar] [Indexed]
- Mohapatra DP, Thakur V, Brar SK. Antibacterial efficacy of raw and processed honey. Biotechnology research international.2011.
[Crossref] [Google Scholar] [Indexed]
- Almasaudi SB, Al-Nahari AA, El Sayed M, Barbour E, Al Muhayawi SM, Al-Jaouni S, et al. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J Bio Sci. 2017;24(6):1255-61.
[Crossref] [Google Scholar] [Indexed]
- Tefera BN, Kim YD. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomedicine. 2019;15(1):1-21.
[Crossref] [Google Scholar] [Indexed]
- Meresa A, Ashebir R, Gemechu W, Teka F. Ethnomedicinal uses, phytochemistry and anti-malarial effect of Croton Ethnomedicinal uses, phytochemistry and anti-malarial effect of Croton macrostachyus (Bisana): A review. Ethno Med uses, Phytochem anti-malarial Eff Crot Ethno Med uses, Phytochem anti- malarial Eff Crot macrostachyus (Bisana) A Rev. 2019.
- Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants: Past history and future perspective. Journal of herbmed pharmacology. 2018;7(1).
- Kieliszek M, Piwowarek K, Kot AM, Błażejak S, Chlebowska-Śmigiel A, Wolska I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci Technol. 2018;71:170-180.
- Nascimento AM, Luz Jr GE. Bee pollen properties: uses and potential pharmacological applications-a review. J Anal Pharm Res. 2018;7(5):513-15.
- Dezmirean DS, Gherman B, Zacharias I. Antimicrobial activity of bee pollen ethanolic and methanolic extracts on Staphylococcus aureus bacterial strain. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj‐Napoca. Anim Sci Biotechnol. 2015;72(1):1-7.
- Atwe SU, Ma Y, Gill HS. Pollen grains for oral vaccination. J Control Release. 2014;194:45-52.
[Crossref] [Google Scholar] [Indexed]
- Komosinska-Vassev K, Olczyk P, Kaźmierczak J, Mencner L, Olczyk K. Bee pollen: chemical composition and therapeutic application. Evid Based Complementary Altern.2015.
[Crossref] [Google Scholar] [Indexed]
- Kocot J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid Med Cell Longev.2018.
[Crossref] [Google Scholar] [Indexed]
- Wasihun AG, Kasa BG. Evaluation of antibacterial activity of honey against multidrug resistant bacteria in Ayder Referral and Teaching Hospital, Northern Ethiopia. SpringerPlus. 2016;5(1):1-8.
[Crossref] [Google Scholar] [Indexed]
- Grecka K, Kuś PM, Worobo RW, Szweda P. Study of the anti-staphylococcal potential of honeys produced in Northern Poland. Molecules. 2018;23(2):260.
- Mama M, Teshome T, Detamo J. Antibacterial activity of honey against methicillin-resistant Staphylococcus aureus: A laboratory-based experimental study. Int J Microbiol.2019.
[Crossref] [Google Scholar] [Indexed]
- Anthimidou E, Mossialos D. Antibacterial activity of Greek and Cypriot honeys against Staphylococcus aureus and Pseudomonas aeruginosa in comparison to manuka honey. J Med Food. 2013;16(1):42-47.
[Crossref] [Google Scholar] [Indexed]
- Roba K, Masresha G, Jemberie W, Nagappan R, Campus M. Journal of Coastal Life Medicine. J Coast Life Med. 2015;3(11):895–900.
- Denisow B, Denisow‐Pietrzyk M. Biological and therapeutic properties of bee pollen: a review. J Sci Food Agric. 2016;96(13):4303-4309.
[Crossref] [Google Scholar] [Indexed]
- Okechukwu EC, Amuta EU, Gberikon GM, Njoku M. Occurrence and Seasonal Variation of Escherichia coli Isolated from Unpasteurised Raw Milk and its Products Sold in Abuja Metropolis, Nigeria. 2019;8(05):1979-1988.
- Bourne JA, Chong WL, Gordon DM. Genetic structure, antimicrobial resistance and frequency of human associated Escherichia coli sequence types among faecal isolates from healthy dogs and cats living in Canberra, Australia. PLoS One. 2019;14(3):e0212867.
[Crossref] [Google Scholar] [Indexed]
- Adjaloo MK, Ankomah AA, Yeboah-Gyan K, Dzomeku BM. Nectar production dynamics in two melliferous plant species. Genetics and Plant Physiology. 2015;5(2):145-161.
- Etikan I, Sulaiman Abubakar Musa RS. Comparison of Convenience Sampling and Purposive Sampling Comparison of Convenience Sampling and Purposive Sampling.2017.
Citation: Roba K, Bedewi Z (2022) Evaluating Croton Macrostachyus: Honey, Nectar, and Pollen Antimicrobial Activities Against Escherichia coli, Shigella boydii, Staphylococcus aureus, and Bacillus subtilis. Clin Microbiol. 11:270.
Copyright: © 2021 Roba K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
