Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2016) Volume 7, Issue 4
Evaluating Physicochemical and Rheological Characteristics and Microbial Community Dynamics during the Natural Fermentation of Cassava Starch
Abstract
The traditional fermentation of cassava starch was investigated by a polyphasic approach combining (i) microbial community identification using conventional and molecular techniques, (ii) analyses of organic acids, volatile compounds, fermentation products and spin-lattice relation time and (iii) evaluation of technological properties, such as pasting properties, water absorption and water solubility indexes. Cassava fermentation microbiota was dominated by bacteria and yeasts genera. Bacteria genera include Lactobacillus, Leuconostoc, Lactococcus and Enterococcus. Lactobacillus was the prevalent genera responsible for the acidification of cassava fermentation by the production of organic acids and also aromatic compounds. Yeast community was dynamically adjusted through the cassava fermentation Pichia kudriavzevii and Issatchenkia orientalis were succeeded by Geotrichum candidum, Clavispora lusitaniae and Rhodotorula mucilaginosa. Candida rugosa, C. pararugosa, C. akabenensis, Cryptococcus albidus, Neurospora crassa and N. intermedia were found exclusively in sour cassava. The acidification of sour cassava was due to the production of acetic, lactic and succinic acids. Volatile compounds, including aliphatic and aromatic hydrocarbons, esters and terpenes contribute to the aroma and correspond to 23% of compounds found after fermentation and sun-drying treatment. The acidification and fermentation process reduced the peak viscosity, paste viscosity, breakdown viscosity and set back viscosity in cassava starch. Solid-state NMR relaxometry measures were associated to the expansion ability and indicated that the fermented and sun-dried products were more inclined to expansion. Loaf expansion ability and pasting temperatures were increased in sour cassava (fermented and sun-dried). The results showed here should be useful to standardize the manufacturing of cassava starch in Brazil, providing homogeneous and high quality products.
Keywords: DNA sequencing; Headspace volatile analysis; Organic acids; Spin-lattice relaxation; Loaf expansion
Introduction
Cassava (Manihot esculenta Crantz), a wood scrub belonging to the Euphorbiaceae family (spurge), is considered an important source of food and dietary calories for large populations of tropical countries in Asia, Africa and Latin America [1]. Originally from Latin America, it is a shrubby plant, made up of a shoot and an underground portion. It is known as “tapioca” in Asian countries, as “mandioca”, “aipim”, “castelinha” and “macaxeira” in Brazil, as “yuca” in Spanish-speaking countries of Latin America, and as “manioc” in French-speaking countries in Africa [2]. In Brazil, cassava production is currently increasing and it is estimated that in the next 30 years the projected production should reach 106 million tons. Cassava starch has various applications in industry, such as in food, paper, and adhesives; however, only a small portion of starch is used in its native state, and mostly it is modified by chemical or physics agents. Cassava fermented and sundried starch or sour (sun-dried) cassava starch (“polvilho azedo” in Brazil or “almidón agrio” in Colombia) is used for the production of special types of gluten-free breads and biscuits that are very popular in some countries of South America [3]. Currently, a large number of individuals in many Western societies adopt a gluten-free diet, avoiding wheat, rye and barley. Although there are at least three clinical glutenrelated conditions recognized-celiac disease, wheat allergy and nonceliac gluten sensitivity-most people change to a gluten-free diet even without any well-defined-medical reason [4]. The market for glutenfree products has been increasing speedily [5], opening opportunities for the development of new technologies using gluten-free ingredients as alternative for traditional manufacturing bakery products [6]. Sour (sun-dried) cassava starch can be used as an adjuvant for bread making or as the main ingredient for gluten-free breads.
Cassava starch fermentation is a common process conducted in small rural cassava starch factories to improve the textural qualities of the starch [7-9]. Natural fermentation is predominantly associated with the fermentative activities of bacteria and yeasts [10]. Cassava fermentation is carried out in tanks for a period of about 30-40 days. The wet acid starch is then sun-dried for a period, depending on the season, generating a non-uniform product. During fermentation considerable amounts of cyanide are removed and antimicrobial compounds are produced including bacteriocins, organic acids, hydrogen peroxide, and other active, low molecular weight metabolites [11].
Frequent variations occur in the quality of the final product from different producers and even from the same producer using raw material from the same origin. This occurs because there is no control parameters applied in the process. During manufacturing, sour cassava starch can be contaminated by unknown microorganisms, which may change the technological characteristics of the product [12]. The physicochemical properties determine the eating and cooking quality of sour cassava starch. A comprehensive analysis was performed using a polyphasic approach and multiple techniques that were simultaneously applied to describe dynamic changes in the physical, chemical, microbiological and rheological characteristics occurring during the natural fermentation of Brazilian cassava starch in the manufacturing of sour cassava starch. The dynamics of the microbial community involved in spontaneous fermentation was evaluated by conducting enumerations in specific culture media followed by the molecular identification of isolated microorganisms such as random amplified polymorphic DNA (RAPD) and partial DNA sequencing. Physical and chemical characteristics, including sugar, organic acid, volatile compound contents, fermentation product contents and the spin-lattice relaxation time were determined by high-performance liquid chromatography (HPLC), head space-solid phase micro-extraction coupled to a chromatograph quadrupole mass spectrometer (HS-SPME/GC-qMS) and low field NMR spectrometry (LF-NMR), respectively. Rheological properties, rapid visco analyzer (RVA) average parameters, water absorption and water solubility indexes were also determined. In view of the need for standardization and improvement of the Brazilian manufacturing process, knowledge and characterization of the manufacturing process and the final product can serve as a basis for planning and obtaining products with adequate physicochemical and pasting characteristics superior to the ones currently marketed in the country.
Materials and Methods
Sour cassava starch (fermented and sun-dried)
Cassava (Manihot esculenta Crantz) cultivated in the Paraná state, Southern Brazil, was processed into sour (sun-dried) cassava starch in accordance to the traditional small-scale processing of Northeastern Brazil. The cassava was washed, peeled and washed a second time for dirt removal. Subsequently, they were grated and the obtained mass was washed again and strained in fine mesh fabric until the water leaching from the cassava was transparent. The wet-extracted starch was sundried for 12h.
The sun-dried extracted starch was transferred to a sour cassava production plant located in the Bahia state, Northeastern Brazil. Natural fermentations were run from June to August 2013. The raw material was put in 1.63 m height polyethylene open tanks with 5,000 liters capacity and covered with a layer of running water of approximately 20 cm and allowed to naturally ferment for 30 days at ambient temperature (average temperature of 18°C). After fermentation, the cassava starch was laid on high density polyethylene (HDPE) black canvas and sun-dried for 12h. Cassava starch from several batches produced during June-August months in 2013 was sampled at (t=1) and (t=30) days alongside the fermentation process and from the final product, the sour (sun-dried) cassava starch, for further analyses.
Microorganism enumeration
Microorganism enumeration was carried out at the beginning of fermentation (t=1), after 30 days of fermentation (t=30) and in the final product, sour (sun-dried) cassava starch. For lactic acid bacteria enumeration and identification, M17 agar plates (HIMEDIA, Mumbai, Índia) were incubated at 30°C for 48 h, lactobacilli MRS agar plates (BD, Le Pont de Claix, France) at 37°C for 4 days under anaerobic jars using a Gaspak anaerobic generator (Becton Dickinson and Company, Franklin Lakes, USA) and Azide Blood Agar Base (Becton Dickinson and Company, Le Pont de Claix, France) at 37°C for 4 days. For yeast enumeration and isolation, malt extract agar (Himedia, Mumbai, India) and YPD 2% (2% peptone, 1% yeast extract and 2% glucose) were used, incubated at 25°C for 7 days. Colonies with distinct morphologies were selected randomly and the cultures were stored in the corresponding isolation broth described above containing 20% glycerol, until further analyses.
Microorganism molecular identification
DNA templates from bacterial and yeast colonies were obtained as described by Sambrook and Russell (2001) [13] and quantified using the Qubit dsDNA HS kit (Invitrogen™, Grand Island, New York, USA). Partial amplification of the 16S rDNA, using the primer pair 27F (5´-AGAGTTTGATCCTGGCTCAG-3´) and 1512R (5´-ACGGCTACCTTGTTACGACT-3´) [14], was performed using Taq DNA polymerase (Invitrogen™, Grand Island, New York, USA) in a DNA thermocycler (MyCycler™, Bio-rad, Hercules, CA, USA).
The amplicons were digested with restriction enzymes ApaI and XhoI (Promega, Madison, USA) and DdeI (Fermentas, São Paulo, Brazil), following the manufacturer’s instructions. The digestion profile was resolved by electrophoresis on 1.2% agarose gels, and electrophoresis was carried out in 1X TAE buffer for 70 min at 100 V and 200 mA. Gels were stained with GelRed (Biotium Inc., Hayward, CA, USA) diluted at 1:10,000 and documented under a MiniBis Pro UV light using the GelCapture software (DNR Bio-Imaging Systems, Hamisha, Israel). Representative profiles of each species observed in the amplified ribosomal DNA restriction analysis were selected for sequencing.
Yeast DNA templates were analyzed by RAPD-PCR using the primer EI1 (5’-CTG GCT TGG TGT ATG -3’) [15]. The band profiles were resolved on 2% gels subsequently stained with GelRed (Biotium Inc., Hayward, CA) diluted at 1:10,000 and documented as described above.
The representative profiles were selected for sequencing and amplified with the primer pairs ITS1 (5´-TCCGTAGGTGAACCTGCGG-3´) and ITS4 (5´-TCCGTAGGTGAACCTGCGG-3´) [16]. PCR products from bacteria and yeast were purified by PCR DNA and the use of the Gel Band Purification kit (GE Healthcare Life Science Inc., Little Chalfont, Buckinghamshire, UK). The sequencing analysis of partial 16S rDNA gene and ITS region was accomplished with a 3130 sequencer (Applied Biosystems Inc., Tokyo, Japan) and subsequently used for identification of the bacteria and yeast, respectively. The identities of the sequences were determined by using the BLASTn algorithm at the GenBank database.
Physicochemical characterization of cassava starch
Moisture content evaluation: The moisture content of the analyzed starch was determined according to the protocol from the American Association of Cereal Chemists (AACC) International Approved Method 44-15.02.
Amylose and amylopectin content: The apparent amylose content was estimated in quadruplicate by iodine-based colorimetry according to the method number 66470 from the International Organization for Standardization. The absorbance was measured on a DU-730 spectrophotometer (Beckman Coulter, Fullerton, USA) at 620nm. The percentage content of amylopectin was estimated by difference (amylopectin percentage content = 100 - apparent amylose percentage content).
Determination of titratable acidity and pH: Titratable acidity and pH were determined according to the 016/IV e 017/IV methods, respectively, from the Adolfo Lutz Institute [17] using a calibrated potentiometer.
Organic acid content determination: The organic acid content of starch was determined as described by Leite et al. [18]. Briefly, 25 mL of H2SO4 45 mmol.L-1 were added to 5 g (dry weight) of starch and homogenized for 1 h on a rotatory shaker at 250 rpm. The supernatant resultant from of a centrifugation at 6,000 g was filtered through 0.45 μm filters (Millipore Corp, Billerica, USA). For carbohydrate analysis, the filtered samples were injected (50 μL) into an HPLC system (Shimadzu Corp., Tokyo, Japan) equipped with an HPX-87H Aminex fermentation monitoring column (150 × 7.8-mm i.d., Bio-Rad Laboratories Inc, Hercules, USA), hydrogen form, 9 μm particle size, 8% cross linkage, pH range 1-3, protected by a cation H+ Micro-Guard cartridge (30 × 4.6-mm i.d.; Bio-Rad Laboratories Inc, Hercules, USA). The mobile phase (isocratic) was 3 mM H2SO4 at a flow rate of 0.7 mL.min-1 at 65°C. Organic acids (lactic, acetic, citric, succinic, butyric and propionic) were quantified by using a diode array detector model SPD-M20A (Shimadzu Corp, Tokyo, Japan), monitoring the absorbance at 210 nm [18]. Chromatograms from the HPLC and compound quantifications were obtained using the LC Solution software (Shimadzu Corp., Tokyo, Japan). Three independent samples each were collected at (t=1) and (t=30) days alongside the fermentation process. Standard curves based on peak area were calculated for the individual concentrations of the determined organic acids, covering a broad range of concentrations, by comparison with standard solutions. Standards (Supelco Analytical, Sigma, St Louis, MO, USA) were prepared in deionized water filtered through 0.45-μm filters (Millipore Corp.). The analyses were performed in triplicate.
Determination of volatile compounds: The volatile compounds present in the headspace samples were extracted using solid phase micro-extraction (SPME) for 30 min at room temperature using a three-phase fiber 50/30 μm DVB/CAR/PDMS into an Agilent 6890 gas chromatograph coupled to an Agilent 5973 N mass selective detector (GC/MS) and a DB-5 (30 m × 0.25 mm × 0.25 mm, J & W Scientific, Folsom, CA). Helium was used as carrier gas at a flow rate of 1.0 mL/ min. The oven temperature was programmed from 50 to 250°C at 5°C/ minute. Injector temperature was kept at 260°C. The mass detector was operated in an electronic ionization mode (70 eV) at 3.15 scan/s with a mass range from 30 μ to 550 μ. Transfer line was kept at 250°C, ion source at 230°C, and an analyzer at 150°C. The compounds were identified according to the Wiley mass spectrometer library (Enhanced data analysis software, Agilent, New York, USA) and the retention index data found in the literature [19]. Samples were analyzed in duplicate.
Determination of the spin-lattice relation time: The analyses were performed using a low field NMR spectrometer (LF-NMR) MARAN Ultra-23 (Oxford Instruments, Tokyo, Japan), operating at 23.4 MHz (for hydrogen) and equipped with an 18 mm variable temperature probe. Hydrogen spin-lattice relaxation times were determined directly by the traditional inversion-recovery pulse sequence (recycle delay - 180º - τ – 90º- acquisition). The 90º pulse, 4.6 μs, was calibrated automatically by the instrument software. The amplitude of the FID was sampled for twenty τ data point, ranging from 0.1 to 5,000 ms, with 4 scans each and 5s of recycle delay. The relaxation values (means of triplicate analysis) and relative intensities were obtained by fitting the exponential data with the aid of the WINFIT 2.4.0.0 software supplied from resonance. Distributed exponential fittings as plots of relaxation amplitude versus relaxation time were performed using the WINDXP software [20].
Technological properties of cassava starch and sour (fermented and sun-dried) cassava starch
Pasting properties: The pasting properties of starch were determined using a Rapid Visco Analyzer 4500 viscometer (Perten Instruments, Hägersten, Sweden) according to method No. 162 of the International Association for Cereal Science and Technology. Starch samples weighing 2.5 g were added to 25 mL distilled water (corrected volume considering 14% moisture content in flour) and the pasting temperature, peak viscosity, peak time, breakdown, minimum viscosity, final viscosity at 50°C and setback were evaluated. The analyses were performed in triplicate and mean values and standard deviations were calculated.
Water absorption index (WAI) and water solubility index (WSI) determination: The WAI and WSI analyses were performed in triplicate, following the method proposed by Anderson, Conway, Pfeifer, and Griffin [21].
Expansion power: The expansion power was determined as described by other researches with modifications [12]. Manual homogenization of 24 g of starch was carried out in 20 g of boiling water. Four portions of this dough, 7g each, were placed in aluminum containers (3.7 cm diameter and 6.8 cm height) and pre-heated in an electric oven at 150°C for 18 min. The expanded cassava starch samples were weighted and covered with Parafilm M®. The apparent volume was determined according to the AACC International Approved Method 10-05.01. The measurements were conducted in quadruplicate and the expansion was determined as the specific volume evaluated by the displacement method of millet seeds and expressed in mL.g1 [22]. The specific volumes were used to classify the sour (fermented and sundried) cassava starch into small (<5.0 mL.g-1), medium (5.0 ≤ x ≤ 10.0 mL.g-1) and large (> 10.0 mL.g-1) [12].
Statistical analyses
Data were expressed as means ± SD and the value significances were analyzed by the GraphPad Prism v.5 software package (San Diego, CA, EUA). Differences between means were compared by a one-way analysis of variance (ANOVA) with a Bonferroni post hoc test. A statistical significance level of 99.9% (p<0.001) was considered for all analyses.
Results and Discussion
Microbiological analyses of the spontaneous fermentation of cassava starch
At the initial (t =1) and final time points of the cassava starch fermentation (t =30), the presumptive count of lactobacilli in MRS agar showed values around 8 log units CFU.g-1, whereas the final product, after sun-drying treatment, showed a reduction of 1.2 log units (Table 1). Previous studies have shown that the Lactobacillus genus is prevalent among the other genera belonging to lactic acid bacteria, being found throughout the manufacturing process of sour (sun-dried) cassava starch [23].
| Sample (fermentation time) |
Bacteria (Log CFU.g-1)* | Yeast (Log CFU.g-1)* | |||
|---|---|---|---|---|---|
| M17 | MRS | Azide blood | YPD 2% | Malt extract | |
| Cassava starch(t=1) (1st day) |
7.6 ± 0.02b | 8.0 ± 0.03a | 7.7 ± 0.04a | 7.9 ± 0.02a | 7.8 ± 0.01a |
| Sour cassava starch (t=30) (30th day) | 8.0 ± 0.02a | 8.2 ± 0a | 7.8 ±0.04a | 6.0 ± 0.02c | 6.2 ± 0.02b |
| Sour (sun-dried) cassava starch | < 2c | 6.8 ± 0.02b | 6.3 ±0.04b | 7.5 ± 0.01b | 7.6 ± 0.06a |
| *Values are expressed as mean ± standard deviation. Bacteria were cultured on M17, MRS and azide blood agar plates. Yeast was cultured on YPD 2% and malt extract agar plates. Samples were harvested at the 1st and 30th days of cassava fermentation and after the sun-dried treatment. (a-c) Means with different superscript letters within a column are significantly different (p < 0.001), according to the Bonferroni post hoc test. |
|||||
Table 1: Microbial enumeration (log CFU.g-1) on selective media of lactic acid bacteria and yeast during cassava starch fermentation.
Regarding the presumptive count for streptococci on azide agar, values for cassava starch at the beginning and end of fermentation were also higher than in the sour (sun-dried) cassava starch, dropping from 7.8 (t =30) to 6.3 log units CFU.g-1 (Table 1). On the other hand, no Lactococcus sp. was detected in sour (fermented and sun-dried) cassava starch, probably because the number of microorganisms was below the limit of detection of the technique (102 CFU.g-1 log).
To identify bacteria, all microorganisms (n=131) isolated from the different batches and media (MRS, M17 and azide blood) were characterized by ARDRA (amplified ribosomal DNA restriction analysis). Eighteen distinct profiles were found and the isolates were identified by partial sequencing of the 16S rDNA. Lactobacillus sp. (42%), Lactobacillus plantarum (14.5%), Leuconostoc citreum (5.3%), Lactococcus sp. (12.2%), Enterococcus sp. (15.3%) and Bacillus sp. (10.7%) were identified based on homology identity (98-100%) searches at GenBank database.
Several studies have shown the involvement of LAB lactic acid bacteria in the spontaneous fermentation of sour cassava starch [23]. These bacteria contribute to the development of characteristic starch properties, such as taste, aroma, appearance, texture, shelf life and safety [10].
The involvement of various Lactobacillus species, mainly L. plantarum and L. fermentum, as the predominant species in sour (fermented and sun-dried) cassava starch manufacturing at two industrial plants in Southeastern Brazil has already been suggested by Lacerda et al. [10]. L. plantarum and other LAB are considered the prevalent microorganisms in the natural fermentation of cassava starch, responsible for the acidification of the product in the tanks during the fermentation process and the production of organic acids and aromatic compounds. Another group of LAB, the genus Leuconostoc has been identified since the beginning of the fermentation process (t =1). Leuconostoc sp has been usually isolated from fermented vegetables, including species that are capable of producing exopolysaccharides (EPS). This group has many applications in the food industry and pharmaceutical field [24].
Leuconostoc sp grow associatively with acid producing Lactococcus sp and can confer aroma and texture to sour (fermented) cassava starch. The associative growth between these two bacteria groups has been described as a synergistic functional relationship [24].
Bacteria belonging to the Bacillus genus were found only in samples collected at the end of the fermentation process (t=30). According to the European Food Safety Authority and ANVISA, RDC under number 263, 09/22/2005, Brazil’s National Health Surveillance Agency, the presence of the Bacillus genus, Gram-positive, ubiquitous, characterized by spore-forming ability and usually present in soil, can be considered as a contamination indicator of fermentation since those opportunistic bacteria can grow due to inadequate hygiene conditions during the production process, which reinforces the need to include the concepts of good manufacturing practices in flour mills.
The yeast count, for both media used in the initial time point (t=1) of the cassava starch fermentation showed higher values (about 2 log units) than at the end of the fermentation process (t =30) (Table 1). On the other hand, in the sour (sun-dried) cassava starch showed an increase in yeast counts, reaching values of around 7.5 log units CFU.g-1 (Table 1). The presence of yeast species predominantly in the advanced stages of the process suggests a higher acid tolerance of these microorganisms [25], which can be considered a technological advantage.
A total of 157 yeasts isolated and analyzed by RAPD were grouped into 19 distinct profiles. The sequencing of the internal transcribed spacer of ribosomal DNA (rDNA ITS) of the 19 representative yeast RAPD profiles, presented homology 98% - 100% to the sequences of the GenBank. Geotrichum candidum (10.8%) Pichia kudriavzevii (33.1%), Issatchenkia orientalis (3.2%), Clavispora lusitaniae (6.4%), Neurospora crassa (1.9%), Neurospora intermedia (1.9%), Rhodotorula mucilaginosa (2.5%), Cryptococcus albidus (8.3%), Candida akabenensis (8.3%), Candida pararugosa (6.4%), Candida rugosa (4.5%) and Geotrichum sp. (12.7%) were identified during both the sour cassava fermentation and sun-drying treatment.
Microbial succession was more evident in the yeast community. Species such as Pichia kudriavzevii and Issatchenkia orientalis are part of natural cassava microbiota and were found only at the beginning of fermentation. Geotrichum candidum, Clavispora lusitaniae and Rhodotorula mucilaginosa were detected during cassava fermentation. Candida rugosa, C. pararugosa, C. akabenensis, Cryptococcus albidus, Neurospora crassa and N. intermedia were found in the final product, the sour cassava (fermented and sun-dried) starch.
It was found that the most common yeast species in sour (fermented and sun-dried) cassava starch when evaluating samples from distinct starches [10]. They identified Galactomyces geotrichum and a species of Issatchenkia, both of which were present throughout the process. Both species occurred at about 5.0 log CFU g-1. The authors also commented that other species appeared only at certain times, especially in the initial phases of the process. However, none of the yeast isolated from the cassava fermentation were able to degrade starch.
In the present study, the identified yeast species have been shown to have high amylolytic activity, thus showing an ecological advantage in fermented sour and sun-dried cassava starch, since they can partially hydrolyze raw starch [26] to provide sugars such as glucose or maltose that can be used as an energy source by other microorganisms, as well as producing enzymes such as linamarase and polygalacturonase, and aldehydes and esters that impart a pleasant aroma to the final product [27].
Characterization and comparison of physical and chemical parameters of the fermentation musts
Moisture content: Cassava starch and sour (sun-dried) cassava starch presented significantly different mean moisture values (p < 0.001), 9.9% ± 0.02 to 12.9% ± 0.04, respectively.
According to the Brazilian Technical Regulation for starch products (ANVISA - RDC under number 263, 09/22/2005), samples of cassava starch and sour (sun-dried) cassava starch should not present significant differences regarding moisture content. The value recommended by Brazilian legislation, for both, is at most 18% w/w, in order to obtain good product preservation, stability, quality and appropriate composition of the final product. Although the 12-hour drying period was observed, the relative humidity in the area is 60%-90%, which may have hampered the drying of the final product. The moisture content of the products are within the limits recommended by the Brazilian legislation (ANVISA - RDC under number 263, 09/22/2005), similar to other commercial products available on the Brazilian market. Drying reduces moisture, volume and cyanide content of roots, thereby prolonging product shelf life. The higher moisture content would indicate that the process of drying the starch under the sun could not be completed within 12 h.
Apparent amylose and amylopectin content: No significant difference was observed between the apparent amylose and amylopectin content (p < 0.001) between the cassava starch and the sour cassava starch samples. Amylose content is an important parameter to study the changes that occur in starch pasting properties, which can affect their industrial applications [28]. Starch paste and thermal properties are negatively regulated by the amylose ratio produced in starchy vegetables [29]. The differences when comparing the results with other studies may be attributed to the use of starches from other sources, as well as the different methods used in the amylose analyses. A more careful and in-depth study demonstrated that amylose content can vary with cultivar conditions or crop planting time [3].
Determination of titratable acidity and pH of the flours during production: There was an increase in titratable acidity from 2.14 ± 0.01 mL of NaOH / 100g to 3.24 ± 0.24 mL NaOH / 100g and a reduction of pH from 4.8 ± 0.05 to 3.25 ± 0.03 for the initial timepoint (t=1d) and final (t=30d), respectively, while the final product, sour (sun-dried) cassava starch showed a titratable acidity of 5.25 ± 0.01 mL of NaOH / 100g and a pH of 3.63. According to the current legislation in Brazil (ANVISA – RDC under number 263, 09/22/2005), cassava starch should remain in the fermentation tank until the product reaches an acidity of about 5.0 mL NaOH / 100g of dry weight. However, in most starch flour in Brazil, the duration of the fermentation process is not controlled by the titratable acidity as a parameter to interrupt fermentation, being only the 30-day fermentation period observed. In the starch evaluated in the present study, there was no control of the endpoint of the fermentation process, with the sour cassava (fermented) starch submitted to sundrying treatment, even with an acidity higher than recommended by the Brazilian regulatory agency.
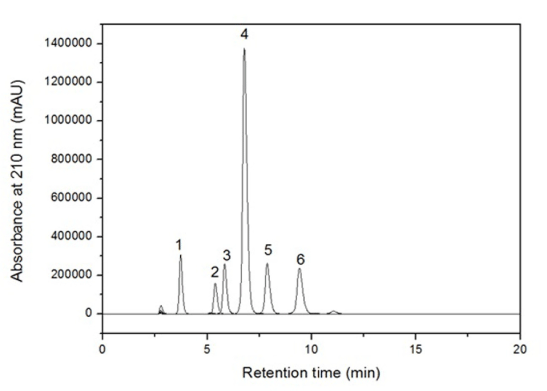
Characterization of organic acids in cassava starch and sour (fermented and sun-dried) cassava starch: Among the organic acids, only acetic, lactic and succinic acids were produced during the fermentation and sun-drying of cassava starch. All of them were detected by HPLC (Figure 1), excepting acetic acid, which was evaluated by HS-SPMS/GC-qMS. The lactic acid concentrations found in the cassava starch and sour (sun-dried) cassava samples were of 0.15 g.L-1 and 0.96 g L-1, respectively. Succinic acid was also detected in the sour (sun-dried) cassava starch samples at a concentration of 0.084 g.L-1. Lactic and succinic acid were detected by HPLC. Acetic acid comprised approximately 40% of the volatile compounds in the headspace of sour (sun-dried) cassava starch, as seen previously in Table 2 and Figure 2. The titratable acidity of sour (sun-dried) cassava starch could not be due to carboxyl groups resulting from residual acids due to degradation of amylose and amylopectin [30], since high molecular weight organic acids were not detected, neither were short-chain and long-chain fatty acids by HS-SPME/GC-qMS.
| Compound | Peak number | Cassava starch Relative area (%) |
Sour (fermented and sun-dried) cassava starch Relative area (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Organic Acids | Acetic acid | 1 | - | 51.2 | |||||
| Aliphatic hydrocarbons and ethers | n-Nonane n-Decane n-Dodecane n-Tridecane n-Tetradecane n-Pentadecane Di- N-octyl ether Nonane, 2,5-dimethyl- Octacosane Heptadecane, 8-methyl- |
2 3 4 5 6 7 8 9 10 11 |
0.7 3.5 1.8 0.1 0.5 0.3 0.3 0.6 - 0.8 |
2.5 9.3 6.6 - - - - 2.0 2.0 - |
|||||
| Ketone | 2,6-ditert-butylcyclohexa-2,5-diene-1,4-dione | 12 | 0.2 | - | |||||
| Aromatic hydrocarbons and aldehydes | Toluene m-Xylene 1,3,5-Trimethylbenzene 1,2,3-Trimethylbenzene 1,2-Dichlorobenzene 1-Methyl-3-(1-methylethyl)benzene 1-Methyl-2-prop-1-en-2-ylbenzene 1-Ethyl-3-methylbenzene 1,2,4-Trimethylbenzene 1-Ethyl-2,3-dimethylbenzene 2-(4-Methylphenyl)propan-2-ol Styrene Benzaldehyde |
13 14 15 16 17 18 19 20 21 22 23 24 25 |
1.5 0.3 1.5 0.6 0.3 15.9 2.2 - - - 0.7 1.1 0.9 |
- - - 1.4 - - - 2.1 3.4 0.7 - - - |
|||||
| Terpenes | Sabinene 2-β-Pinene β-Myrcene δ-Carene Llimonene α-Terpinolene p-Mentha-1(7),8-dien-2-ol α-Terpineol Dihydrocarvone Trans-2-caren-4-ol Carvotanacetone p-Mentha-1,8-dien-7-al Carvacrol Neryl acetate Geranyl acetate α-Bergamotene β-Bisabolene β-Damascenone Caryophyllene α-Ionone |
26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 |
0.4 1.0 3.1 0.2 44.6 1.1 0.4 0.7 0.8 1.3 2.2 0.3 0.3 1.0 0.3 1.2 1.2 - - - |
- - - - 8.6 - - - - - - - - - - - - 1.5 6.6 2.1 |
|||||
| Alcohol | 2-Ethyl-1-hexanol | 46 | 5.1 | - | |||||
| Aldehyde | Decanal | 47 | 1.0 | - | |||||
| TOTAL | 100.0 | 100.0 | |||||||
| The metabolites were all confirmed by comparison of retention times (Rfs) and mass spectra (MS) of authentic substances. Arbitrary units (peak area) were used. Mean values of duplicate identification. |
|||||||||
Table 2: Volatile compounds identified in the “headspace” samples of cassava and sour (sun-dried) cassava starches by HS-SPME/GC-qMS (extraction temperature 25°C; extraction time, 30 min, using three-phase fiber 50/30μm DVB/CAR/PDMS).
Although in previous studies the increase in total acidity was ascribed to the production of organic acids, mainly lactic acid and substantial amounts of acetic and butyric acids [31]. The sour (fermented and sun-dried) cassava starch may have traces of propionic acid, without the butyric or propionic acid presence [32].
In regions with average temperatures around 18°C, fermentation is slow, with the predominance of lactic microbiota, mainly Lactobacillus plantarum, while in regions withaverage temperatures around 35°C, fermentation is quicker and butyric microbiota is predominant, mostly Clostridium butyricum [10].
Taken together, the data regarding pH, titratable acidity, acetic, lactic and succinic acid content and low room temperature indicate that fermentation was interrupted before completion during sour cassava starch manufacturing. Additionally, no Clostridium butyricum was found among the natural microbiota microorganisms. Succinic acid is a dicarboxylic acid produced as an intermediate of the tricarboxylic acid (TCA) cycle or as the major product of anaerobic fermentation by certain microorganisms [33]. Several yeast species found in the microbiota of sour cassava fermentation should contribute to succinic acid production during the oxidative metabolism of starch [34].
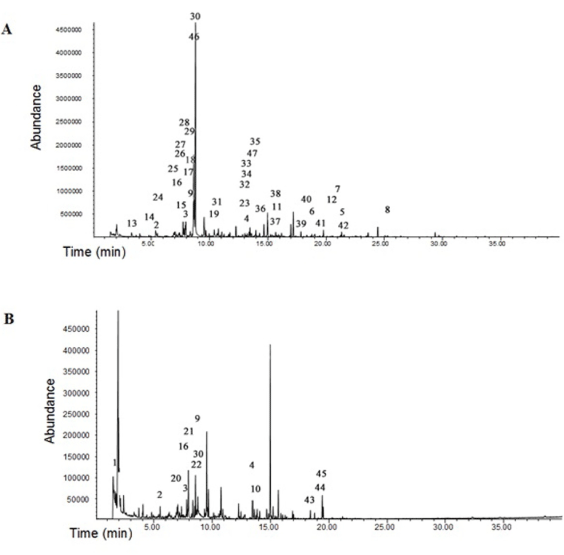
Characterization of volatile compounds and in cassava starch and sour (fermented and sun-dried) cassava starch: A higher number of distinct volatile compounds were found in the cassava starch in comparison to the sour (fermented and sun-dried) cassava starch (Figures 2A and 2B). This may be due to the sun-drying treatment, which may have caused the loss of some of the volatile compounds (Table 2). Aliphatic and aromatic hydrocarbons and terpenoidic compounds were found in a higher amount in cassava starch than in the sour (fermented and sun-dried) cassava starch. Sour (fermented and sun-dried) cassava starch has acetic acid comprising 40% (Table 2) of the volatile compound, and seems to protect the natural fermentation against contamination by spoilage microorganisms. A reduction of the profile diversity of terpenes and aromatic hydrocarbons was observed.
Terpenoids are the main representative class in the volatile compounds in both starches (Table 2). They can be released by yeast α-glycosidases during the fermentation process, contributing to the aroma of the final product [35]. Limonene confers a fresh, citrus taste and odor, β-damascenone brings a sweet fruity smell and α-ionone, a tropical fruity and flowery smell which are all present in considerable amounts in the final product (http://theleafonline. com/c/science/2014/09/terpene-profile-limonene), contributing to unique flavors, such as the pleasant aroma and taste of fermented cassava. Aromatic hydrocarbons had their relative content decreased in the sour (fermented and sun-dried) cassava starch and some of them like methylbenzene, 1-3-dimethylbenzene, 1,3,5-trimethylbenzene, 1,2-dichlorobenzene, 1-methyl-3-(1-methylethyl)benzene, 1-methyl- 2-prop-1-en-2-ylbenzene, 2-(4-methylphenyl)propan-2-ol, styrene and benzaldehyde were removed by fermentation (Table 2).
Lactic acid bacteria and yeast present in cassava starch fermentation are able to produced volatile compounds formed via primary and secondary metabolism (MVOCs). Furthermore, moisture and temperature influence MVOC emission, and a prolonged growth phase due to a lower temperature may influence the production of certain compounds and extend the time for maximum production [36]. Other environmental factors such as substrate pH, light and CO2 or O2 levels can probably also influence the MVOC pattern. In the food industry, volatile organic acids are used as flavorings, preservatives and inhibitors of microbial growth.
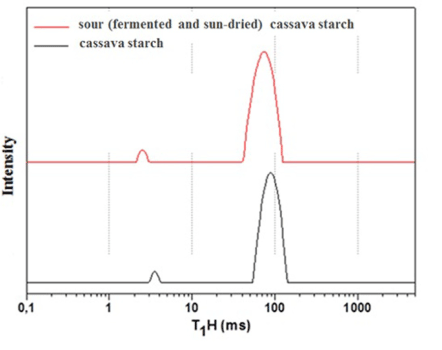
H-NMR relaxometry information of cassava starch and sour (fermented and sun-dried) cassava starch: Solid-state NMR relaxometry allowed for the monitoring of the changes in cassava starch during fermentation and sun-drying processing, where the longitudinal relaxation time (T1H) parameter provided information about the molecular dynamics of the starch before and after fermentation (Figure 3). The T1H parameter showed a great variation in the proton spin-lattice relaxation times, from 83.3 to 69.3 ms, for cassava starch and sour (fermented and sun-dried) cassava starch, respectively. The shortest spin-lattice relaxation time T1H (p < 0.001) indicates that the fermented and sun-dried product is more inclinable to expansion, since there is a population of hydrogen atoms in a lower confinement (or greater mobility) and greater heterogeneity in sour (fermented and sun-dried) cassava starch [20], as demonstrated by the line width and the peak intensity that changed during processing (Figure 3).
Figure 3: Distribution curves of domain relaxation times for cassava starch and sour (fermented and sun-dried) cassava starch obtained by LF-NMR. The relaxation values (means of triplicate analysis) and relative intensities were obtained by fitting the exponential data with the aid of the WINFIT 2.4.0.0 software supplied by resonance equipment.
Technological properties of cassava starch and sour (sun-dried) cassava starch
Pasting properties: The sour (fermented and sun-dried) cassava starch presented a different pasting profile (p < 0.001) than cassava starch (Table 3). The sour cassava starch pastes presented lower viscosity at high temperature (lower peak viscosity), lower agitation stability (higher breakdown) and lower retrogradion tendency (lower setback) than the cassava starch. This cassava starch pasting profile alteration following fermentation and sun-drying treatment has also been observed in other studies [12,31,37]. Photochemical and enzymatic modifications occur during cassava starch manufacturing [37]. Starch molecules (amylose and amylopectin) in the amorphous regions of the granules were partially depolymerized by the amylolytic enzymes and organic acids produced by microorganisms from the natural environment and by UV irradiation (mainly UVB and UVC irradiation) during sun drying to the size-reduced starch molecules [37,38]. Besides depolymerization, starch molecules present carbonyl and carboxylate groups, which indicate that oxidation of the amylose and amylopectin hydroxyl groups is observed, in a mechanism involving free radicals [12]. The weakened granule organization caused by oxidative depolymerization during sour cassava starch fermentation [39] makes the starch granules show little swelling and, therefore, lower peak viscosity. The more weakened granule organization also causes the starch granules to readily disintegrate, which leads to higher breakdown. After being oxidatively depolymerized, amylose and amylopectin presented lower molecular weights, and a lower retrogradation tendency is observed (lower setback).
| Parameters | Cassava starch | Sour (sun-dried) cassava starch |
|---|---|---|
| Final viscosity at 50ºC (RVU)* | 172.5 ± 0.2a | 55.8 ± 0.2b |
| Pasting temperature (ºC)* | 68.6 ± 0.1ns | 70.2 ± 0.1ns |
| Peak viscosity (RVU)* | 283.31 ± 3.1a | 216.4 ± 2.4b |
| Peak time (min)* | 3.7 ± 0ns | 3.7 ± 0ns |
| Trough (RVU)* | 123.3 ± 3.7a | 37.3 ± 0.9b |
| Breakdown (RVU)* | 160.0 ± 5.0b | 179.2 ± 1.5a |
| Setback (RVU)* | 49.2 ± 3.1a | 18.5 ± 0.7b |
| WAI (g.g-1) | 2.0 ± 0.1a | 2.1 ± 0a |
| WSI (%) | 0.2 ± 0a | 0.6 ± 0,1a |
| Expansion (mL.g-1) | 1.4 ± 0.4b | 4.5 ± 0.4a |
| Amylose (%) | 20.4 ± 0.4a | 19.9 ± 0.3a |
| Amylopectin (%) | 79.6 ± 0.4a | 80.1 ± 0.3a |
|
Means and standard deviation of triplicate measurements. |
||
Table 3: Technological characteristics of cassava starch and sour (sun-dried) cassava starch: pasting properties, water absorption, solubility indexes, amylose and amylopectin contents.
Water Absorption Index (WAI) and Water Solubility Index (WSI): Cassava starch and sour cassava starch (fermented and sun-dried) showed similar water absorption and water solubility index values, indicating that there were no effects following fermentation and sun drying (Table 3).
The water absorption index (WAI) is related to the degree of starch swelling or gelatinization. As the WAI was performed at 30°C, starches were not swollen because they did not reach the minimum energy required for the gelation process. Neither the cassava starch nor the sour cassava starch showed significant water uptake at this temperature (Table 3). The oxidative depolymerization of sour cassava starch was not efficient enough to prompt starch granules solubility in cold water, not assisting them to their pre-gelatinization state, since only pre-gelatinized starch granules can absorb water at ambient temperature, increasing starch viscosity [40]. The water solubility index (WSI) is a parameter that measures the total degree of degradation of the starch granule. Among the changes observed during the fermentation process of sour cassava starch (fermented and sun-dried), an increase in starch solubility is expected [37]. Herein, a significant increase in starch solubility was observed for sour cassava starch (fermented and sun-dried). Variations in the quality of the final product of a same producer are frequent due to the lack of control parameters in sour cassava starch processing. Therefore, the search for improvements in the technological process to obtain a final product with better quality and standardization is a major challenge for the sector. Generally, sour (sun-dried) cassava starch has a higher WSI value than its native starches. Studies have shown that cassava starch shows lower solubility than sour cassava starch, due to the presence of non-solubilized amylose in the crystalline region of the native granule, while the amylose of the fermented starch is already partially released [41].
Expansion properties: The bread making ability of both starches is represented by the loaf expansion values displayed in Table 3. There was a significant 3.2 fold increase in loaf expansion after starch fermentation and sun-drying treatment, although the production of organic acids during fermentation was discrete, as discussed previously, but still enough to promote physical changes in the granules, enhancing their ability to swell and solubilize in water [42].
The hydrolysis of the glycosidic bonds in the amorphous region of the granules by acids, enzymes and UV irradiation resulted in increased mobility and greater heterogeneity of the hydrogen molecules (relaxometry analysis), which probably results in the development of the expansion property. When comparing the specific volumes obtained to the loaf expansion indexes established [41], it can be observed that both starches showed low loaf expansion indexes, lower than 5 mL.g-1 (Table 3). However, the loaf expansion values shown herein are similar to those observed for distinct genetic varieties of cassava [3]. A superior loaf expansion can be obtained, where a specific volume between 5 and 10 mL g-1 can be reached after 83 days of fermentation [32].
The loaf expansion of cassava starch may be due to its high swelling capacity and solubility resulting from molecular degradation after acidification and irradiation [42]. Maximizing the expansion can depend on the degree of sour starch polymerization, the number of carboxyl and hydroxyl groups, pH, granule density, and other parameters that show significant correlation, whether positive or negative, with the expansion of the dough and its characteristics after cooking, as well as with its storage after cooking [37]. Although not fully established, the mechanism of sour cassava starch expansion may be similar to the one for extruded products, where the driving force would be water evaporation, and cell expansion would be governed mainly by dough-crust viscosity [41]. Partial depolymerization of sour cassava starch during fermentation and sun-drying provided small linear fragments and facilitated the development of an amorphous matrix structure of starch dough [38] reducing dough viscosity during expansion, aiding in the bubble expansion. However, other phenomena besides depolymerization could improve loaf expansion at different baking stages, including mass transfers, such as CO2 or water displacement from the surrounding matrix to the expanding bubbles, inertia and surface tension [3].
Conclusions
Sour (sun-dried) cassava starch has great potential as a more economical and sustainable alternative to wheat flour in gluten-free bread production around the world. The results showed herein can be applied in order to supply the market with high-quality and homogeneous sour cassava starch. To control of several parameters in the manufacturing process is necessary to increase the efficiency of the sour (sun-dried) cassava starch production process. The fermentation lifetime should be controlled by the physical and chemical parameters that can be an indicator of the quality of the final product. Formation of organic acids and volatile compounds as terpenoids and esters, and molecular relaxometry measures should be evaluated in order to achieve technological properties, which guarantee optimum quality of expansion of the final product and desirable flavor. Furthermore, changes in the ambient temperature and relative humidity should be minimized, since they may affect the efficiency of the fermentation process. The aim is to achieve ideal viscosity parameters, since this is the major quality technological importance that defines the acceptance and application of the product in the food industry, mainly associated with the production of cheese bread, where a mixture of cassava starch and sour cassava starch are associated with cured cheese powder, resulting in an appreciated and widely consumed product throughout the country.
Acknowledgement
The authors acknowledge the financial support from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) and FAPESB (Fundação de Amparo à Pesquisa do Estado da Bahia). We also thank MSc Andrea Mattos and Dr Patrícia Ribeiro Pereira for fruitful discussions.
References
- Olsen K, Schaal B (2001) Microsatellite variation in cassava (Manihotesculenta, Euphorbiaceae) and its wild relatives: further evidence for a southern Amazonian origin of domestication. Am J Bot 88: 131-142.
- Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresource Technol 74: 81-87.
- Alvarado PM, Grosmaire L, Dufour D, Toro AG, Sánchez T, et al. (2013) Combined effect of fermentation, sun-drying and genotype on breadmaking ability of sour cassava starch. Carbohydr Polym 98: 1137-1146.
- Lundin KE (2014) Non-celiac gluten sensitivity - why worry? BMC Med 12: 86.
- Gallagher E, Gormley TR, Arendt EK (2004) Recent advances in the formulation of gluten-free cereal-based products. Trends Food Sci Tech 15: 143-152.
- Schober TJ (2009) Manufacture of gluten-free speciality breads and confectionery products. In E. Gallagher (ed.), Gluten-free food science and technology (pp. 130-180). Oxford: Wiley-Blackwell.
- Oluwasola O (2010) Stimulating rural employment and income for cassava (Manihot sp.) processing farming households in Oyo State, Nigeria through policy initiatives. J DevAgric Econ 2: 18-25.
- Lacerda IC, Miranda RL, Borelli BM, Nunes AC, Nardi RM, et al. (2005) Lactic acid bacteria and yeasts associated with spontaneous fermentations during the production of sour cassava starch in Brazil. Int J Food Microbiol 105: 213-219.
- Holzapfel WH (2002) Appropriate starter culture technologies for small-scale fermentation in developing countries. Int J Food Microbiol 75: 197-212.
- Demiate IM, Dupuy N, Huvenne JP, Cereda MP, Wosiacki G, et al. (2000) Relationship between baking behavior of modified cassava starches and starch chemical structure determined by FTIR spectroscopy. Carbohydr Polym 42: 149-158.
- Sambrook J, Russel DW (2001) Molecular Cloning: A Laboratory Manual. (3rdedn), Cold Spring Harbor Laboratory Press, New York.
- Wang X, Haruta S, Wang P, Ishii M, Igarashi Y, et al. (2006) Diversity of a stable enrichment culture which is useful for silage inoculant and its succession in alfalfa silage. FEMS MicrobiolEcol 57: 106-115.
- de Barros Lopes M, Soden A, Henschke PA, Langridge P (1996) PCR differentiation of commercial yeast strains using intron splice site primers. Appl Environ Microbiol 62: 4514-4520.
- Naumova ES, IvannikovaIuV, Naumov GI (2005) Genetic differentiation of the sherry yeasts Saccharomyces cerevisiae. PriklBiokhimMikrobiol 41: 656-661.
- Adolfo Lutz Institute, (2008) Analytical Standards Institute Adolfo Lutz. (3rdedn), Sao Paulo.
- Leite AM, Leite DC, Del Aguila EM, Alvares TS, Peixoto RS, et al. (2013) Microbiological and chemical characteristics of Brazilian kefir during fermentation and storage processes. J Dairy Sci 96: 4149-4159.
- Adams RP (1995) Identification of essential oil components by gas chromatography mass spectroscopy. Illinois: Allured Publishing Corporation.
- Brito LM, Tavares MI (2012) Evaluation of the influence of nanoparticles' shapes on the formation of poly(lactic acid) nanocomposites obtained employing the solution method. J NanosciNanotechnol 12: 4508-4513.
- Anderson RA, Conway HF, Pfeifer VF, Griffin EL (1969) Gelatinization of corn grits by roll- and extrusion-cooking. Cereal Sci Today 14: 47.
- Faubion JM, Hoseney RC (1982) High-temperature short time extrusioncooking of wheat starch and flour. I. Effect of moisture and flour type on extrudate properties. Cereal Chem 59: 529-533.
- Ampe F, Sirvent A, Zakhia N (2001) Dynamics of the microbial community responsible for traditional sour cassava starch fermentation studied by denaturing gradient gel electrophoresis and quantitative rRNA hybridization. Int J Food Microbiol 65: 45-54.
- Khue NT, Ngoc NH (2013) Exopolysaccharide in Lactobacillus rhamnosus Pn04 after co-culture with Leuconostocmesenteroides Vtcc-B-643. JPAS 3: 14-17.
- Halm M, Hornbaek T, Arneborg N, Sefa-Dedeh S, Jespersen L (2004) Lactic acid tolerance determined by measurement of intracellular pH of single cells of Candida krusei and Saccharomyces cerevisiae isolated from fermented maize dough. Int J Food Microbiol 94: 97-103
- Rodriguez-Sanoja R, Morlon-Guyot J, Jore J, Pintado J, Juge N, et al. (2000) Comparative characterization of complete and truncated forms of Lactobacillus amylovorus a-amylase and role of the C-terminal direct repeats in raw-starch binding. Appl Environ Microbiol 66: 3350-3356.
- Oyewole OB (2001) Characteristics and significance of yeasts' involvement in cassava fermentation for 'fufu' production. Int J Food Microbiol 65: 213-218.
- Ascheri DPR, Boêno JA, Bassinello PZ, Ascheri JLR (2012) Correlation between grain nutritional content and pasting properties of pre-gelatinized red rice flour. Revista Ceres 59: 16-24.
- Luo J, Jobling SA, Millar A, Morell MK, Li Z, et al. (2015) Allelic effects on starch structure and properties of six starch biosynthetic genes in a rice recombinant inbred line population. Rice (NY) 8: 15.
- Silva GO, Takizawa FF, Pedroso RA, Franco CML, Leonel M, et al. (2006) Physicochemical characteristics of modified food starches commercialized in Brazil. Food SciTechnol 26: 188-197.
- Adegunwa MO, Sanni LO, Maziya-Dixon B (2011) Effects of fermentation length and varieties on the pasting properties of sour cassava starch. Afr J Biotechnol 10: 8428-8433.
- Aquino ACMS, Pereira JM, Watanable LB, Amante ER (2013) Standardization of the sour cassava starch reduces the processing time by fermentation water monitoring. Int J Food Sci Tech 48: 1892-1898.
- Lee PC, Lee WG, Kwon S, Lee SY, Chang HN, et al. (2000) Batch and continuous cultivation of Anaerobiospirillumsucciniciproducens for the production of succinic acid from whey. ApplMicrobiolBiotechnol 54: 23-27.
- Chaves-López C, Serio A, Grande-Tovar CD, Cuervo-Mulet R, Delgado-Ospina J, et al. (2014) Traditional fermented foods and beverages from a microbiological and nutritional perspective: The Colombian heritage. Compr Rev Food Sci F 13: 1031-1048.
- Calleja A, Falqué E (2005) Volatile composition of Mencia wines. Food Chem 90: 357-363.
- Sunesson AL, Nilsson CA, Carlson R, Blomquist G, Andersson B (1997) Influence of temperature, oxygen and carbon dioxide levels on the production of volatile metabolites from Streptomyces albidoflavus cultivated on gypsum board and tryptone glucose extract agar. Ann OccupHyg 41: 393-413.
- Marcon MJA, Vieira GCN, Simas KN, Santos K, Vieira MA, et al. (2007) Effect of the improved fermentation on physicochemical properties and sensorial acceptability of sour cassava starch. Braz Arch BiolTechnol 50: 1073-1081.
- Vatanasuchart N,Naivikul O, Charoenrein S, Srirot K (2005) Molecular properties of cassava starch modified with different UV irradiations to enhance baking expansion. CarbohydPolym 61: 80-87.
- Putri WDR, Marseno HDW, Cahyanto MNC (2012) Role of lactic acid bacteria on structural and physicochemical properties of sour cassava starch. APCBEE Procedia 2: 104-109.
- Silva PA, Assis GT (2011) Development and characterization of an extruded breakfast cereal from cassava enriched with milk whey protein concentrate. Braz J Food Technol 14: 260-266.
- Gomes AMM, Silva CEM, Ricardo NMPS (2005) Effects of annealing on the physicochemical properties of fermented cassava starch (polvilhoazedo). CarbohydPolym 60: 1-6.
- Dias ARG, Zavareze ER, Elias MC, Helbig E, Silva DO, et al. (2011) Pasting, expansion and textural properties of fermented cassava starch oxidized with sodium hypochlorite. Carbohydr Polym 84: 268-275.
- Nunes OLG, Cereda MP (1994) Effects of drying process on the development of expantion in Cassava starch hydrolyzed by lactic acid.
- Bertolini AC, Mestres C, Lourdin D, Valle GD, Colonna P (2001) Relationship between thermomechanical properties and baking expansion of sour cassava starch (PolvilhoAzedo). J Sci Food Agr 81: 429-435.
Copyright: © 2016 Karine Rebouças H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.