Indexed In
- Open J Gate
- Academic Keys
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- CABI full text
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 11, Issue 4
Equine Epizootic Lymphangitis: A Synopsis
Fatma Graiban A Mheiri* and Ulrich WerneryReceived: 09-May-2023, Manuscript No. JTD-23-21292; Editor assigned: 12-May-2023, Pre QC No. JTD-23-21292 (PQ); Reviewed: 26-May-2023, QC No. JTD-23-21292 ; Revised: 02-Jun-2023, Manuscript No. JTD-23-21292 (R); Published: 09-Jun-2023, DOI: 10.35241/2329-891X.23.11.396
Abstract
Equine Epizootic Lymphangitis (EEL) is a fungal disease that affects horses, mules, and donkeys in Asia, South America, Africa, mainly in Ethiopia, with the epicenter being in Bishoftu (Debre Zeit) south of the capital Addis Ababa. The disease is prevalent in rural areas, where equids have a significant impact on the socio-economic well-being of Ethiopian families, who rely on these animals as a source of income. Various studies have been conducted on the prevalence of EEL in Ethiopia.
This paper summarizes the findings of various studies conducted by Ethiopian scientists and others on the prevalence of EEL in different regions of the country, explains its association with altitude and climate. It also summarizes the history of the Histoplasma capsulatum and highlights the different forms of the disease.
The authors also discuss the devastating effects of EEL on the welfare of equids and their owners, as well as the lack of available treatment. The paper concludes with a call to action for the Ethiopian government to address this important welfare issue and provide support for affected equid populations. Overall, this paper provides important insights into the impact of EEL on the livelihood of many Ethiopians and underscores the urgent need for effective solutions.
Keywords
Equine Epizootic Lymphangitis (EEL); Epizootic lymphangitis; Fungal disease; Histoplasma capsulatum variety Farciminosum (HCF); Equine disease
Introduction
The world population of equids is estimated to be 112 million, including approximately 60 million horses, 43 million donkeys and 10 million mules [1]. In Africa, the estimated population of equids is around 25 million.
Working equids make up the majority of the world's equid population, and most of them reside in low-income countries. These beasts of burden have significant socioeconomic importance and a wide-ranging impact on the livelihoods of millions of families and individuals. They play a vital role in reducing poverty, promoting gender equality, enhancing rural development, and providing food security. Losing an animal can be devastating for an entire family. Unfortunately, working equids are often neglected, as they are not typically included in governmental vaccination programs, eradication campaigns, or legislation, despite being susceptible to diseases that are notifiable to the world organization for animal health [2], such as African Horse Sickness (AHS), glanders, and Equine Epizootic Lymphangitis (EEL). Ethiopia currently has the world's second-largest equid population, with a total of 11.3 million equids, including the largest population of donkeys worldwide, with a total of 8.7 million. These animals are used for transport, as cart and riding animals, and in the agricultural sector [3].
Equine Epizootic Lymphangitis (EEL) is considered the most significant infectious disease affecting horses in many parts of Ethiopia [3-8]. According to Ameni EEL is the second most prevalent disease in Ethiopian horses after African Horse Sickness (AHS). EEL is caused by Histoplasma, a thermally dimorphic fungus found primarily in soil contaminated with bird droppings or excrements of bats. Although the fungus is known to exist worldwide, it is more common in tropical regions where it thrives in damp soil. The fungus has two forms: mycelia or mold form, which grows in soil or culture medium at ambient temperature of 25°C, and the yeast form, which grows in bodies of mammals and humans and in culture at a temperature of 37°C. The estate mycelia develop asexual spores as tuberculate macro and microconidia. Animals and humans contract the disease by inhaling the spores, leading to a condition known as Darling’s disease. The spores then transform into the yeast form in animals and humans, which reproduces through budding. Histoplasma Capsulatum (HC) has three recognized varieties: Histoplasma capsulatum var. capsulatum (HCC), which is a pathogen found in the New World; Histoplasma Capsulatum var. Duboisii (HCD), an African pathogen affecting humans; and Histoplasma Capsulatum var. Farciminosum (HCF), is an old-world pathogen affecting equines [9]. Interestingly, phylogenetic studies have identified at least eight different clades, indicating that the three recognized varieties are phylogenetically meaningless [10,11].
Equine Epizootic Lymphangitis (EEL) is a highly infectious and contagious systemic disease caused by the fungus Histoplasma capsulatum var. farciminosum that can affect all Equidae but mainly horses and mules [12]. Donkeys can also be infected, but less, while in zebras and other wild equids the disease have not been reported [9,13,14]. Various animal species have been found to be susceptible to HCF, including bovines, Bactrian and dromedary camels, canines, felines and wildlife [15-20].
Additionally, laboratory animal species such as guinea pigs, mice, and rabbits have also been experimentally infected [21,22]. A subspecies of Histoplasma, known as Histoplasma Capsulatum var. Capsulatum (HCC) is a common cause of histoplasmosis in humans. The primariy route of infection in humans for Histoplasma capsulatum var. capsulatum is through inhalation of the organism from the environment. Although interhuman transmission of this fungus is rare, it can affect immune compromised individuals. However, Histoplasma capsulatum var. Farciminosum (HCF) the causative agent of EEL is not considered a zoonotic fungus. EEL is a non-listed disease under the World Organisation for Animal Health (WOAH), despite its significant socioeconomic impact in countries where it is endemic.
Literature Review
Past and present
In 1903, Samuel taylor darling graduated from the college of physicians and surgeons of baltimore in the US. In 1905 during his internship at Ancon Hospital in Panama [23], he performed autopsies when he first discovered Histoplasma. He believed that it was a type of protozoan which he named Histoplasma capsulatum, because it occupied the cytoplasm of histiocyte-like cells and was enveloped by a capsule [24]. Since then Histoplasmosis became known as “Darling’s Disease” [25]. In 1912, Henrique da Rocha-Lima, conducted further studies on tissues of Darling’s Panama patients and suggested that the organism is associated with organisms of Leishmaniasis. However, later he proved the similarity of this organism to Cryptococcus farciminosum (Rivolta), the cause of epizootic lymphangitis in horses [26]. Here concluded, that Histoplasma capsulatum was a yeast-like fungus rather than a protozoan [24]. In 1933 Dr. William A. De Monbreun successfully grew the first culture of the organism. In 1930s-to 1950, the Giemsa and Methenamine silver stains were introduced, which helped to diagnose cases correctly, that were mistakenly diagnosed as malaria, yellow fever or tuberculosis. In 1955, Amphotericin B, an affective fungicide, was produced from the extract of Streptomyces bacterium by the Squibb Institute for Medical Research New Brunswick, Canada. From that time onward it was used in clinics in 1958 to control and treat fungal diseases including EEL [26].
Equine Epizootic Lymphangitis (EEL) was already known around 1850 under many different names, like African glanders, Blastomycosis, Farcin d' Afrique, Mal de verme, Farcin de revière and the scientific name was lymphangitis epizootica. Already in 1883 Rivolta and Micellone detected the disease in Algeria and France and Rivolta recognized it in 1873 from horses’ ulcers as a fungal yeast causing infections of the lymph vessels. He clearly differentiated it from glanders. In 1891 Nocard, then Tokishige in 1896 and later Bierbaum in 1919 and Bouquet and Negre in 1920 investigated this disease in more detail [27].
Equine Epizootic Lymphangitis (EEL) has been eradicated from many countries, but it still exists in some countries of the Mediterranean region, also India, Pakistan, Japan, North Africa and East Africa, mainly Ethiopia [28]. There seem to be some endemic areas in the US, like Mississippi-Ohio, but this is disputed by who stated that EEL has not been reported in horses in the United States [29]. EEL occurs much more frequently in tropical and subtropical areas than in temperate zones. The WOAH classified it as a List B disease which possesses an economical and public health importance to countries that are involved in the trade of animals, but removed it recently from its list for unknown reason [2].
Ethiopia’s endemic EEL status
Equine Epizootic Lymphangitis (EEL) is often seen in different parts of Ethiopia in horses, mules, and donkeys. A number of studies have been published about cases in the past two decades by Ethiopian scientists. The epicenter of the disease is in Debre Zeit (Bishoftu) south of Addis Ababa, where Addis Ababa University (AAU), College of Veterinary Medicine is located. The college collaborates with the Society for the Protection of Animals (SPANA), equine hospital and The Donkey Sanctuary under the administration of Addis Ababa University College of Veterinary Medicine in Bishoftu Ethiopia. The equine clinic is conveniently located in the Veterinary Faculty of the Ethiopian University. This was a good opportunity for the authors to visit the faculty and clinic several times over the last years to receive on-site information about EEL in this area. The last visit was in spring 2022, when also samples were collected and brought back to CVRL. Various studies on the prevalence of EEL in Ethiopia have been conducted and none in other countries. Ameni conducted a study on EEL in carthorses, one of the main working equids activity in rural parts of Ethiopia, but also used as transportation in some towns [30]. Three towns in specific Debre-Zeit, Mojo and Nazareth, where mainly male equids are used, highlight the importance of cart-horses. This also reflects on the economy and the socio-economic importance as well. It shows a lack of infrastructure in rural parts of Ethiopia. The topography of the country is still not ready for modern transportation technologies. Ameni and Siyoum carried out a cross-sectional study on 19,000 cart horses in 28 Ethiopian towns and found an overall EEL prevalence of approximately 20% [3]. He found an association between the altitude of the study towns and prevalence of EEL. EEL cases were significantly higher in towns. The fungal disease was also more prevalent in humid and hot areas ranging from above 1500 m sea level to 2300 m altitude. EEL was low in cold, windy, and dry areas. It is interesting to note that EEL in equines can be cured by moving horses to areas over 2300 m. Losses of cart horses, mules, or donkeys are often devastating to the owner and his family, as often these animals are the only income source for an Ethiopian family. Horses that are so badly infected by the fungus that they cannot work any longer are abandoned by the owners in remote areas, where they die and are eaten by different species of vultures (Figure 1). Equids and specially cart horses face many constraints that are threatening their survival. Infected horses rarely respond to antibiotics and antifungal drugs are not available in Ethiopia. Seeing these poor creatures is an important welfare issue which should be addressed by the Ethiopian government.

Figure 1: SAbandoned incurable EEL horse, eaten by vultures.
Aetiology
The aetiological agent of this disease is a dimorphic fungus called Histoplasma capsulatum var. farciminosum, previously known as Cryptococcus farciminosus. It is considered a variant of Histoplasma capsulatum var. Capsulatum (HCC) and Histoplasma capsulatum var. duboisii acquired by equines, originating from South America [8,11]. HCF infects equids only, whereas HCC can infect both, horses and human beings and other animal species. It is important to know that the fungus has two distinct phases, the mycelial form found in the environment and the yeast form growing in infected organs. This fungus forms a mycelium in the environment with hyphae which have macro and microconidia spores at the end of the hyphae which spread into the air and infects the host by inhalation. It can also be transmitted through infected fomites which then infect various tissues and organs of the host. To identify the fungus properly, the colonies have to convert from the mold form to the yeast form. In the laboratory, the mycelial form favors a humid and wet environment. It can persist in soil for many months. Environmental contamination with the fungus may also occur through discharging open abscesses or nasal, lacrimal discharge. Survival of the HCC pathogen has so far only been investigated in vitro. Bardelli and Ademollo reported that the fungus remained viable after desiccation in a laboratory for 25 months [31].
Diagnostics of the fungus including staining, culturing, skin tests and serology are well described by Scantlebury and Reed [13]. Here we can confirm that culture of HCF is difficult as the organism is very slow growing despite using different fungal agars. Care must also be taken to reduce overgrowth by contaminants as samples originate mainly from abscesses, mucopurulent discharge, scabs and tissues. It is also obvious, that the mycelia grown on culture media can be converted into the yeast phase in the laboratory by altering the culture conditions [32,33].
Immunity
H. capsulatum, is an intracellular pathogen, which survives and thrives in its host. The pathogen is capable of infecting macrophages through phagocytosis, but only after breaching the skin to gain access to cutaneous extracellular space. The pathogen weakens the immune response by impairing Langerhans cells and expressing the Heat Shock Protein 60 (Hsp60) to avoid macrophage activation upon internalization [33]. H. capsulatum alters its cell wall components to prevent recognition by leukocytes and avoid immune response induction, producing α-glucans to decoy Dectin-1 and glucanases to conceal β-glucans on its surface [33]. After phagocytosis, the pathogen produces catalases to resist reactive oxygen and nitrogen species and overcomes challenges in the macrophage by producing hydroxamates as siderophores and calcium binding protein (Cbp1) to acquire calcium in a calcium-restricted microenvironment [34,35]. The pathogen has a reactivation capacity, leading to chronic cutaneous granulomas, and polymicrobial infection is common with other bacteria in the lesion. The immune response to H. capsulatum is complex and varies between individual hosts. Further investigation is required to understand the elasticity of the immune response to H. capsulatum.
Discussion
Different forms of equine epizootic lymphangitis
The EEL forms are well described by several scientists [3,4,8,13,35]. Equine epizootic lymphangitis is a chronic pyogranulomatous multifocal disease of the skin, lymph vessels and lymph nodes of the limbs and neck of Equidae. One of the oldest descriptions of EEL was conducted in 1945 and displayed as a drawing (Figure 2). The clinical conditions are divided into 4 different forms:
1. Cutaneous EEL
2. Ocular (keratoconjunctivitis) EEL
3. Respiratory (multifocal pulmonary lesions) EEL
4. Asymptomatic EEL
Clinical manifestations of EEL in equids have been reported by many researchers [3,4,33,36-39].
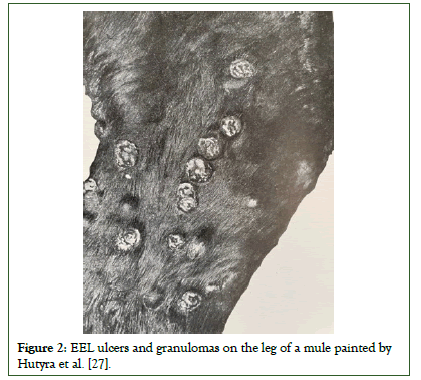
Figure 2: EEL ulcers and granulomas on the leg of a mule painted by Hutyra et al. [27].
Cutaneous EEL
This is the most common and widely reported form, which is characterized by subcutaneous, freely transferrable nodules which originate from infected superficial lymph nodes which progress along the lymphatic system. These alterations of the lymph nodes, skin and lymphatic vessels are described by Buxton and Fraser [40]. The lesions develop within weeks up to 3 months to form. Nodules may appear in any part of the body, but mainly in the lower limbs, neck, chest and face (Figure 3).
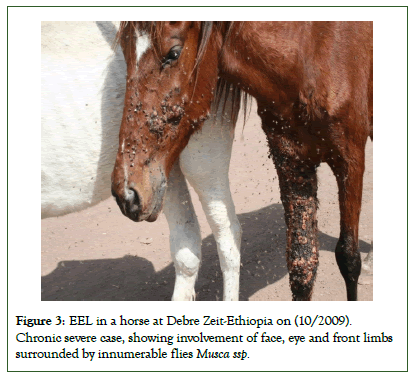
Figure 3: EEL in a horse at Debre Zeit-Ethiopia on (10/2009). Chronic severe case, showing involvement of face, eye and front limbs surrounded by innumerable flies Musca ssp.
First, there will be an intradermal swelling that develops into a small lump, followed by several nodules along the lymphatics. These nodules rupture and discharge thick pus, leaving ulcerated skin lesions. This process frequently undergoes alternating periods of discharge and ulceration. The skin covering these ulcers become thick and fuse with the subcutaneous tissues (Figures 4 and 5).

Figure 4: EEL of an Ethiopian horse in Debre Zeit (10/2009) showing severe multiple ulcerated nodules on the right front leg.
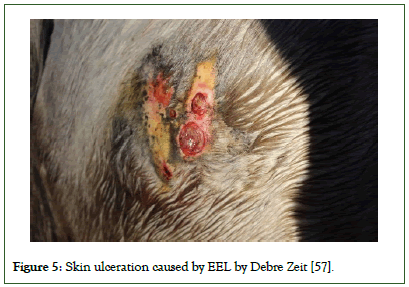
Figure 5: Skin ulceration caused by EEL by Debre Zeit [57].
The nodules are round pyogranulomatous with a thick fibrous capsule that contains thick hypogenous exudate. They eventually coalesce along the lymphatic vessels and the pathogen migrates to the nearby lymph nodes which become enlarged and contain pus from which the fungus disseminates to other areas of the body. The fungus can affect any part of the body, but needs entry through wounds. In cart horses, trauma through poor harnessing is one of the major factors of chest, neck and leg wounding which facilitates the entrance of the organisms [41] and the spread to other parts of the body. Therefore, The Donkey Sanctuary in Debre Zeit (Bishoftu) established a very simple easy to understand guide to educate people how harness should work properly. To ease the education process and to help the community, the Donkey Sanctuary hired Ethiopian women to produce equine harnesses which are made from soft material to avoid any injury to the animal skin (Figure 6).

Figure 6: Proper equine harness equipment produced at SPANA, Ethiopia 2016.
In summary: the cutaneous EEL is characterized by chronic, suppurative, ulcerating, pyogranulomatous dermatitis and lymphangitis with repeated cycles of nodule development and discharge and ulceration. Healing of the ulcers results in scar formation. A similar pattern of lesions as described in horses have also been observed in donkeys. The Donkey Health and Welfare Project (DHWP) in Ethiopia treated 34 cases of donkey EEL predominantly of the cutaneous form [42]. In some parts of Egypt lacrimal histoplasmosis is a common manifestation and chronic cases are sometimes seen with purulent nasal discharge from fistulation of the lacrimal duct [43,44].
Ocular keratoconjunctivitis EEL
The ocular form is characterized as a serous to mucopurulent discharge including granulomatous reaction of the lacrimal duct (Figure 7). These lesions may reach to the external skin and affects the canthus. Often there is also swelling of the palpebrae. The fungal infection may also affect the lacrimal ducts which can lead to nodule development of the skin of the face. The ocular form has been reported mainly in donkeys in Egypt, but also has been seen in horses and mules in Ethiopia (Wernery, 2022). The severity of this infection can cause partial or complete loss of vision or eye in horses as shown in Figure 8 [45].

Figure 7: Ocular form of EEL [57].
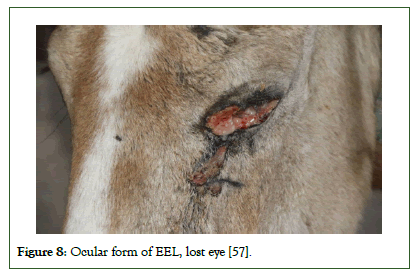
Figure 8: Ocular form of EEL, lost eye [57].
Flies play an important role of the transmission of the fungus to other parts of the animal or other equids. The flies are such a nuisance that infected horses are often found in the middle of the road. The wind created by passing vehicles gives them some relief from this pest (Figure 9). Singh showed that the normal horse flies Musca and Stomoxys spp. were able to carry HCF in their digestive tract for 20 days and then transmit the disease [22]. Ameni and Terefe believe that the tick Boophilus may also play a role [46].

Figure 9: An emaciated EEL horse in the middle of a road receiving relief by passing vehicles [57].
In summary: The ocular EEL form is a severe infection of the eye with keratoconjunctivitis, corneal ulceration, lacrimal duct infection which may predispose bacterial infection and myiasis.
Respiratory EEL
The respiratory form causes multifocal pulmonary lesions described by Ainsworth and Austwick, which either occurs through inhalation of the fungal spores from the environment or from lesions of the naso-lacrimal duct and from nodules of the nasal mucosa and external nares [47]. It is rarely reported as badly infected horses are abandoned and no necropsy is being performed.
Granulomas are present in the conchae, trachea and lungs. Mucopurulent nasal discharge is common with coughing, increased respiratory distress and weakness.
In summary: The respiratory EEL is a serious, debilitating disease involving nose, conchae, trachea and lungs. It resembles Glanders and therefore proper laboratory investigations are necessary.
Asymptomatic EEL
It is not entirely clear if asymptomatic EEL in equids exists, as there is a lack of information concerning immunological studies investigating the existence and role in these animals.
However, this form has been reported by Al-Ani [48]. The fungus induces both, humoral and cell-mediated immune responses [48]. Serological tests, like antibody ELISA should be established to investigate asymptomatic carriers as well as antibody levels after treatment and eventual vaccination. As an alternative to testing antibodies, “histofarcin” similar to mallein may be used for the diagnosis of EEL in equids [49]. Ameni who investigated the sensitivity and specificity of histofarcin produced in Ethiopia concluded, that histofarcin could play a significant role in detecting early infection of EEL [50]. However, standard test validation procedures are required before histofarcin can be safely applied for the diagnosis of EEL, as 31% of false positives were reported in endemic districts [49].
In summary: The asymptomatic form is not well described and therefore serological investigations are necessary to define the existence of this form.
Differential diagnosis of epizootic equine lymphangitis:
1. Glanders caused by Burkholderia mallei
2. Strangles caused by Streptococcus equi var. equi
3. Sporotrichosis caused by Sporothrix schenckii
4. Melioidosis caused by Burkholderia pseudomallei
5. Caseous lymphangitis caused by Corynebacterium pseudotuberculosis
6. Cryptococcosis caused by Cryptococcus neoformans
7. Ophthalmic and cutaneous Habronemiasis caused by Habronema muscae, H. microstoma and Draschia megastoma
Transmission
Equine epizootic lymphangitis zoonotic capability is not yet fully understood, but human infection has been described [5,19,33,50,]. Animal to animal transmission can occur through inhalation, ingestion, flies, fomites and through direct contact. The skin of equids gets infected by the fungus through direct contact by an open wound, which is the most common route of infection. This way of transmission was confirmed by Singh through experimental infection experiments in India [22,51]. The organism requirs a point of trauma to gain access through the skin reproduced EEL experimentally in 2 naive horses, one horse was injected with pus aspirated from an unruptured abscess of an EEL horse into the prescapular and prefemoral lymph nodes, 0.2 ml each [52]. Additionally, also 0.2 ml of pus were brushed on scarified skin of the inner hindleg, nasal membrane and also dropped onto the conjunctiva. The second horse was infected with mycelial form of HCF grown in vitro. HCF which was grown in vitro was injected into the same lymph nodes and brushed on the same scarified skin as well as dropped onto the conjunctiva as performed in the first case. Typical EEL lesions appeared after 4 weeks at many sides of the first horse’s body, infected with the yeast form of the fungus, whereas similar lesions appeared after three months, only at the infected lymph nodes and scarified areas. HCF was re-isolated from both horses, confirming the agreement of Koch’s postulate. This experiment has opened the way for a vaccine production.
The disease spreads frequently when large populations of equids are stabled together in a small area. It is reported that the fungus can stay viable in stable dust for a month [13]. Poor hygiene and poor stable management reinforce the spread of the fungus.
Flies are considered as vector for EEL and HCF and have been isolated from the alimentary tract of biting flies which had fed on open lesions. The disease developed in horses three miles away from nearest cases [53,54]. EEL lesions were also found on genitals of mares and therefore it is believed that the disease can be transmitted venereally [55]. EEL can be transmitted during mounting from the stallion to the mare and mare’s milk may infect suckling foals [27].
When testing samples from equids or laboratory infected animals, the yeast form is isolated only, as it grows at temperatures above 300°C. Therefore, in histology samples obtained from abscesses or biopsies, the yeast form of HCF is found. The mycelia or mold form lives in moist soil and beddings at lower temperatures for example in horse stables where spores are produced. These spores may be inhaled producing the pneumonic EEL form or may enter skin wounds when animal roll on the ground. In lungs and skin wounds, the fungus converts into yeast form.
Treatment and prevention
No completely satisfactory treatment is known yet. Surgical excision of lesions combined with antifungal drugs could be used, but these treatments are limited due to financial constraint and availability of the drug. SPANA, Ethiopia has developed a protocol for the treatment of infected equids and also advises horse owners how to react in controlling the disease. The details of these strategies are laid down by Scantlebury and Reed [13]. In Ethiopia and other African countries where the disease occurs, disease control measures were laid down by SPANA, which includes educating horse owners to regularly clean horse equipment, fly control, preventing wounds by proper harnessing, routine deworming and protecting their horses from attacks of feral dogs and hyenas. Horses are often housed on mud flooring which is the major source of contamination. Therefore, floors must be regularly cleaned and if possible disinfected.
The OIE, WOAH also has previously recommended, how to control EEL, but particularly in developing countries these recommendations are often impracticable as most of the equid owners are very poor and cannot afford any financial restraint [2]. WOAH recommendation includes culling of infected horses and application of stringent hygiene practices to prevent the spread of the fungus as well as movement restrictions. Many treatment types have been tried with disappointing results [58]. Differences in response to treatment between cases in early and advanced stages were also reported [58,59]. N-butanal extracts from berries of the plant Phytolacca dodecandra were also used and showed antifungal effects in laboratory experiments Hadush, et al. Evaluated the efficacy of Sodium Iodide (NaI), Potassium Iodide (KI) and phytochemical (Endod) extract as well as Penstrip for horses with EEL. They found statistically significant difference in the therapeutic effect of this treatment.
Two vaccines which are not commercially available have been developed some time ago. A live attenuated vaccine which was tested in China may protect 75% of horses vaccinated. The fungus for the vaccine production was isolated from an infected horse in Manchuria, Mongolia and named T21. More than 70 subcultures were made using 14 different temperature changes for the growth which took in total 620 days. Small rough colonies were selected from each subculture which all the time produced small rough and large fungal colonies when subcultured. When 71 subcultures were reached, no more subcultures were produced. For the vaccination experiment, fungal subcultures were used from different subcultures, but it is not clear if both colony morphological strains were used for the attenuated vaccine. However, the best results were achieved with passage 71 of strain T21 with a protection of 75.5% immunized horses, subcutaneously administered with a dose of 3 ml.
The second vaccine which is also not commercially available was produced by Al-Ani [33]. It is a formalinized inactivated vaccine, prepared from yeast form of the fungus. It was administered subcutaneously at a dose of 5 ml once a year with good results. No information was given how this vaccine was produced.
Conclusion
Equine Epizootic Lymphangitis (EEL) is a fungal, chronic pyogranulomatous multifocal disease of the skin, lymph vessels, and lymph nodes of limbs and neck of Equidae, caused by a dimorphic fungus Histopasma Capsulatum var. Farciminosum (HCF). Apart from a significant health issue for the horse, it has a severe socio-economic impact on many Ethiopian families living in the lowlands relying solely on cart horses as their source of income. EEL is a disease that is prevalent in areas with high humidity and temperature and it is very difficult to control it. The disease is particularly prevalent in the Debre Zeit region located in Bishoftu, south of the Ethiopian capital Addis Ababa. During multiple visits to Bishoftu, a team of scientists from the Central Veterinary Laboratory (CVRL) in Dubai, UAE made themselves acquainted with the highly prevalent EEL in this region. Based on a Cooperation Agreement between CVRL and Addis Ababa University (AAU), College of Veterinary Medicine, more than hundred samples were collected from infected equids by the CVRL team at the Society for the Protection of Animals (SPANA), equine hospital and the Donkey Sanctuary. These specimens were then shipped to CVRL for fungal culture with the aim of eventually producing a vaccine against EEL. Additionally, an antibody ELISA is under development to evaluate the prevalence of the disease in Ethiopia and assess the immune response after vaccination. This collaboration holds promise for the development of effective measures to control EEL and to reduce the disease impact on the equid population, improving the livelihood of Ethiopian families, depending on healthy carthorses.
Acknowledgments
The authors are indebted to the ruler of Dubai and vice president of the United Arab Emirates, HH. Sheikh Mohammed Bin, Rashid Al Maktoum and Dr. Ali Ridha, Director General of the Central Veterinary Research Laboratory, Dubai for their financial and scientific support conducting this research project. We also thank the team from the Ethiopian Donkey Trust (SPANA) in Bishoftu (Debre Zeit), Ethiopia especially Dr. Getachew Mulugeta and Dr. Gezahegne Mamo and Dr. Hanna Zewdu for allowing and helping us with specimen collection from EEL infected equids. A special thanks go to the Dean of the Veterinary Faculty in Bishoftu Prof. Hika Waktole.
References
- Stringer A, Lunn DP, Reid S. Science in brief: report on the first Havemeyer workshop on infectious diseases in working equids, Addis Ababa, Ethiopia, November 2013. Equine Vet J. 2015;47:6-9.
[Crossref] [Google Scholar] [PubMed]
- Stear, M.J. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees) 5th Edn. Volumes 1 & 2. World Organization for Animal Health 2004. ISBN 92 9044 622 6.€ 140. Parasitology. 2005;130(6):727-727.
- Ameni G, Siyoum F. Study on histoplasmosis (epizootic lymphangitis) in cart-horses in Ethiopia. J of Vet Sci. 2002;3:135-139.
[Crossref] [Google Scholar] [PubMed]
- Ameni G. Epidemiology of equine histoplasmosis (epizootic lymphangitis) in carthorses in Ethiopia. J VET J. 2006;172(1):160-165.
[Crossref] [Google Scholar] [PubMed]
- Guerin C, Shibru S, Touati F. Lymphangite epizootique du cheval en Ethiopie. J Mycol. 1992;2:1-5.
- Scantlebury CE, Zerfu A, Pinchbeck GP, Reed K, Gebreab F, Aklilu N, et al. Participatory appraisal of the impact of epizootic lymphangitis in Ethiopia. Prev Vet Med. 2015;120(3-4):265-276.
[Crossref] [Google Scholar] [PubMed]
- Stringer AP, Bell CE, Christley RM, Gebreab F, Tefera G, Reed K, et al. A cluster-randomised controlled trial to compare the effectiveness of different knowledge-transfer interventions for rural working equid users in Ethiopia. Prev Vet Med. 2011;100(2):90-99.
[Crossref] [Google Scholar] [PubMed]
- Weeks RJ, Padhye AA, Ajello L. Histoplasma capsulatum variety farciminosum: A new combination for Histoplasma farciminosum. Mycologia. 1985;77:964-970.
- Markey B, Leonard F, Archambault M, Cullinane A, Maguire D. Clinical Veterinary Microbiology. 2013;2:497-504.
- Kasuga T, White TJ, Koeing G et al. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol. 2003;12:3383-3401.
[Crossref] [Google Scholar] [PubMed]
- Kasuga T, Taylor JW, White TJ. Phylogenetic relationships of varieties and geographical groups of the human pathogenic fungus histoplasma capsulatum darling. J Clin Microbiol.1999;37:653-663.
- Ameni G. Pathology and clinical manifestation of epizootic lymphangitis in cart mules in Ethiopia. J Equine Sci. 2007;18:1-4.
- Aiello SE, Moses MA, Allen DG. The Merck Veterinary Manual:Epizootic Lymphangitis (11thed). Wiley. 201;6:639-640.
- Wernery U. Caseous lymphadenitis (Pseudotuberculosis) in camelids. J Camel Pract Res. 2012;19(1):21-27.
- Al-Ani FK. Epizootic lymphangitis in horses: A review of the literature. Rev Sci Tech. 1999;18:691-695.
[Crossref] [Google Scholar] [PubMed]
- Dalling T, Robertson A, Bodie GF, Spruell JSA. Diseases of camels proceedings of the international encyclopedia of veterinary medicine, ed. Green W and Son; Edinburgh.1966.
- Chandel BS, Kher HN. Occurrence of histoplasmosis like disease in camel (Camelus dromedarius). Indian Vet. J. 1994;71:521-523.
- Purohit NR, Chouhan DS, Choudhary RJ. Lymphangitis in the camel (two cases). Agr Practice. 1985;6:23-24.
- Murata Y, Sano A, Ueda Y, Inomata T, Takayama A, Poonwan N, et al. Molecular epidemiology of canine histoplasmosis in Japan. Sabouraudia. 2007;45(3):233-247.
[Crossref] [Google Scholar] [PubMed]
- Ueda Y, Sano A, Tamura M, Inomata T, Kamei K, et al. Diagnosis of histoplasmosis by detection of the internal transcribed spacer region of fungal rRNA gene from a paraffin-embedded skin sample from a dog in Japan. Vet Microbiol. 2003;94:219-224.
[Crossref] [Google Scholar] [PubMed]
- Herve V, Le Gall-Campodonico P, Blanc F, Improvisi L, Dupont B, Mathiot C, et al. Histoplasmose a Histoplasma farciminosum chez un cheval Africain. J Mycologie Med.1994;4:54.
- Singh T. Studies on epizootic lymphangitis. Study of clinical cases and experimental transmission. Indian Vet J. 1966;36(1):45-59.
- Chaves-Carballo E. The tropical world of Samuel Taylor Darling: Parasites, pathology and philanthropy. Sus Acad Press.2007;260.
- Gossel P. Bulletin of the History of Medicine:Medical Sciences. Johns Hopkins University.2004;503-504.
- Daniel TM, Baum GL. Drama and discovery: the story of histoplasmosis. Greenwood Publishing Group. 2002.
- Lukela JR. Histoplasmosis. JAMA. 1961;178:321-322.
- Hutyra JV, Marek J, Manninger R. Spezielle Pathologie and Therapie der Haustiere. Lymphangioitis epizootica Gustav Fischer Verlag, Jena. 1945;631-641.
- OIE Update for epizootic lymphangitis. USA: Center for Food Security and Public Health (CFSPH), and Institute for International Cooperation in Animal Biologics, an OIE Collaborating Centre. 2009.
- Science in brief: report on the first Havemeyer workshop on infectious diseases in working equids, Addis Ababa, Ethiopia, November 2013
- Ameni G. Equine epizootic lymphangitis in cart-horses in Ethiopia: Clinical, epidemiological and economic features. Revue Scie et Tecn. 2002;21:711-719.
[Crossref] [Google Scholar] [PubMed]
- Bardelli P, Ademollo A. Sulla resistenza delle Conture di Cryptococcus farciminosus. Rivolta agli agenti fisici e chimici. Annual Igiene. 1927;37:81-85.
- Bullen JJ. The yeast-like form of Cryptococcus farciminosus (Rivolta): (Histoplasma farciminosum). J Pathol Bacteriol. 1949;61:117-120.
- Habich C, Kempe K, Gomez FJ, Lillicrap M, Gaston H, van der Zee R, et al. Heat shock protein 60: identification of specific epitopes for binding to primary macrophages. FEBS letters. 2006;580(1):115-120.
[Crossref] [Google Scholar] [PubMed]
- Garfoot AL, Dearing KL, VanSchoiack AD, Wysocki VH, Rappleye CA. Eng1 and Exg8 Are the Major α-Glucanases Secreted by the Fungal Pathogen Histoplasma capsulatum. J Biol Chem. 2017;292(12):4801-4810.
[Crossref] [Google Scholar] [PubMed]
- Sebghati TS, Engle JT, Goldman WE. Intracellular parasitism by Histoplasma capsulatum: Fungal virulence and calcium dependence. Science. 2000;290(5495):1368-1372.
[Crossref] [Google Scholar] [PubMed]
- Selim SA, Soliman R, Osman K, Padhye AA, Ajello L. Studies on histoplasmosis farciminosi (epizootic lymphangitis) in Egypt: Isolation of Histoplasma farciminosum from cases of histoplasmosis farciminosi in horses and its morphological characteristics. Eur J Epidemiol. 1985;1:84-89.
[Crossref] [Google Scholar] [PubMed]
- Ajello L. Comparative morphology and immunology of members of the genus histoplasma: A review. Mycoses. 1968;11(7):507-514.
[Crossref] [Google Scholar] [PubMed]
- Gabal MA, Hassan FK, Said AA, Karim KA. Study of equine histoplasmosis and characterization of Histoplasma farciminosum. Sabouraudia. 1983;21:121-127.
[Crossref] [Google Scholar] [PubMed]
- Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actin Omycetes. (3rdedn). WB Sounders Company, Philadelphia, USA. 1988;325-352.
- Buxton A, Fraser G. Animal Microbiology:Immunology, bacteriology, mycology, diseases of fish and laboratory methods.(1stvol), Blackwell Sci. Publications, London. 1977;310.
- Gabal MA, Hennager S. Study on the survival of Histoplasma farciminosum in the environment/experimentelle untersuchungen zur lebensfähigkeit von Histoplasma farciminosum. Mycoses. 1983;26(9):481-487.
[Crossref] [Google Scholar] [PubMed]
- Svendsen ED, Duncan J, Hadrill D. Epizootic lymphangitis in: The Professional Handbook of the Donkey. The Donkey Sanctuary, 2008;10.
- Saleh M. The Professional Handbook of the Donkey:Lacrimal Histoplasmosis in Donkeys. (2ndedn). ed. ED Svendsen. Whihet Book Limited, London. 1989.
- Heragy AM. Lacrimal histoplasmosis in the working donkeys of Luxor village. Proceedings of the International Colloquium on Working Equines, Al Baath University, Hama, Syria. 2003;20-26.
- Ameni G, Terefe W. A cross-sectional study of epizootic lymphangitis in cart-mules in western Ethiopia. Prev Vet Med. 2004;66(1-4):93-99.
[Crossref] [Google Scholar] [PubMed]
- Ameni G, Siyoum F. Study on histoplasmosis (epizootic lymphangitis) in cart-horses in Ethiopia. J Vet Sci. 2002;3(2):135-139.
[Crossref] [Google Scholar] [PubMed]
- Gabal MA, Khalifa K. Study on the immune response and serological diagnosis of equine histoplasmosis (epizootic lymphangitis). Zentralbl Veterinarmed B. 1983;30(1‐10):317-321.
[Crossref] [Google Scholar] [PubMed]
- Ameni G, Terefe W, Hailu A. Histofarcin test for the diagnosis of epizootic lymphangitis in Ethiopia: development, optimisation, and validation in the field. The Vet J. 2006;171:358-362.
[Crossref] [Google Scholar] [PubMed]
- Chandler FW, Kaplan W, Ajello L. Histoplasmosis duboisii. A Colour Atlas and Textbook of the Histopathology of Mycotic Diseases. Wolfe Medical Publications.1980:67-69.
- Singh T. Studies on epizootic lymphangitis: Clinical cases and experimental transmission. Indian J Vet Sci. 1966;36:45-59.
- Ameni G. Preliminary trial on the reproducibility of epizootic lymphangitis through experimental infection of two horses. Vet J. 2006;172(3):553-555.
[Crossref] [Google Scholar] [PubMed]
- Treviño GS. Canine blastomycosis with ocular involvement. Pathol Vet. 1966;3(6):652-658.
[Crossref] [Google Scholar] [PubMed]
- White EG, Jordan FT. Veterinary Preventive Medicine. (4thedn). Williams and Wilkins, Baltimore, US.1963.
- Jubb KV, Kennedy PV. Pathology of domestic animals: integumentary system.(1stvol). Academic Press, New York. 1970:656.
- Mekonnen N, Makonnen E, Aklilu N, Ameni G. Evaluation of berries of Phytolacca dodecandra for growth inhibition of Histoplasma capsulatum var. farciminosum and treatment of cases of epizootic lymphangitis in Ethiopia. Asian Pac J Trop Biomed.2012;2:505-510.
[Crossref] [Google Scholar] [PubMed]
- Endebu B. Epidemiology of Epizootic and Ulcerative Lymphangitis in Ethiopia: Retrospective Analysis and Cross-sectional Study and Treatment Trial at Debre-Zeit and Akaki, DVM Dissertation, Addis Ababa University. 1996;1-84.
- Siyoum F, Debre Zeit. Histoplasmosis farciminosi (Epizootic lymphangitis): study on the epidemiology, socioeconomic importance and treatment trials; 2001.
- Hadush B, Ameni G, Medhin G. Equine histoplasmosis: Treatment trial in cart horses in Central Ethiopia. Trop Anim Health Prod. 2008; 40:407-411.
[Crossref] [Google Scholar] [PubMed]
- Zhang WT, Wang ZR, Liu YP, Zhang DL, Liang PQ, Fang YZ, et al. Attenuated vaccine against epizootic lymphangitis of horses. Chin J vet Sci Technol. 1986;7:3-5.
Citation: Mheiri FGA, Wernery U (2023) Equine Epizootic Lymphangitis: A Synopsis. J Trop Dis. 11:396.
Copyright: &©; 2023 Mheiri FGA et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

