Indexed In
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2022) Volume 6, Issue 1
End-Stage Heart Failure in Becker’s Muscular Dystrophy: A Case Report of Perioperative Management for Oncological Surgery during COVID-19 Pandemic
Piovano Chiara1*, Savi M1,2, Babbini M1, Greco M1,2, Monzani R1 and Cecconi M1,22Department of Anesthesiology and Intensive Care, Humanitas University, Milan, Italy
Received: 15-Dec-2021, Manuscript No. JSA-21-14991; Editor assigned: 17-Dec-2021, Pre QC No. JSA-21-14991 (PQ); Reviewed: 31-Dec-2021, QC No. JSA-21-14991; Revised: 03-Jan-2022, Manuscript No. JSA-21-14991 (R); Published: 10-Jan-2022, DOI: 10.35248/2684-1606.22.05.165
Abstract
Background: Becker’s Muscular Dystrophy (BMD) is an X-linked recessive disease caused by dystrophin deficiency and characterized by progressive skeletal muscle weakness and functional impairment especially involving proximal muscles of the lower limbs while resulting in several comorbidities, including restrictive respiratory insufficiency and dilated cardiomyopathy.
Materials and methods: We report a case of successful intraoperative management in a 63- year- old ASA IV patient affected by BMD associated with several comorbidities who presented with ingravescent dyspnea and dysphagia due to a supraglottic laryngeal carcinoma. He is also affected by a dilated cardiomyopathy with congestive heart failure (ACC/AHA stage C), chronic atrial fibrillation and pulmonary hypertension; he carries also a Cardiac-Resynchronization Therapy-Defibrillator (CRT-D).
Results: Total intravenous anaesthesia with ketamine allowed us to avoid propofol-induced cardiodepressant effects and spare perioperative high doses of opioids, granting hemodynamic stability and optimal pain control throughout the procedure. A challenging intraoperative management was implemented by using an intravenous strategy to solve the specific problems presented by this case.
Conclusion: This case highlights why it is useful for anesthetists to be familiar with multiple drugs in combination to prevent deleterious events on a potential critical patient who eventually did not require intensive care support after surgery, saving precious ICU resources especially scarce during COVID-19 surge.
Keywords
Becker’s muscular dystrophy; Ketamine; Perioperative management; Heart congestive failure
Introduction
Becker’s muscular dystrophy is a progressive neuromuscular degenerative disorder due to mutations of DMD gene with a milder clinical evolution compared to Duchenne’s muscular dystrophy thanks to the major availability of functional dystrophin. Concerns regarding the perioperative management of such patients have been raised due to the potential harmful consequences of general anaesthesia in the perioperative period. Cases of volatile anaesthetic- induced rhabdomyolysis are reported in literature, therefore a total intravenous strategy has to be adopted and tailored upon the patient’s history and comorbidities [1-3].
Case Presentation
Our patient is an ASA IV 63-year-old man, obesity grade 1 (95 kg, 174 cm-BMI 31.4) affected by a supraglottic laryngeal carcinoma provoking severe dysphagia for solid food and mild dyspnoea. At the age of 29 he was diagnosed with Becker’s myopathy leading to the permanent loss of lower limbs motility and cardiogenic heart failure (ACC/AHA Stage C). Since 2000, he was diagnosed with an ingravescent dilated cardiomyopathy; at transthoracic echocardiography, severe systolic dysfunction (EF=25%) and grade III diastolic dysfunction (EDV=290 ml) were detected, as well as pulmonary hypertension (mild tricuspidal valve regurgitation, estimated PAPs=56+5 mmHg). As he developed chronic Atrial Fibrillation (AF), a CRT-D was implanted in 2011. Respiratory function was preserved (Tiffeneau index>70, VFC>50%). A perioperative evaluation was performed two weeks before surgery. A neck and thorax CT scan and routine lab test were performed (Figure 1).
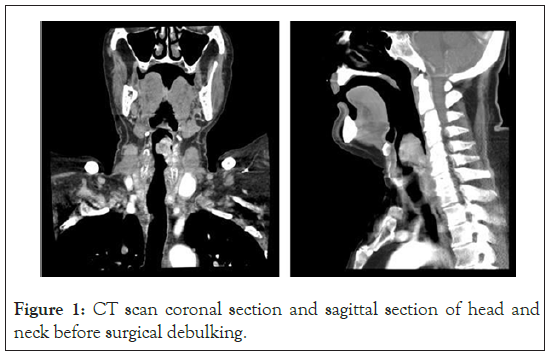
Figure 1: CT scan coronal section and sagittal section of head and neck before surgical debulking.
At his arrival at the surgical unit, the patient was monitored; two large-bore venous lines and a radial arterial line were placed. A magnet was positioned over the CRT-D. AF was the background rhythm. In the operating room, a surgical tracheostomy in local anaesthesia was performed and a tracheal cannula was placed. Then continuous infusion on remifentanil (0.05 mcg.kg-1 IBW) and ketamine (2-4 mcg.kg-1 min IBW) were started and a bolus of lidocaine (0.5 mg.kg-1 IBW) and dexamethasone (0.1 mg.kg-1 ) were delivered, followed by the induction of general anaesthesia combining a bolus of midazolam (0.08 mg.kg-1 IBW) and ketamine (0.5 mcg.kg-1 IBW). Neuromuscular blockade was obtained by administering rocuronium (0.3 mg.kg-1 LBW) and monitored through train-of-four stimulation; afterwards laser-tracheal tube was placed through the tracheostomy. Maintenance was conducted by administering ketamine (2-4 mcg.kg-1 .min IBW) and remifentanil (0.03-0.08 mcg.kg-1 .min IBW); anaesthetic depth was assessed by Bispectral Index Monitoring (BIS), ranging from 50 to 60 throughout the procedure. To ensure stability of the anaesthetic plan, micro doses of midazolam 0.03-0.05 mg/ kg-1 has been titled approximately every 60’ (the last micro dose 40 min before the end of the surgery). A protective mechanical ventilation strategy was adopted (tidal volume=6 ml.kg-1 PBW and positive-expiratory end pressure=5 cm H2O, respiratory rate=12 breaths min-1 ). Therefore, the tumor debulking was performed through micro direct laryngoscopy. Magnesium sulphate (2 g) was administered as adjuvant for pain control; mean arterial pressure target was set equal or above 65 mmHg, for lower values boluses of noradrenaline (10 mcg) were administered. Two blood gas analyses were performed during the procedure; lactates never raised above 1,4 mmol.L-1. Acetaminophen (1 g), ibuprofen (600 mg) and tramadol (50 mg) were administered as pain relievers starting at the induction and continuing during procedure, as well as metoclopramide (10 mg) added to dexamethasone for post- operative nausea and vomiting prophylaxis. After surgery, which lasted 150 minutes, sugammadex (2 mg.kg-1 ) was administered to reverse the neuromuscular blockade and the patient was awakened from anaesthesia and extubated. We did not register any delay to awake.
Results
He was kept under observation in the recovery room for 4 hours, spontaneously breathing with low- flow oxygen (4 l.min-1) delivered by tracheomask, then he was transferred to the ordinary ward where telemetry monitoring was set for the following 24 hours. During hospital stay he did not complain of pain or discomfort. He was discharged from the hospital 3 days after surgery. We conducted a monthly follow-up phone call for three months, to monitor his quality of life and functional capacity. No worsening in his cardiopulmonary function was detected after discharge.
Discussion
Our perioperative management allowed us to preserve hemodynamic stability and optimal pain control throughout the procedure. It is essential to develop different strategies for the maintenance of anaesthesia since the dystrophic patients who are exposed to inhaled anaesthetics may develop disease-related cardiac complications, or rarely, a malignant hyperthermia-like syndrome characterized by rhabdomyolysis, which can occur intraoperatively or postoperatively.
We adopted therefore a total intravenous strategy, focusing on the versatile properties of ketamine both on anaesthesia and analgesia and its favorable profile on hemodynamic status [4]. We decided to avoid propofol use because of its intense hemodynamic impact, reducing vascular resistances both on venous and arterial side, possibly affecting mean arterial pressure. Relatively common and generally benign events (transient hypotension, positive-pressure mechanical ventilation, modest hypercarbia) can have potentially serious consequences in a patient at high risk of acute pathological derangements during general anaesthesia. In hemodynamically unstable patients, ketamine has shown to improve heart rate, systolic blood pressure and significantly reduce fluid requirements, which is pivotal in case of severe EF reduction.
The presence of heart failure has been described as the main independent risk factor for predicting perioperative cardiac morbidity and mortality, leading to the development of postoperative complications, including atelectasis and pneumonia. One of our main goals was to promote an adequate left-sided filling and contractility to grant systemic perfusion and avoid sudden increases both in left ventricular preload and afterload. Ketamine continuous infusion allows an important reduction of opioids administration, enhances their analgesic properties without decreasing the endogenous sympathetic tone [5]. It has been demonstrated in vivo that opioids inhibit T-lymphocyte proliferation and suppress NK cell cytotoxicity; postoperative acute pain also seems to have an immunosuppressive and pro- metastatic effect, therefore every effort has to be done to control pain with alternative drugs [6].
Ketamine administration in case of pulmonary hypertension is still debated. suggested to avoid its use for potential increase in pulmonary vascular resistances but their review was based on studies conducted in spontaneously breathing, healthy volunteers [7]. Recent studies under more controlled conditions have reported no increase in Pulmonary Vascular Resistances (PVR) and an excellent safety profile in patients with pulmonary hypertension. Under high sympathetic tone conditions, ketamine actually may dilate the pulmonary vascular smooth muscle, as shown in several animal models [8]. Although the potential deleterious effects of ketamine on PVR remain a concern, they must be balanced against the benefits in terms of analgesia and hypnosis in absence of significant myocardial depression and vasodilation.
We did not observe any worsening in terms of respiratory, cardiac and neuromuscular function during hospital stay. According to his caregiver (namely, his sister), his quality of life and functional capacity improved after discharge, thanks to the resolution of dyspnoea and an extreme reduction of his dysphagia, passing from a totally liquid diet to a soft-solid diet [9].
Conclusion
In conclusion, this case report offers a different perspective for the intraoperative management of a frail patient with ketamine infusion. As reported by, COVID-19 pandemic has caused an important delay in diagnosis and surgical treatment of oncological patients, with reduction in 5-year net survival consequently. Our hospital effort is to maintain a safe pathway of oncological patient in order to mitigate the deleterious effect of the COVID-19 pandemic. The transferral to the ordinary ward allowed us to save resources such as ICU bed and staff without deferring surgery.
Conflict of Interest
The authors declare the following conflicts of interest:
P.C., S.M., B.M.A., G.M. and M.R.E. declare no external funding and no competing interests.
C.M. reports consulting for Edwards Lifesciences, Directed Systems, and Cheetah Medical.
Acknowledgments
This case report was published with the written consent of the patient. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments.
REFERENCES
- Carenzo L, Costantini E, Greco M, Barra FL, Rendiniello V, Mainetti M, et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia. 2020; 75(7): 928-934.
[Crossref], [Google Scholar], [PubMed]
- Poole TC, Lim TY, Buck J, Kong AS. Perioperative cardiac arrest in a patient with previously undiagnosed Becker's muscular dystrophy after isoflurane anaesthesia for elective surgery. Br J Anaesth. 2010; 104(4): 487-489.
[Crossref], [Google Scholar], [PubMed]
- Gurnaney H, Brown A, Litman RS. Malignant hyperthermia and muscular dystrophies. Anesth Analg. 2009; 109(4): 1043-1048.
[Crossref], [Google Scholar], [PubMed]
- Kamp J, Olofsen E, Henthorn TK, Van Velzen M. Ketamine Pharmacokinetics. Anesthesiology. 2020; 133(6): 1192-1213.
- Gupta A, Devi LA, Gomes I. Potentiation of μ-opioid receptor-mediated signaling by ketamine. J Neurochem. 2011; 119(2): 294-302.
[Crossref], [Google Scholar], [PubMed]
- Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010; 105(2): 106-115.
[Google Scholar], [PubMed]
- Strumpher J, Jacobsohn E. Pulmonary hypertension and right ventricular dysfunction: Physiology and perioperative management. J Cardiothorac Vasc Anesth. 2011; 25(4): 687-704.
[Crossref], [Google Scholar], [PubMed]
- Kaye AD, Banister RE, Fox CJ, Ibrahim IN, Nossaman BD. Analysis of ketamine responses in the pulmonary vascular bed of the cat. Crit Care Med. 2000; 28(4): 1077-1082.
[Crossref], [Google Scholar], [PubMed]
- Sud A, Torr B, Jones ME, Broggio J, Scott S, Loveday C, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol. 2020; 21(8): 1035-1044.
[Crossref], [Google Scholar]
Citation: Chiara P, M Savi, M Babbini, M Greco, R Monzani, M Cecconi (2022) End-Stage Heart Failure in Becker’s Muscular Dystrophy: A Case Report of Perioperative Management for Oncological Surgery during COVID-19 Pandemic. J Surg Anesth. 6:165.
Copyright: © 2022 Chiara P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
