Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2021) Volume 9, Issue 2
En-Bloc Kidney Transplant Using an Aortic Extension from a Paediatric Multi-Visceral Donor to a Paediatric Recipient
Sergio Assia-Zamora1,2*, Chris Callaghan1,2, Ioannis Loukopoulos1,2, Martin Drage1,2, Michael Ramage1,2, Steve Marks2,3, Jelena Stojanovic2, Francis Calder1,2 and Nicos Kessaris1,22Department of Paediatric Nephrology, Great Ormond Street Hospital for Children NHS Foundation Trust, Great Ormond Street, London, WC1N 3JH, UK
3NIHR Great Ormond Street Hospital Biomedical Research Centre, University College London Great Ormond Street Institute of Child Health, London, WC1N 1EH, UK
Received: 15-Feb-2021 Published: 08-Mar-2021, DOI: 10.35248/2329-6925.21.9.408
Abstract
Renal transplantation is the gold standard treatment for end stage kidney disease (ESKD) in children. EBKT from small paediatric donors under five years of age and weighing less than 20 kg can be a good option for selected paediatric renal transplant recipients. EBKT refers to transplantation of both kidneys from the same donor into a single recipient. The utilisation of these organs has a higher risk of vascular thrombosis, stenosis and ureteral leak in children.
We present the case of a successful EBKT, which was performed with an aortic extension using a segment of the aortic arch since the donor operation included multivisceral donation requiring the patch of the Superior Mesenteric Artery (SMA). This is the first description of utilising the thoracic aorta as an extension graft for EBKT in paediatric multivisceral donor for a paediatric recipient.
The aorta was reconstructed and the en-bloc kidneys were successfully implanted into the left iliac fossa onto the iliac vessels.
Keywords
Renal transplantation; Paediatric; Kidneys; Vascular thrombosis; EBKT
Introduction
Renal transplantation is the gold standard treatment for end stage kidney disease (ESKD) in children. It improves quality and quantity of life as well as physical and cognitive development. Dialysis is associated with six time’s lower patient survival, poor physical growth and lowers than average neurodevelopment [1].
En-bloc kidney transplantation (EBKT) was first described by Carrel in 1908 in a xenograft model [2]. EBKT from small paediatric donors under five years of age and weighing less than 20 kg can be a good option for selected paediatric renal transplant recipients. EBKT refers to transplantation of both kidneys from the same donor into a single recipient [3]. The utilisation of these organs has a higher risk of vascular thrombosis, stenosis and ureteral leak in children [4,5]. Recently, better outcomes have been demonstrated with the improvement of anticoagulation protocols and refinement of surgical techniques [3].
We present the case of a successful EBKT, which was performed with an aortic extension using a segment of the aortic arch since the donor operation included multivisceral donation requiring the patch of the Superior Mesenteric Artery (SMA). The aorta was reconstructed and the en-bloc kidneys were successfully implanted into the left iliac fossa onto the iliac vessels.
Case Description
Surgical procedure
The donor after brain death (DBD) was a two-year old, 15 kilogram child with irreversible hypoxic brain injury secondary to drowning, with good renal function, without co-morbidities. The procurement was carried outby the cardiothoracic retrieval team, a liver team as well as arenal retrieval team. The heart and lungs were procured as well as the liver and small bowel for a multivisceral transplant. The thoracic aorta, coeliac trunk and superior mesenteric artery (SMA) were retrieved as a whole tube. The renal arteries were retrieved beyond that as a second tube all the way to the bifurcation of the iliac arteries. As the recipient team, we were aware of the high risk of stenosis secondary to proximal closure of the aortic tube so an additional aortic arch segment was retrieved to reconstruct the suprarenal aorta on the back bench. Donor kidneys were recovered en-bloc with aorta, inferior vena cava and bilateral ureters in continuity to the bladder insertion. The Inferior vena cava (IVC) was procured including both renal veins and divided 5 mm above the confluence of the right renal vein to the IVC. All of the organs were perfused with University of Wisconsin (UW) solution and packed in an organ-transport-box at 0-4°C.
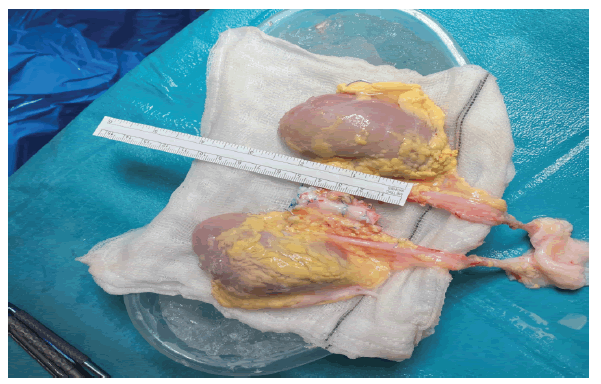
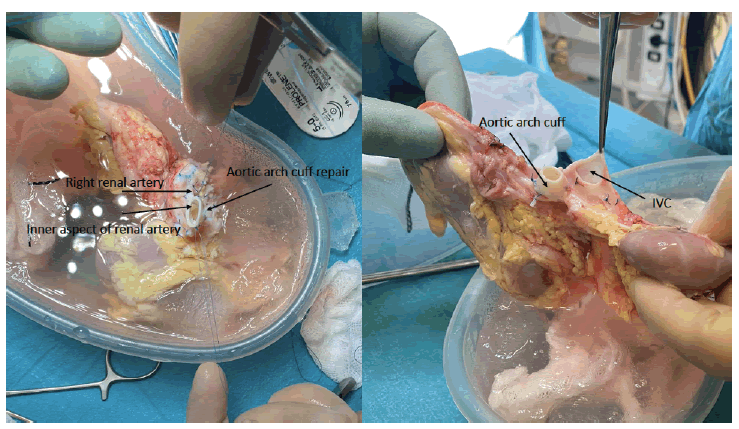
The en-bloc kidneys measured 7 and 7.5 cm. Back bench surgery was performed after removing additional fat and tissue adherent to the kidneys, all the branches of the aorta and IVC were ligated except for the renal blood supply (Figure 1). The suprarenal aorta and part of the proximal right renal artery were missing due to the nature of the multivisceral retrieval, therefore, these were reconstructed by the addition of a short portion of the aortic arch in an end-to-end fashion with 6-0 prolene running suture top end was closed with 5-0 prolene suture. Similarly, the top end of the IVC was closed with 5-0 prolene running suture (Figure 2). Both ureters were divided from the bladder.
Figure 1: En-block Kidneys on the back table. Both kidneys and the aorta have been prepared for the implant. Both ureters are joined onto the donor bladder.
Figure 2: Photos showing the upper aspect of the aorta with its aortic cuff extension and inferior vena cava. The inner aspect of the right renal artery is also shown following the repair.
An EBKT was performed following informed consent from the recipient family, acknowledging that there might be higher risk of technical complications. At the time of recipient assessment, special attention was taken into consideration including favourable vascular anatomy, adequate bladder capacitance and function, no history of thrombophilia, adequate cardiac function, absence of either significant pulmonary or systemic hypertension, no orthostatic or history of hypotension, absence of high-risk of recurrence of kidney disease.
The recipient was a 13 year old male with ESKD on peritoneal dialysis secondary to bilateral renal dysplasia and posterior urethral valves with previous valve resection, bladder augmentation and Mitrofanoff formation. The decision to implant into the left iliac fossa was taken as the Mitrofanoff was in the right iliac fossa and close to the anterior superior iliac spine. A Gibson incision was performed followed by careful dissection of the extraperitoneal space to the iliac vessels and augmented bladder.
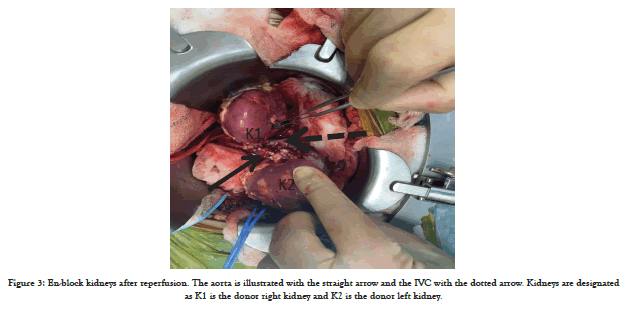
The en-bloc kidneys were implanted laterally in the extraperitoneal space. The en-bloc graft was resting on the psoas muscle; subsequently anastomosing the donor distal aorta end-to-side to the common iliac artery with 6-0 prolene running suture and the donor IVC to the external iliac vein end-to-side. Intravenous heparin was administered prior to reperfusion and the kidneys perfused well (Figure 3). There was a strong palpable thrill in the graft aorta and both renal arteries. Urine output was visible on the table from both kidneys.
Figure 3: En-block kidneys after reperfusion. The aorta is illustrated with the straight arrow and the IVC with the dotted arrow. Kidneys are designated as K1 is the donor right kidney and K2 is the donor left kidney.
The position of the kidneys were placed laterally and always taking extra caution in the definitive position to avoid any perfusion risk. The blood inflow was from the common iliac artery, through the distal aorta and then to both renal arteries, reason why the proximal closure was performed with the aortic extension. The outflow was performed from the renal veins to the inferior vena cava and then to the external iliac vein. It is important to take into account the final position of the EBKT, since there is a high risk of kinking of both renal arteries and veins.
The ureteric anastomosis was performed by spatulating the distal ends of both ureters and anastomosing the medial walls with 4-0 PDS running suture prior to the implantation onto the bladder. Two 6-french stents were introduced into both ureters and the bladder; subsequently the ureters were anastomosed to the bladder with 4-0 PDS running suture before closure.
While still in theatre and before waking up the patient, a Doppler ultrasound was performed. This showed good perfusion in one kidney but poor perfusion in the medial organ. Immediately the wound was reopened and a kinking was observed in the renal artery due to the limited retroperitoneal space. The decision to open the peritoneum was taken to release the pressure on the enbloc kidneys. A second ultrasound was performed demonstrating adequate flow into both kidneys after closure.
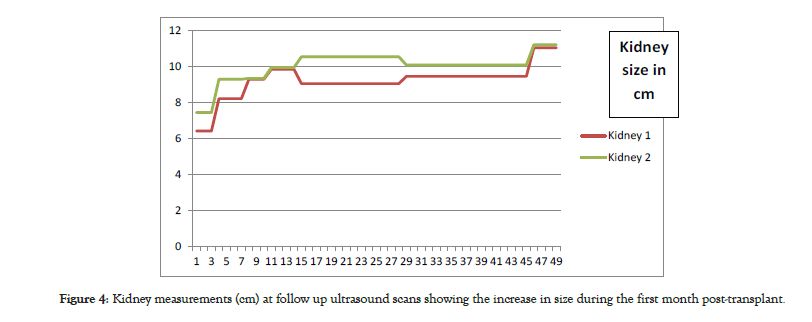
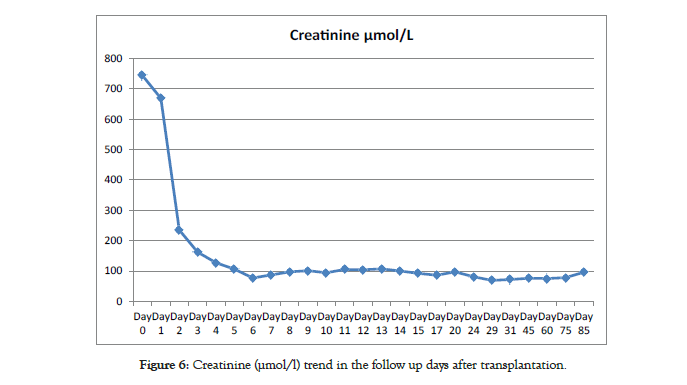
The patient was sent to recovery and finally back to the ward following satisfactory progress. Subcutaneous heparin was started as prophylaxis against graft thrombosis until discharge. Renal allograft function improved with decreasing plasma creatinine from 744 μmol/l pre-transplant to 235 μmol/l the day after transplant and then 97 μmol/l on day 8 post-transplant giving estimated glomerular filtration rate of 117.33 mls/min/1.73 m2. He was discharged from hospital at one week on aspirin 75 mg once daily [6]. Subsequent Doppler ultrasounds were satisfactory and measurements of the EBKT were performed (Figure 4).
Figure 4: Kidney measurements (cm) at follow up ultrasound scans showing the increase in size during the first month post-transplant.
Follow up
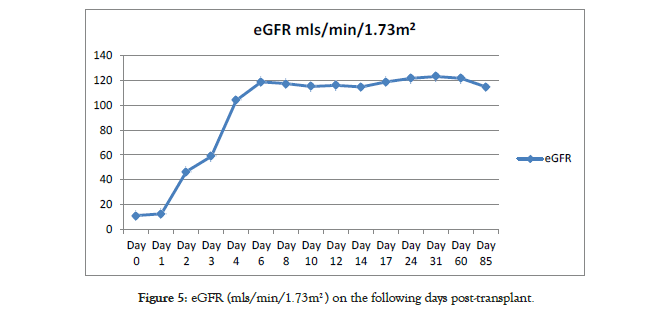
Immunosuppression included basiliximab 20 mg on day 0 and day 4, tacrolimus 0.15 mg/kg twice daily, mycophenolate mofetil 600 mg/m2 twice daily and rapid weaning of corticosteroids over five days. Both stents were removed 28 days after the surgery. The patient is followed up in the post-transplant clinic as per our follow up protocol showing satisfactory progress and stable renal allograft function (Figures 5 and 6).
Figure 5: eGFR (mls/min/1.73m2 ) on the following days post-transplant.
Surgical procedure
The donor after brain death (DBD) was a two-year old, 15 kilogram child with irreversible hypoxic brain injury secondary to drowning, with good renal function, without co-morbidities. The procurement was carried outby the cardiothoracic retrieval team, a liver team as well as arenal retrieval team. The heart and lungs were procured as well as the liver and small bowel for a multivisceral transplant. The thoracic aorta, coeliac trunk and superior mesenteric artery (SMA) were retrieved as a whole tube. The renal arteries were retrieved beyond that as a second tube all the way to the bifurcation of the iliac arteries. As the recipient team, we were aware of the high risk of stenosis secondary to proximal closure of the aortic tube so an additional aortic arch segment was retrieved to reconstruct the suprarenal aorta on the back bench. Donor kidneys were recovered en-bloc with aorta, inferior vena cava and bilateral ureters in continuity to the bladder insertion. The Inferior vena cava (IVC) was procured including both renal veins and divided 5 mm above the confluence of the right renal vein to the IVC. All of the organs were perfused with University of Wisconsin (UW) solution and packed in an organ-transport-box at 0-4°C.
The en-bloc kidneys measured 7 and 7.5 cm. Back bench surgery was performed after removing additional fat and tissue adherent to the kidneys, all the branches of the aorta and IVC were ligated except for the renal blood supply (Figure 1). The suprarenal aorta and part of the proximal right renal artery were missing due to the nature of the multivisceral retrieval, therefore, these were reconstructed by the addition of a short portion of the aortic arch in an end-to-end fashion with 6-0 prolene running suture top end was closed with 5-0 prolene suture. Similarly, the top end of the IVC was closed with 5-0 prolene running suture (Figure 2). Both ureters were divided from the bladder.
Figure 1: En-block Kidneys on the back table. Both kidneys and the aorta have been prepared for the implant. Both ureters are joined onto the donor bladder.
Figure 2: Photos showing the upper aspect of the aorta with its aortic cuff extension and inferior vena cava. The inner aspect of the right renal artery is also shown following the repair.
An EBKT was performed following informed consent from the recipient family, acknowledging that there might be higher risk of technical complications. At the time of recipient assessment, special attention was taken into consideration including favourable vascular anatomy, adequate bladder capacitance and function, no history of thrombophilia, adequate cardiac function, absence of either significant pulmonary or systemic hypertension, no orthostatic or history of hypotension, absence of high-risk of recurrence of kidney disease.
The recipient was a 13 year old male with ESKD on peritoneal dialysis secondary to bilateral renal dysplasia and posterior urethral valves with previous valve resection, bladder augmentation and Mitrofanoff formation. The decision to implant into the left iliac fossa was taken as the Mitrofanoff was in the right iliac fossa and close to the anterior superior iliac spine. A Gibson incision was performed followed by careful dissection of the extraperitoneal space to the iliac vessels and augmented bladder.
The en-bloc kidneys were implanted laterally in the extraperitoneal space. The en-bloc graft was resting on the psoas muscle; subsequently anastomosing the donor distal aorta end-to-side to the common iliac artery with 6-0 prolene running suture and the donor IVC to the external iliac vein end-to-side. Intravenous heparin was administered prior to reperfusion and the kidneys perfused well (Figure 3). There was a strong palpable thrill in the graft aorta and both renal arteries. Urine output was visible on the table from both kidneys.
Figure 3: En-block kidneys after reperfusion. The aorta is illustrated with the straight arrow and the IVC with the dotted arrow. Kidneys are designated as K1 is the donor right kidney and K2 is the donor left kidney.
The position of the kidneys were placed laterally and always taking extra caution in the definitive position to avoid any perfusion risk. The blood inflow was from the common iliac artery, through the distal aorta and then to both renal arteries, reason why the proximal closure was performed with the aortic extension. The outflow was performed from the renal veins to the inferior vena cava and then to the external iliac vein. It is important to take into account the final position of the EBKT, since there is a high risk of kinking of both renal arteries and veins.
The ureteric anastomosis was performed by spatulating the distal ends of both ureters and anastomosing the medial walls with 4-0 PDS running suture prior to the implantation onto the bladder. Two 6-french stents were introduced into both ureters and the bladder; subsequently the ureters were anastomosed to the bladder with 4-0 PDS running suture before closure.
While still in theatre and before waking up the patient, a Doppler ultrasound was performed. This showed good perfusion in one kidney but poor perfusion in the medial organ. Immediately the wound was reopened and a kinking was observed in the renal artery due to the limited retroperitoneal space. The decision to open the peritoneum was taken to release the pressure on the enbloc kidneys. A second ultrasound was performed demonstrating adequate flow into both kidneys after closure.
The patient was sent to recovery and finally back to the ward following satisfactory progress. Subcutaneous heparin was started as prophylaxis against graft thrombosis until discharge. Renal allograft function improved with decreasing plasma creatinine from 744 μmol/l pre-transplant to 235 μmol/l the day after transplant and then 97 μmol/l on day 8 post-transplant giving estimated glomerular filtration rate of 117.33 mls/min/1.73 m2. He was discharged from hospital at one week on aspirin 75 mg once daily [6]. Subsequent Doppler ultrasounds were satisfactory and measurements of the EBKT were performed (Figure 4).
Figure 4: Kidney measurements (cm) at follow up ultrasound scans showing the increase in size during the first month post-transplant.
Follow up
Immunosuppression included basiliximab 20 mg on day 0 and day 4, tacrolimus 0.15 mg/kg twice daily, mycophenolate mofetil 600 mg/m2 twice daily and rapid weaning of corticosteroids over five days. Both stents were removed 28 days after the surgery. The patient is followed up in the post-transplant clinic as per our follow up protocol showing satisfactory progress and stable renal allograft function (Figures 5 and 6).
Figure 5: eGFR (mls/min/1.73m2 ) on the following days post-transplant.
Figure 6: Creatinine (µmol/l) trend in the follow up days after transplantation.
Results and Discussion
There is evidence showing that EBKT has better long-term outcomes compared with adult standard criteria deceased donor kidney transplant (DDKT) [7,8]. In the case of living donor kidney transplants (LDKT), the long-term outcomes between EBKT and LDKT are similar [9,10]. We have reported the use of En-Bloc kidneys from a multivisceral donor utilising the thoracic aorta as an extension and therefore being able to utilise both kidneys for an EBKT. This is the first report in the literature using this type of graft in order to preserve both kidneys with the aorta.
EBKT was originally developed to increase the transplanted nephron mass and to overcome the technical challenges of small calibre vessels in paediatric donors. While it has made the technical aspects of procuring and transplanting small paediatric kidneys easier, challenges are still present and surgical experience and technique has been shown to greatly affect outcomes [7,11]. It is important to highlight that EBKT can be procured from donors less than 15 kg with acute kidney injury [12]. Consequently, EBKT has become more widely accepted and has been extended to include donation after cardiocirculatory death (DCD) donors; [13] and infant donors <5 kg body weight [14].
The most common causes for early renal allograft failure are vascular complications, with reported rates of vascular thrombosis between 2.5 to 12.5% in small paediatric donors, considerably higher than thrombosis rates for standard adult donors [8,15,16,10]. Surgical technique, peri-operative blood pressure management, vessel calibre, vessel or kidney torsion, hypercoagulable states, haematomas, lymphocytes and acute rejection have all been suggested as causes for thrombosis [15,17]. The absence of an aortic patch during Single Kidney Transplant (SKT) in paediatric donors less than 12 months with EBKT is also a risk factor for renal allograft thrombosis.
Paediatric small kidney donors, 10-14 kg donors, should not be considered as marginal donors. The difficulty, however, is now in determining when it is more appropriate to perform a paediatric EBKT as opposed to splitting and performing two DDKT. Unfortunately, there are no widely accepted guidelines to direct clinicians but instead based on earlier registry analysis, less than 5 years or 20 kg is being crudely used as the cut-off where EBKT is preferential to SKT [18]. Paediatric donors less than 15 kg demonstrated no significant outcome difference transplanting enbloc compared to SKT in one-year survival [15].
Renal allograft survival of ideal standard criteria donors was similar from DDKT from donors weighing >35 kg and EBKT from donors >10 kg. Donor weights between 10-14 kg performed as EBKT had superior outcomes over standard criteria donor kidneys. However, the protective benefit was less than half as compared to single kidney transplants. Authors concluded that from a resource perspective, splitting kidneys from donors 10-14 kg would increase the availability of organs and overall total graft survival years to the recipient population [16].
Renal allograft failure is a major concern for all paediatric donors, EBKT or SKT, with most single centre studies and transplant registries reporting early renal allograft failure at higher rates than those of standard adult donors. After approximately 12 months post-transplant, survival outcomes with EBKT equal that of SCDT, with studies showing superiority of EBKT over living donor kidneys [8,10].
The use of EBKT has gained growing acceptance with favourable outcomes, patients receiving such allografts have an increased incidence of vascular thrombosis [14,8,19]. Renal Ultrasonography (US) is the reference standard for post-operative evaluation of renal transplant recipients and is usually performed early after transplantation. Post-operative ultrasound involves evaluation of peri-transplant fluid collection, spectral analysis of the donor aorta, inferior vena cava, renal arteries, and veins and intrarenal arterial Resistive Indexes (RI) of each kidney [20].
Previous research on intrarenal arterial RIs and on transplanted arterial velocity has been focused largely on the clinical importance of elevated RIs and velocity. The list of differential diagnoses for increased RI in the immediate postoperative setting includes venous thrombosis [21].
Thrombosis does not necessarily occur at once at a discrete time point. Rather, it may have to be regarded as a continuous process that evolves throughout a definite time span [20]. The initial thrombotic event may occur at the level of the capillaries or venules before propagation into the main arteries or veins [22]. It is also considered that decreased RIs, is associated with a higher rate of thrombosis [20].
Thrombosis does not necessarily occur at once at a discrete time point. Rather, it may have to be regarded as a continuous process that evolves throughout a time span. The initial thrombotic event may occur at the level of the capillaries or venules before propagation into the main arteries or veins [22].
Non-selective use of antiplatelet and anticoagulation medication may decrease the risk of developing thrombosis [23,24]. However, post-operative antiplatelet and anticoagulation medication can increase the risk of haematoma development, reoperation, and transfusion requirements [24].
Previous studies have suggested that paediatric EBKT should be performed for donors <10 kg whereas single kidneys for use in two recipients is appropriate when the donor is >20 kg in size [25,16,26]. However, donors weighing between 10 and 20 kg represent an undetermined area in achieving the proper balance between utilisation and outcomes [27,28]. In a large retrospective UNOS registry analysis of donors <10 years of age from 1995-2007, reported that kidneys from donors with a 15-19, 10-14 and <10 kg body weight were used for EBKT in 40%, 65% and 86% of adult recipients [16]. In a subsequent UNOS registry analysis of donors <10 years of age span 1987-2007, it was reported that kidneys from donors with a 10-13, 13-15, 15-20 and >20 kg body weight were used for EBKT 64%, 49%, 24% and 4% of adult recipients [29]. In addition, they noted that although paediatric EBKT functioned better than single kidneys for all paediatric donor weight groups studied, acceptable graft outcomes could be achieved with single KT from donors >10 kg because the graft failure risk declined above this donor size.
It was considered that the risk of renal allograft failure may be higher when transplanting kidneys from small paediatric donors into paediatric recipients [25,16,30,15,10]. However, recent studies demonstrate improving results as size-matching between donors and recipients from a functional and growth perspective [14,31].
This case is particularly important since the use of an aortic extension on the proximal aorta has never been described before in the literature. The only description in the literature in the case the kidneys are procured en-bloc in multivisceral donors requiring a graft on an aortic patch including the SMA and coeliac axis origins. It has been described before by using a 1 cm segment of the distal abdominal aorta, opened it longitudinally, and fashioned a single aortic patch with which the proximal aorta was closed [32]. However, in this case the 1 cm required aorta was obtained from the aortic arch; this way the infrarenal aorta could be kept long enough to successfully perform the anastomosis. The availability of the distal abdominal aorta and IVC for inflow and outflow confers better graft positioning rather than short vascular cuffs. It is important to highlight the importance of using autologous aorta compared to using venous grafts, in order to prevent aneurysmatic vein dilatation and rupture after long-term exposure to arterial pressure [33-39].
Conclusion
Recipient selection and donor assessment is a cornerstone to success in KT from small paediatric donors. We demonstrate the feasibility of the en-bloc procedure and the use of an aortic extension as an alternative for vascular reconstruction with good outcome. The retrieving team should be aware of the potential reconstruction challenges, especially in the case of multi-visceral donors.
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR) Biomedical Research Centres based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London as well as Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
REFERENCES
- Chavers BM, Molony JT, Solid CA, Rheault MN, Collins AJ. One-year mortality rates in US children with end-stage renal disease. Am J Nephrol. 2015;41:121-128.
- Carrel A. Transplantation in mass of the kidneys. J Exp Med. 1908;10:98-140.
- Damji S, Callaghan, CJ, Loukopoulos I, Kessaris N, Stojanovic J, Marks SD, et al. Utilisation of small paediatric donor kidneys for transplantation. Pediatric Transplantation. 2019;23:135-140.
- Harmon WE, Alexander SR, Tejani A. The effect of donor age on graft survival in pediatric cadaver renal transplant recipients. Transplantation. 1992;54:232-237.
- Van Heurn E, De Vries E. Kidney transplantation and donation in children. Pediatr Surg Int. 2009;25:385-393.
- Al Midani A, Rudarakanchana N, Nagra A, Fidan K, Tugtepe H, Matthias M, et al. Low-dose aspirin reduces the rate of renal allograft thrombosis in pediatric renal transplant recipients. Exp Clin Transplant. 2020;18:157-163.
- Sanchez-Fructuoso AI, Prats D, Pérez-ContÃn MJ, Marques M, Torrente J, Conesa J, et al. Increasing the donor pool using en bloc pediatric kidneys for transplant. Transplantation. 2003;76:1180.
- Bhayana S, Kuo YF, Madan P, Mandaym S, Thomas PG, Lappin JA, et al. Pediatric en bloc kidney transplantation to adult recipients: More than suboptimal? Transplantation. 2010;90:248.
- Sharma A, Fisher, RA, Cotterell AH. En bloc kidney transplantation from pediatric donors: comparable outcomes with living donor kidney transplantation. Transplantation. 2011;92:564-569.
- Sureshkumar KK, Reddy CS, Nghiem DD. Superiority of pediatric en bloc renal allografts over living donor kidneys: a long term functional study. Transplantation. 2006;82:348-353.
- Varela-Fascinetto G, Bracho E, Bracho E, Valdés R, Romero B, Medeiros M, et al. En bloc and single kidney transplantation from donors weighing less than 15 kg into pediatric recipients. Transplant Proc. 2001;33:2034-2037.
- Troppmann C, Chandrasekar S, Kathrin T, Ghaneh F. Does acute kidney injury contraindicate transplantation of kidneys from very small pediatric donors? single-center analysis of 68 en bloc kidney transplants from donors =15 kg. Transplantation. 2018;102:S175-S176.
- Jones HE, Marks SD, Koffman G, Drage M. Successful paediatric en bloc renal transplant into a 15 year-old child: A tale of two kidneys. Arch Dis Child. 2012;97:A165-A166.
- Zhao WY, Zhang L, Zhu YH, Chen Y, Zhu FY, Shen Q, et al. En bloc kidneys transplanted from infant donors less than 5kg into pediatric recipients. Transplantation. 2014;97:555-558.
- Mohanka R, Basu A, Shapiro R, Kayler LK. Single versus en bloc kidney transplantation from pediatric donors less than or equal to 15 kg. Transplantation. 2008;86:264-268.
- Kayler LK, Magliocca J, Kim RD, Howard R, Schold JD. Single kidney transplantation from young pediatric donors in the United States. Am J Transplant. 2009;9:2745-2751.
- Unal B, Piskin T. En bloc and dual kidney transplantation: Two initial cases from a new kidney transplantation center. Transplant Proc. 2012;44:1700-1702.
- Mwipatayi B. En Bloc kidney transplant from an 18-months-old donor to an adult recipient: Case report and literature review. Int J Surg Case Rep. 2013;4:948-951.
- Bent C, Fananapazir G, Tse G, Corwin MT, Vu C, Santhanakrishnan C, et al. Graft arterial stenosis in kidney en bloc grafts from very small pediatric donors: Incidence, timing, and role of ultrasound in screening. Am J Transplant. 2015;15:2940-2946.
- Fananapazir G, Tse G, Corwin MT, Santhanakrishnan C, Perez RV, McGahan JP, et al. Pediatric En bloc kidney transplants: Clinical and immediate postoperative us factors associated with vascular thrombosis. Radiology. 2015;279:935-942.
- Lockhart ME. Reversed diastolic flow in the renal transplant: Perioperative implications versus transplants older than 1 month. AJR Am J Roentgenol. 2008;190:650-655.
- Gok MA, Shenton BK, Buckley PE, Peaston R, Cornell C, Soomro N, et al. How to improve the quality of kidneys from non-heart-beating donors: A randomised controlled trial of thrombolysis in non-heart-beating donors. Transplantation. 2003;76:1714-179.
- Robertson AJ. Low dose aspirin as prophylaxis against renal-vein thrombosis in renal transplant recipients. Neprhol Dial Transplant. 2000;15:1865-1868.
- Eng M, Brock G, Li X, Chen Y, Ravindra KV, Ravindra KV, et al. Perioperative anticoagulation and antiplatelet therapy in renal transplant: is there an increase in bleeding complication? Clin Transplant. 2011;25:292-296.
- Dharnidharka VR, Stevens, G, Howard RJ. En-Bloc kidney transplantation in the United States: an analysis of united network of organ sharing (UNOS) data from 1987 to 2003. Am J Transplant. 2005;5:1513-1517.
- Laurence JM. Utilisation of small pediatric donor kidneys: A decision analysis. Transplantation. 2011;91:1110-1113.
- Pelletier SJ, Guidinger MK, Merion RM, Englesbe MJ, Wolfe RA, Magee JC, et al. Recovery and utilization of deceased donor kidneys from small pediatric donors. Am J Transplant. 2006;6:1646-1652.
- Maluf DG. Optimizing recovery, utilization and transplantation outcomes for kidneys from small, <20 kg, pediatric donors. Am J Transplant. 2013;13:2703-2712.
- Surechkumar KK. When is it reasonable to split pediatric en bloc kidneys for transplantation into two adults? Transplant Proc. 2010;42:3521-3523.
- Groschl I, Wolff T, Gürke L, Eugster T, Hopfer H, Steiger J, et al. Intermediate-term outcome of single kidney grafts from pediatric donors weighing 10-14 kg in adult recipients. Clin Transplant. 2013;27:E302-E307.
- Zhao WY, Zhu YH, Zhu FY, Zhang L, Chen Y, Shen Q, et al. Single kidneys transplanted from small pediatric donors less than 15 kilograms into pediatric recipients. Transplantation. 2014;98:e97-100.
- Troppmann C, Perez R. Transplantation of pediatric en bloc kidneys when the proximal vascular cuff is too short: The aortorenal aortic lid. Transplantation. 2007;84:1064-1065.
- Hayes JM. The transplantation of difficult donor kidneys and recipients: Helpful surgical techniques. J Urol. 1993;149:250.
- Travis JA. Aneurysmal degeneration and late rupture of an aortorenal vein graft: Case report, review of the literature, and implications for conduit selection. J Vasc Surg. 2000;32;612.
- Horrow, MM. Immediate postoperative sonography of renal transplants: vascular findings and outcomes. Am J Roentgenol. 2013;201:W479-W486.
- Don S, Kopecky KK, Filo RS, Leapman SB, Thomalla JV, Jones JA, et al. Duplex doppler US of renal allografts: causes of elevated resistive index. Radiology. 1989;171:709-712.
- Rifkin MD, Needleman L, Pasto ME, Kurtz AB, Foy PM, McGlynn E, et al. Evaluation of renal transplant rejection by duplex Doppler examination: Value of the resistive index. AJR Am J Roengenol. 1987;148:759-762.
- Siskind, E, Lombardi P, Blum M, Tyrell R, Villa M, Kuncewitch M, et al. Signiicance of elevated transplant renal artery velocities in the postoperative renal transplant patient. Clin Transplant. 2013;27:E157-E160.
- Idrovo, JP, Teo TY, Shah KG, Wang P, Bhaskaran MC, Pellerito J, et al. Transitory peaked waveforms with elevated velocities in Doppler sonograpy after renal transplant. AJR Am J Roentgenol. 2011;9:421-424.
Citation: Assia-Zamora S, Callaghan C, Loukopoulos I, Drage M, Ramage M, Marks S, et al. (2021) En-Bloc Kidney Transplant Using an Aortic Extension from a Paediatric Multi-Visceral Donor to a Paediatric Recipient. J Vasc Med Surg. 9:408.
Copyright: ©2021 Assia-Zamora S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.