Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 3
In-vitro Antibacterial Efficacy and Phytochemical Profiles of Cinnamomum Tamala Leaf Extracts against Some Pathogenic Bacteria
Rahul Kumar Chandel1, Zeba Khan2* and Shubham Gambhir12Department of Agricultural Sciences, Aligarh Muslim University, Uttar Pradesh, India
Received: 04-Sep-2024, Manuscript No. JNDT-24-26879; Editor assigned: 06-Sep-2024, Pre QC No. JNDT-24-26879(PQ); Reviewed: 20-Sep-2024, QC No. JNDT-24-26879; Revised: 27-Sep-2024, Manuscript No. JNDT-24-26879(R); Published: 04-Oct-2024, DOI: 10.35248/2161-0509.24.14.300
Abstract
Background: Protein Energy Wasting (PEW) is highly prevalent among dialysis patients, leading to significant morbidity and mortality. Protein enriched dairy products may provide a feasible nutritional intervention to overcome the discrepancy between protein requirement and intake. We investigated the impact of an additional daily protein enriched yogurt on nutritional parameters in Hemodialysis (HD) patients with hypoalbuminemia. Methods: A prospective, single center, cross-over pilot study. Patients treated with HD and hypoalbuminemia, defined as blood albumin below 38 g/L, were considered eligible. After enrollment, all patients started a 4-months control period of strict dietitian surveillance followed by 4-months intervention period that consisted of an additional 20 g protein-enriched yogurt daily. Primary endpoint was increase in protein intake measured by normalized Protein Catabolic Rate (nPCR). Results: Ten hemodialysis patients were recruited. Mean albumin at baseline was 35 g/dL. Seven patients entered control period, 5 started intervention. Four patients completed intervention period. There was no difference in nPCR between screening to control or intervention period (mean nPCR 1.06, 1.10 and 1.26 in screening, control and intervention period respectively, p=0.273). Albumin level significantly increased from screening (34.5 g/L) to end of control period (38 g/L), p=0.012; with no further increase at the end of intervention period (36.35 g/L, p=0.196 between intervention and control period). The yogurt was well tolerated. Conclusion: Protein enriched yogurt did not increase nPCR in a limited cohort of dialysis patients with hypoalbuminemia, however it was well tolerated. Larger-scale studies may provide more information about its role in PEW management in this patient population.
Keywords
Antibacterial activity; Cinnamomum tamala; Mineral elements; Phytochemicals
Abbreviations
GC: Gas Chromatography; MS: Mass Spectroscopy
Introduction
Evolution of new strains of disease-causing agents and spread of antibiotic resistance and are of great concern to the global health community. Medicinal plant of India have been found of immense global importance in treatment because of an adverse effect of synthetic drug had created varied types of complicated diseases, besides causing resistance to synthetic drugs. Thus, global attention has been shifted towards finding new sources, specifically herbal extracts, for the development of therapeutic drugs. There is need to develop a Nobel herbal antibacterial formulation to get rid of resistance and also to investigate newer drugs with less resistance as well as cheap, easily available and eco-friendly. The pictorial organisms over a period of time change their antibiotic sensitivity pattern and develop resistance against commonly used therapeutic agents. A feasible way to overcome the problem of microbial resistance is the development of new antibacterial agents for substitution with ineffective ones. Herbal medicines have been used from centuries to cure human diseases, theses medicinal properties are due to the presence of phytochemicals in the plants. Various health related effects such as anticarcinogenic, antibacterial, antifungal, antimutagenic, antithrombotic, anticarcinogenic and vasodilatory activities, are possed by phytochemicals.
A wide variety of plants have been found to possess antibacterial activity. Tejpat (Cinnamomum tamala, Buch-Ham) belongs to Lauraceae family and is used in Indian system of traditional medicines in various ayurvedic formulations. It has been used in traditional medicines as an astringent, stimulant and carminative. The leaves are used as a spice but can be employed with myrobalans during dyeing and in the manufacture of vinegar. Its leaves are widely used as an important spice throughout the world since ancient times [1]. Leaves and bark have aromatic, astringent, stimulant and carminative qualities and used in rheumatism, colic, diarrhoea, nausea and vomiting. Essential oil extracted from the leaves known as Tejpat oil is medicinally important and used as a carminative and various heart and kidney disorders. It is also used in pharmaceutical preparation because of its hypoglycemic, stimulant and carminative properties. The essential oil from Cinnamomum tamala exhibits antidermatophytic, antibacterial, antifungal, antihyperglycaemic and (antihypercholesterolaemic) effects. Thus the present study was carried out to examine the antibacterial effects of aqueous and methanolic leaf extracts of Cinnamomum tamala against major pathogenic bacteria.
Materials and Methods
Plant material
Leaves of C. tamala were collected from the local market of Delhi, India.
Extract preparation
The collected plant material was washed and surface sterilized with 0.1% HgCl2. Spice material was dried out in hot air oven at 35°C-40°C for 2-3 days and was powdered using a grinder mixer and extracts were prepared following the methodology given below:
Aqueous extraction
10 g of powdered plant part was mixed thouroughly with 100 ml distilled water. The solution was kept at room temperature for at least 24 hr and then filtered using a muslin cloth. The filtrate was again filtered using Whatman's filter paper no.1 under strict aseptic conditions [2]. Finally the filtrate was concentrated by evaporation in a water bath to make the final volume 1/10th of the original volume.
Methanolic extraction
Powdered plant material was macerated in 80% methanol to obtain the hydro-alcoholic crude extract at room temperature. After 3 days (72 hr), the filtrate was separated by Whatman No. 1. insoluble materials obtained after extraction were re-macerated. The extract was concentrated by heating on the dry oven at 40°C to evaporate the alcohol from the filtrate. Finally the extract was stored at 4°C until the actual experiment is done.
Gas Chromatography-Mass Spectroscopy (GC/MS) analysis
The separation and identification of compounds in C. tamala were done by using Shimadzu QP 2010 Ultra GC-MS equipped with Rtx-5ms column measuring 30 × 0.25 mm and NIST14 library. Helium with flow rate 1 ml/min was used as a carrier gas. 1 μl volume of each sample was utilized. The injection temperature was maintained at 250°C. The oven temperature programme was set with an initial temperature of 50°C and then it was increased to 250°C with 4°C/min ramping rate. The temperature for ion source was maintained at 200°C and the interface at 250°C.
Test microorganisms
The test bacterial cultures were obtained from a microbial type culture collection, IM-Tech, Chandigarh. Stock culture of Staphylococcus aureus, Pseudomonas aeruginosa, E.coli, Citrobacter and Klebsiella pneumonia were grown in nutrient broth at 37°C and were subcultured and maintained in nutrient broth at 4°C.
Antibacterial activity by disc diffusion method
Screening for antibacterial activity of extract was done following disc diffusion method. Inoculation of the plate with the test organisms by streaking the swab in a back and forth motion, or by streaking (zigzag) method, manual placement of discs on the nutrient agar plate by forceps and measurement of the zone of inhibition was done in mm [3].
Phytochemical analyses
Total phenol was determined by Folin-Ciocalteau reagent, following the protocol proposed by Ramamoorthy and Bono. Saponin content was determined following Obdona and Ocheko. Alkaloid content was determined by the protocol given by Helrich. Flavonoids were determined by Shinoda test, steroids and triterpenoids were quantified by Salkowski test.
Results
The study of antibacterial properties of Cinnamomum tamala against various pathogens was conducted. The antibacterial activities of the plant were studied against five bacterial strains Staphylococcus aureus, Pseudomonas aeruginosa, E.coli, Citrobacter and Klebsiella pneumonia. Evaluated aqueous and methanolic extracts showed a variable degree of inhibition zones against different bacterial species. The methanolic extract was found comparatively more effective with zone sizes ranging from 07.00 mm to 29.10 mm (Table 1) [4]. The aqueous extract showed inhibition in the range of 1.62 mm to 28.66 mm, slightly lower than that of methanolic extract. The result showed that both extracts (methanol and aqueous) possess antibacterial activity against tested microorganisms i.e., Staphylococcus aureus, Pseudomonas aeruginosa, E.coli, Citrobacter and Klebsiella pneumonia. The methanolic extract of the leaf of Cinnamomum tamala showed pronounced inhibition against all the test microorganisms i.e., Staphylococcus aureus, Pseudomonas aeruginosa, E.coli, Citrobacter and Klebsiella pneumonia. The order of inhibition (in mm) was S. aureus and E.coli> K. pneumoniae> C. bacter> P. aeruginosa. Zone of inhibition for aqueous extract of the leaf of Cinnamomum tamala followed the order K. pneumoniae>S. aureus>P. aeruginosa>C. bacter>E.coli.
Phytochemical analysis of different fraction
Phytochemical screening of methanol and aqueous extract of Cinnamomum tamala leaves was done using different organic solvent was reported in Table 1. The methanol and aqueous extract of Cinnamomum tamala leaves showed a positive result for the presence of alkaloids, triterpenoids, glycosides and tannin and the results were negative for carbohydrate and saponin (Tables 2 and 3) (Figures 1-5) [5].
| Pathogens | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 200 mg/ml | 100 mg/ml | 50 mg/ml | 25 mg/ml | 12.5 mg/ml | 6.25 mg/ml | 3.12 mg/ml | Drug | |
| S. aureus | 20.33 ± 0.51 | 16.08 ± 0.89 | 16.08 ± 0.34 | 14.16 ± 0.49 | 13.66 ± 0.86 | 11.75 ± 0.27 | 8.75 ± 0.12 | 29.10 ± 0.54 |
| P. aerruginosa | 18.50 ± 0.23 | 16.66 ± 0.65 | 14.00 ± 0.48 | 10.91 ± 0.21 | 9.33 ± 0.65 | 9.16 ± 0.10 | 7.00 ± 0.54 | 23.00 ± 0.36 |
| E. coli | 21.50 ± 0.56 | 18.58 ± 0.89 | 15.41 ± 0.49 | 13.16 ± 0.24 | 11.33 ± 0.81 | 9.16 ± 0.25 | 8.03 ± 0.65 | 21.00 ± 0.59 |
| Citrobacter | 17.50 ± 0.87 | 16.00 ± 0.52 | 14.85 ± 0.44 | 12.75 ± 0.28 | 11.25 ± 0.79 | 10.91 ± 0.25 | 9.00 ± 0.21 | 26.00 ± 0.69 |
| K. pneumoniae | 21.16 ± 0.26 | 17.33 ± 0.54 | 14.83 ± 0.15 | 12.66 ± 0.65 | 11.16 ± 0.49 | 9.58 ± 0.57 | 7.58 ± 0.68 | 24.80 ± 0.48 |
Table 1: Antibacterial activity of methanolic extract of Cinnamomum tamala against different pathogens (Mean ± SE).
| Pathogens | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 200 mg/ml | 100 mg/ml | 50 mg/ml | 25 mg/ml | 12.5 mg/ml | 6.25 mg/ml | 3.12 mg/ml | Drug | |
| S. aureus | 21.00 ± 0.59 | 17.50 ± 0.57 | 15.25 ± 0.36 | 13.62 ± 0.12 | 13.37 ± 0.26 | 11.12 ± 0.29 | 10.25 ± 0.47 | 27.53 ± 0.65 |
| P. aerruginosa | 16.87 ± 0.06 | 14.37 ± 0.29 | 14.50 ± 0.69 | 13.25 ± 0.50 | 8.50 ± 0.62 | 8.50 ± 0.55 | 6.75 ± 0.88 | 23.37 ± 0.59 |
| E. coli | 11.75 ± 0.59 | 11.25 ± 0.25 | 9.75 ± 0.55 | 8.37 ± 0.71 | 5.75 ± 0.69 | 3.62 ± 0.25 | 2.50 ± 0.39 | 25.62 ± 0.54 |
| Citrobacter | 13.75 ± 0.82 | 13.25 ± 0.45 | 11.75 ± 0.11 | 8.50 ± 0.44 | 7.00 ± 0.25 | 5.25 ± 0.35 | 2.50 ± 0.64 | 14.50 ± 0.21 |
| K. pneumoniae | 18.75 ± 0.19 | 15.75 ± 0.63 | 13.25 ± 0.45 | 12.00 ± 0.18 | 8.00 ± 0.54 | 6.25 ± 0.27 | 1.62 ± 0.69 | 28.66 ± 0.51 |
Table 2: Antibacterial activity of aqueous extract of Cinnamomum tamala against different pathogens (Mean ± SE).
| Plant | Extract | Alkaloids | Glycoside | Carbohydrate | Tanin | Flavonides | Terpenoids | Saponins |
|---|---|---|---|---|---|---|---|---|
| C. tamala (Leaf extract) |
Aqueous | + | + | - | - | + | - | - |
| Methanol | + | + | - | + | + | + | - |
Table 3: Phytochemical analysis of methanol and aqueous leaves fractions of Cinnamomum tamala.
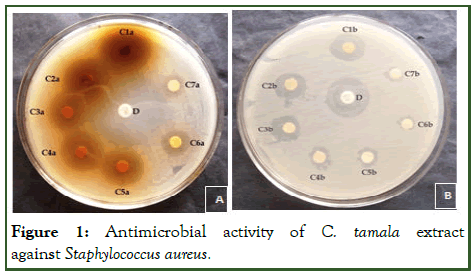
Figure 1: Antimicrobial activity of C. tamala extract against Staphylococcus aureus.
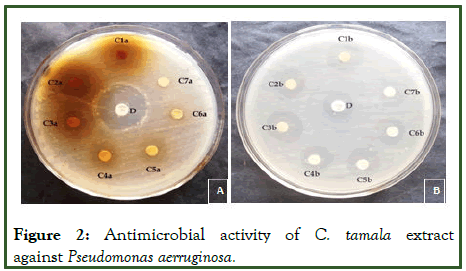
Figure 2: Antimicrobial activity of C. tamala extract against Pseudomonas aerruginosa.
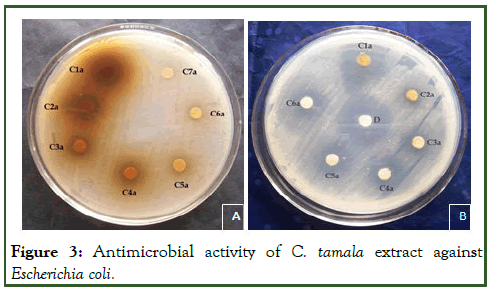
Figure 3: Antimicrobial activity of C. tamala extract against Escherichia coli.
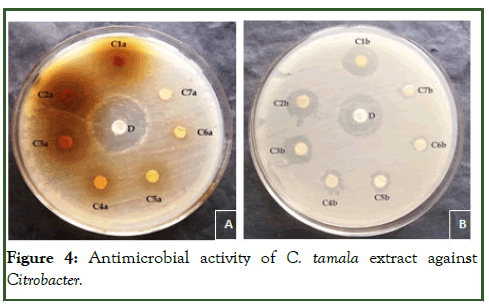
Figure 4: Antimicrobial activity of C. tamala extract against Citrobacter.
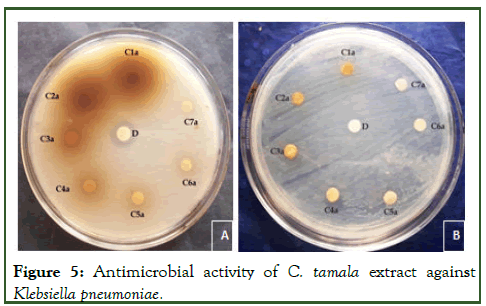
Figure 5: Antimicrobial activity of C. tamala extract against Klebsiella pneumoniae.
Chemical composition of Cinnamomum tamala essential oil
Thirty-one compounds were identified in the Cinnamomum tamala essential oil extract. These include cinnamaldehyde (42.898%), tans-cinnamyl acetate (24.327%), ascabin (14.249%), hydro cinnamyl acetate (3.384%), beta-caryophyllene (1.669%) and alpha-copaene (1.414%) as major components [6]. Chemical composition of essential oils of different eucalyptus species have earlier been elucidated. Among the chemical constituents of Cinnamomum tamala oil, cinnamaldehyde (42.898%), tanscinnamyl acetate (24.327%), ascabin (14.249%) were identified as major components (Table 4) [7].
| Peak | Compound | *RT(Min.) | Area (%) |
|---|---|---|---|
| 1 | Alpha-pinene | 3.621 | 0.095% |
| 2 | Camphene | 3.864 | 0.037% |
| 3 | Benzaldehyde | 4.05 | 1.222% |
| 4 | Beta-pinene | 4.312 | 0.034% |
| 5 | p-cymene | 5.16 | 0.065% |
| 6 | Beta-Phellandrene | 5.276 | 0.106% |
| 7 | Acetophenone | 6.15 | 0.044% |
| 8 | Linalool | 6.853 | 0.442% |
| 9 | Beta-phenylpropionaldehyde | 8.608 | 0.856% |
| 10 | Phenetol | 8.775 | 1.109% |
| 11 | Benzofuran | 9.006 | 0.185% |
| 12 | Acrolein | 10.285 | 0.286% |
| 13 | Cinnamaldehyde | 10.886 | 42.898% |
| 14 | 2-propenal, 3-phenyl cinnamaldehyde | 13.249 | 1.087% |
| 15 | Eugenol | 14.473 | 0.078% |
| 16 | Hydro cinnamyl acetate | 14.836 | 3.384% |
| 17 | Alpha-copaene | 14.98 | 1.414% |
| 18 | Pivalic acid | 15.34 | 0.129% |
| 19 | Cinnamyl acetate | 15.45 | 0.091% |
| 20 | Methyl eugenol | 15.909 | 0.107% |
| 21 | Beta-caryophyllene | 16.329 | 1.669% |
| 22 | Valecene | 16.91 | 1.089% |
| 23 | Tans-cinnamyl acetate | 17.172 | 24.327% |
| 24 | Alpha-humulene | 17.365 | 1.636% |
| 25 | Bicyclogermacrene-lepdozene | 18.658 | 0.192% |
| 26 | Naphthalene | 19.455 | 0.066% |
| 27 | Spathulenol | 21.108 | 0.780% |
| 28 | Caryophyllene oxide | 21.23 | 0.135% |
| 29 | Alpha-patchoulene | 21.547 | 1.090% |
| 30 | Humulene oxide | 22.993 | 1.097% |
| 31 | Ascabin | 24.584 | 14.249% |
Note: *RT: Retention time obtained by chromatogram
Table 4: Chemical compounds identified in chronological order using GCMS analysis of Cinnamomum tamala essential oil (major compounds are marked in the bold form).
Discussion
The inhibition zone in five bacterial strains Staphylococcus aureus, Pseudomonas aeruginosa, E.coli, Citrobacter and Klebsiella pneumoniae by the aqueous extract was less as compared to other organic fractions [8]. This may be because the same active substances were present in aqueous extracts, but in low concentration, substances were soluble in organic solvents and therefore, not present in aqueous extracts [9].
Methanolic extract of Cinnamomum tamala was found more effective against all test pathogen as compared to aqueous it was confirmed from the study of Mishra et al. Cinnamomum tamala extract can be considered as a potential antimicrobial agent for the treatment of various infectious diseases [10]. Methanolic extract of Cinnamomum tamala shows maximum antibacterial activity against Staphylococcus aureus and E.coli followed by Pseudomonas aeruginosa, Citrobacter and Klebsiella pneumoniae.
Aqueous extract of Cinnamomum tamala shows maximum antibacterial activity against Staphylococcus aureus and Klebsiella pneumonia followed by Pseudomonas aeruginosa, E.coli and Citrobacter. The results of the present investigation also encourage the use of organic solvents in the preparation of plant extracts as compared to aqueous extracts. The polarity of antibacterial compounds make them more readily extracted by organic solvents In the current investigation phytochemical screening of Cinnamomum leaf extract revealed that it possesses at least three to four of the following classes of secondary metabolites: Phenols, alkaloids, flavonoids, terpenoids, tannins and saponins [11]. Phenols are the aromatic compounds having hydroxyl groups, which occur almost in all parts of plants and are widespread among the plant kingdom and offer resistance to diseases and pests in plants.
There are also studies showing that alkaloids and flavonoids are the responsible compounds for the antibacterial activities in higher plants.
The antibacterial effects of C. tamala cinnamaldehyde (42.898%), tans-cinnamyl acetate (24.327%), ascabin (14.249%), hydro cinnamyl acetate (3.384%) have been demonstrated by other researchers too. Some researchers found cinnamaldehyde (42.898%), to be highly toxic to bacteria. While, in some of the studies indicate that tans-cinnamyl acetate (24.327%), ascabin (14.249%), to possess potent antibacterial and toxic effects. Among various compounds of Cinnamomum tamala essential oil, cinnamaldehyde (42.898%), tans-cinnamyl acetate (24.327%) and ascabin (14.249%) was found as major ones [12]. The above findings suggest that acute toxicity of C. tamala essential oil and its constituent compounds especially include cinnamaldehyde (42.898%) and tans-cinnamyl acetate (24.327%) are quite promising and have antibacterial effects against gram-negative and gram-positive bacteria.
The possible mechanism of action may be due to the blockage of protein synthesis by these compounds either at transcriptional or at the translational level and in turn peptidoglycan synthesis inhibition at membrane level. Presence of these phytochemicals justifies the observed antibacterial activities in the current study The result suggests that the plant extract could be used as a potential alternative for the development of an efficient and effective drug from natural sources that can be used for the treatment of infectious diseases and will help in combating the diseases caused by pathogenic bacterial strains.
Conclusion
It can be concluded that leaf extracts of the plant under study have great potential as antibacterial compounds against bacteria. And they can be used in the treatment of infectious disease caused by resistant bacteria. Such screening of various natural organic compounds and identification of active agents in one need of the hour because the successful prediction of lead molecule and drug discovery will pay off late in drug development. The present study reveals that the selected plants would exert several beneficial effects by antibacterial activity could be harnessed as drug formulation. The obtained results provide support for the use of these plants in medicine and suggest their further advance investigation.
Further investigations are needed to more clearly understand the therapeutic values and curative effects of this plant.
Conflict of Interest
There is no conflict of interest among the authors or anybody else.
References
- Chang ST, Chen PF, Chang SC. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J Ethnopharmacol. 2001;77(1):123-127.
[Crossref] [Google Scholar] [PubMed]
- Chang ST, Cheng SS. Antitermitic activity of leaf essential oils and components from Cinnamomum osmophleum. J Agric Food Chem. 2002;50(6):1389-1392.
[Crossref] [Google Scholar] [PubMed]
- Chanotiya CS, Yadav A. Enantioenriched (3S)-(+)-linalool in the leaf oil of Cinnamomum tamala Nees et Eberm. from Kumaon. J Essent Oil Res. 2010;22(6):593-596.
- Cordell GA, Quinn‐Beattie ML, Farnsworth NR. The potential of alkaloids in drug discovery. Phytother Res. 2001;15(3):183-205.
[Crossref] [Google Scholar] [PubMed]
- Govindarajan R, Vijayakumar M, Singh M, Rao CV, Shirwaikar A, Rawat AK, et al. Antiulcer and antimicrobial activity of Anogeissus latifolia. J Ethnopharmacol. 2006;106(1):57-61.
[Crossref] [Google Scholar] [PubMed]
- Khan ZS, Nasreen S. Phytochemical analysis, antifungal activity and mode of action of methanol extracts from plants against pathogens. J Agric Technol. 2010;6(4):793-805.
- Kumar S, Sharma S, Vasudeva N. Chemical compositions of Cinnamomum tamala oil from two different regions of India. Asian Pac J TropDis. 2012;2:S761-S764.
- Manandhar S, Luitel S, Dahal RK. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Trop Med. 2019;2019:1895340.
[Crossref] [Google Scholar] [PubMed]
- Rana VS, Langoljam RD, Verdeguer M, Blazquez MA. Chemical variability in the essential oil of Cinnamomum tamala L. leaves from India. Nat Prod Res. 2012;26(14):1355-1357.
[Crossref] [Google Scholar] [PubMed]
- Singh V, Gupta AK, Singh SP, Kumar A. Direct analysis in real time by mass spectrometric technique for determining the variation in metabolite profiles of Cinnamomum tamala Nees and Eberm genotypes. ScientificWorldJournal. 2012;2012(1):549265.
[Crossref] [Google Scholar] [PubMed]
- Srivastava B, Sagar A, Dubey NK. Evaluation of Cinnamomum tamala oil and its phenylpropanoid eugenol for their antifungal and antiaflatoxigenic activity. Food Anal Methods. 2011;4:347-356.
- Thongson C, Davidson PM, Mahakarnchanakul W, Weiss J. Antimicrobial activity of ultrasound‐assisted solvent‐extracted spices. Lett Appl Microbiol. 2004;39(5):401-406.
[Crossref] [Google Scholar] [PubMed]