PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2019) Volume 11, Issue 2
In vitro Study of Antibacterial Activity by Citrus aurantifolia Fruit Peel, Citrus limetta Fruit Peel and Citrus aurantifolia Leaves against Oral Pathogens
Preeti Singh*Received: 19-Mar-2019 Published: 12-Apr-2019
Abstract
Peels and leaves of citrus fruit have shown the various effects against bacteria present in oral cavity. They have also shown various pharmacological activities against bacteria responsible for dental caries. The values of minimum inhibitory concentration (MIC) were determined, the maximum zone of inhibition (16 mm) was observed in the combination of fruit peel and leaf waste. Scanning Electron Microscopy results also showed the effect of fruit peel and leaf waste extracts on bacteria. Peel and leaf waste extract effectively inhibit the growth of bacteria. The natural agents for antimicrobial activity have been detected in Citrus aurantifolia fruit peel and leaves.
Introduction
To prevent oral diseases, oral hygiene should be maintained carefully. Dentistry has mainly focused the value of preventative measure for oral diseases and need to use tooth brushes [1]. Preventive measures for maintain oral health from pathogenic organisms can be adopted by using various natural methods.
The human oral cavity is colonized by a large variety of bacteria flora than any other anatomic area, more than 700 species of bacteria have already been identified 400 of which were found in the periodontal pocket adjacent to teeth [2]. Organisms not normally associated with oral flora also have been isolated from toothbrushes, including Enterobacteria, Pseudomonas [3]. So the infectious microorganisms remaining on the brush can re-infect our mouth teeth, some of them can even spread to the rest of our body and cause serious health problems, including heart disease, stroke, arthritis, haematogenous, bacteremia and chronic. There are many ways allows the bacteria bread and grow on toothbrushes, spray from flushing toilet, a damp environment, a single toothbrush can be the breeding ground for trillions of bacteria [4]. Brushes of healthy individual yielded various types of organisms as Pseudomonas, Staphylococcus epidermidis, Staphylococcuc aureus [5]. The objective of this study was to identify anti-microbacterial properties present in fruit waste and peel of citrus plants.
Fruit and leaf waste
Peels of various fruits are generally considered as waste product and are normally thrown away by us [6]. Different studies conducted on peels revealed the presence of important constituents, which can be used for pharmacological or pharmaceutical purpose [7]. Various number of components having activities like antioxidant, antimicrobial, anti-inflammatory, anti-proliferative etc. have been isolated from different peels [8].
Materials and Methods
Materials
Waste material: The peelings, leaves and pulp of fruits were collected. Solvents: Petroleum ether, chloroform, Methanol, Acetone, Hexane, Ethanol was obtained from Merck Chemicals. Chemicals: Ferric chloride, Lead acetate, Mollish reagent, HCl, H2SO4, NaCl, NaOH, Fehling’s A and B, acetic acid, HNO3 were obtained from Hi- Media and Sigma. Instruments: TLC (Thin Layer Chromatography), Scanning electron microscopy (SEM), RAMAN (Thermo scientific DXRxi Raman imaging microscope) Spectroscopy.
Methods
Preparation of bacterial culture from Saliva: 1 ml of saliva was collected and immersed into 50 ml of distilled water in different beakers for 24 hours. 1 ml of contaminated water (saliva) was added in the nutrient broth. The microbial growth was observed after 24 hours of incubation.
Preparation of the fruit and leaf waste extract: The fruit wastes were rinsed with distilled water to remove dirt and sterilized with HgCl2 and then rinsed with distilled water and shade dried. Electrical grinder was used to powder the shade dried peels. The Powder was stored in refrigerator until further analysis.
Extraction: The 80% ethanol and methanol extracts were prepared by soaking 1 gm of samples in 10 ml of these solvents separately. The extracts were filtered after 24 hours through Whatman filter paper.
Phytochemical screening: Methanol and 80% ethanol extract of the fruit waste was screened for presence of various secondary metabolites with phytochemical screening.
Alkaloids
Dragendorff’s test: 1 ml extract was treated with 1% HCl and Dragendorff’s reagent. Appearance of red precipitate indicated presence of alkaloid.
Flavonoid
Ferric chloride test: 1 ml extract was treated with few drops of ferric chloride solution. Appearance of greenish black colour indicated presence of flavonoid.
Saponins
Frothing test: 1 ml extract was treated with 10 ml distilled water and tube was shaken vigorously. Appearance of bubbles indicated presence of saponins.
Tannins
1 ml extract was treated with 15% ferric chloride solution. Appearance of blue colour indicated presence of tannin.
Coumarins
1 ml extract was treated with 1 ml 10% sodium hydroxide solution and 1 ml chloroform. Appearance of yellow colour indicated presence of coumarins.
Steroids
1 ml extract was treated with 0.5 ml chloroform and 0.5 ml concentrated sulphuric acid.
Proteins
1 ml extract was treated with concentrated nitric acid. Appearance of yellow colour indicated presence of proteins.
Carbohydrates
1 ml extract was treated with 1 ml Fehling A and B and heat. Appearance of brick red precipitate indicated presence of carbohydrates.
Preparation of media and sterilization
For microbial culture the source of growth will be media on which they will grow and proliferate. Nutrient agar media was weighed in 100 ml flask was autoclaved at 121°C at 15 psi pressure for 20 minutes. The laminar was sterilized with ethanol and then UV must be switched on for 20 minutes prior pouring.
Isolation and identification of microbial colonies
Microbial colonies were isolated from the saliva under defined media. Sterile media were inoculated with the distilled water from saliva in different conical flasks. Colonies of bacteria were obtained after 24 hours of incubation.
Identification by gram staining
Heat fixed smear slide was placed on staining tray. Smear was flooded gently with crystal violet and left for 1 minute. The slide tilted slightly and gently rinsed with tap water or distilled water using a wash bottle. Smear was flooded gently with Gram’s iodine and left for 1 minute. The slide tilted slightly and gently rinsed with tap water or distilled water using a wash bottle. The smear was appearing as a purple circle on the slide. Decolourization was done by using 95% ethyl alcohol or acetone. Slide was then tilted slightly and alcohol applied drop by drop for 5 to 10 seconds until the alcohol runs almost clear and immediately rinsed with water and flooded gently with safranin to counter-stain and stand for 45 seconds. Slide was then tilted slightly and gently rinse with tap water or distilled water using a wash bottle. Slide was dried and visualized the smear using a light-microscope under oil-immersion. By this procedure some gram positive and gram negative bacteria were confirmed.
Antimicrobial activity of the waste extract
The methanolic extracts of fruit and leaf waste were screened for antimicrobial activity by agar well diffusion method. Agar surface was cut with the help of sterile cork borer having a diameter of 6.0 mm size. All bacterial strains were grown in nutrient broth (NB) for 4-6 hours at 27°C. After solidification of the agar, appropriate wells were made on agar surface by using sterile cork borer (3 wells per 90 mm diameter plate). Different volumes of the extracts were prepared and 30 ul-45 μl of each volume was added to the wells. Bacterial cultures were incubated at 27°C for 24 hours. Antimicrobial activity was determined by measuring the zone of inhibition surrounding the well. The assays were carried out under aseptic conditions. Antibiotic: Streptomycin (0.005 mg/ml) was used. Methanol used as controls for bacteria. Each concentration included triplicates.
Isolation and purification of the secondary metabolites from fruit waste
Thin layer chromatography (TLC)
TLC is simple chromatographic technique and wide variety of the solvent system was used for the separation of phytochemicals present in the fruit waste extract. It was carried on Merk silica 20*20 Aluminium sheets and the presence of the, flavonoid were detected using standards quercetin, spraying reagent ninhydrin reagent, iodine fumes. Solvent system used for the flavonoid was methanol: chloroform (7:3).
Purification of waste extracts using silica gel column chromatography
The combination of fruit waste extract was separated with silica gel column chromatography. The solution of different polarity were used for the elution of the compound on the basis of the polarity from non-polar to polar solvent and the eluted fractions are collected at a definite period of time. Gradation of the chloroform and methanol were used for the elution of the compounds [9].
Raman spectroscopy
Raman spectroscopy is a method where a sample is radiated with monochromatic visible, ultra-visible or near infrared light emitted from a laser, which probes molecular vibrations (stretching, bending, deformation) and the structure of chemical components present in the investigated sample is gathered. Functional groups of the molecules can be identified by their unique pattern of light scattering, called Raman spectrum.
Scanning electron microscopy (SEM)
The microstructure of bacterial sample isolated from saliva was studied using SEM (TESCAN) analysis. The sample (powder) was mounted on a double coated conductive carbon tape that holds the sample firmly to the stage surface and can be used as a ground strap from the sample surface to sample holder.
SEM analysis was done using fine powder of bacteria culture isolated from saliva. In the Scanning Electron Microscopy an electron beam was focused into the affine probe and subsequently raster scanned over a small rectangular area. The energy of the electron beam was continuously adjusted to suit the examination in progress.
Results and Discussion
Extraction of fruit and leaf waste
All phytochemical compounds from fruit waste material were extracted by the two solvents i.e. ethanol and methanol.
Phytochemical analysis
The phytoconstituents determination of 80% ethanol and methanol extracts of fruit waste (A,B,C) were carried out (Table 1). Preliminary screening of methanol extract showed presence of steroid, saponins, flavonoids, phenols, terpenoid, carbohydrates and proteins. However 80% ethanol extract showed the presence of carbohydrates and proteins. A is Citrus aurantifolia fruit peel extract, B is Citrus aurantifolia leaf extract, C is Citrus limetta fruit peel extract.
| Test | Chemicals | Result | 80% ethanol | Methanol | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |||
| Steroid | 1 ml extract + 0.5 ml CHCl3 +0.5 ml conc. H2SO4 | Red color | + | + | + | ++ | ++ | + |
| Saponins | 1 ml extract + 5 ml water and shake it. | Formation of stable foam | ++ | + | + | ++ | + | ++ |
| Alkaloid | 1 ml extract + 1% HCl | Red precipitate | + | + | + | ++ | + | ++ |
| Coumerin | 1 ml extract +1 ml 10% NaOH+ CHCl3 | Yellow color | - | + | + | + | + | + |
| Flavanoid | 1 ml extract + 1 ml 2% NaOH | Yellow color+dil.HCL, The yellow colour disappears | ++ | + | + | +++ | +++ | ++ |
| Terpenoid | 1 ml extract + 1 ml conc. H2SO4 | Reddish brown colour | + | + | + | ++ | + | + |
| Glycosides | 1 ml extract + 0.5 ml CHCl3 +0.5 ml conc. H2SO4 | Red brown color | _ | _ | _ | _ | _ | _ |
| Protein | 1 ml extract + conc. HNO3 | Yellow color | ++ | + | + | ++ | + | ++ |
| Carbohydrate | 1 ml extract + 1 ml Fehling A and B and heat | Brick red precipitate | + | + | + | + | + | + |
Table 1: Phytoconstituents present in the fruit waste extracts (A, B, C).
Antimicrobial screening
Antimicrobial assay of fruit and leaf waste extracts (A, B, C) were done against bacteria and the technique employed was agar well diffusion method at varying volumes. Present data (Tables 2 and 3) obtained from the study has shown a strong dose-dependent antimicrobial activity [10].
| Samples | 30 µl | 35 µl | 40 µl |
|---|---|---|---|
| Antibiotics (streptomycin) | 15 mm | 15 mm | 15 mm |
| Control (methanol) | 1 mm | 1 mm | 1 mm |
| Fruit waste extract A | 10 mm | 11 mm | 12 mm |
| Fruit waste extract B | 12 mm | 12 mm | 14 mm |
| Fruit waste extract C | 11 mm | 12 mm | 14 mm |
Table 2: Zone of inhibition of waste extract (without combination) against bacteria present in saliva.
| Samples | 30 µl | 35 µl | 40 µl |
|---|---|---|---|
| Antibiotic (streptomycin) | 20 mm | 20 mm | 20 mm |
| Control (methanol) | 1 mm | 1 mm | 1 mm |
| Fruit waste extract Combinations(A,B,C) |
14 mm | 15 mm | 16 mm |
Table 3: Zone of inhibition of waste extract (with combination) against bacteria present in saliva.
In antimicrobial analysis Methanol was used as control and antibiotic (streptomycin) was used. The concentration of antibiotic was 0.005 mg/ml whereas the volume of the waste extract ranged upto 30 to 40 μl. The concentration of the fruit peel powder and leaves powder were 0.2 gm/mL
Identification, purification and characterization of fruit waste extract
Thin layer chromatography (TLC): Thin Layer Chromatography is widely used in natural product extract analysis as a finger print which can be used for TLC identification of the biomolecules present in the plant. The prepared methanolic extract of fruit and leaf waste was separated on aluminium-coated TLC plates. The TLC plate was developed under saturated conditions with solvent systems. Developed TLC plates were visualized by using ethanolic potassium hydroxide. Quercetin was used as the flavonoid standard for the identification of the flavonoids. The retention factor (Rf) of methanolic extract of fruit and leaf waste extracts i.e. A, B, C were 0.76, 0.77, 0.77 respectively and that of quercetin was 0.77.
Purification with silica gel column chromatography: Fruit waste extract were purified for the presence of flavonoid with silica gel column chromatography. Gradients of the hexane and methanol were used for the elution of the compounds. Fractions were eluted from non-polar to polar solvent and were collected at a definite period of time.
Fractions of the combination obtained in column chromatography had the same Rf (Retardation Factor) as that of standard quercitin, hence it showed the presence of quercitin in the fractions.
Raman spectroscopy: In the standard Raman spectra, the unique pattern of light intensity i.e., Raman shift was observed around the 1450 cm-1 while the methanolic extract of fruit waste also showed the Raman shift around the 1450 cm-1. This confirmed the presence of Quercetin in waste extract.
SEM analysis of the samples: The structure of bacterial sample isolated from saliva as a control and treated bacterial samples were studied using SEM (scanning electron microscopy) (TESCAN) analysis. The typical SEM image of bacteria showing the ruptured bacterial cell after addition of combination of fruit waste extracts of methanol. The sample in powdered form was mounted on double coated conductive carbon tape that holds the sample surface to sample holder. The sample was coated with Palladium for creating the charge on the sample surface.
The images of the bacteria were analysed by observing the surface, in control bacteria of saliva without treatment showed the smooth surface without any rupture. The bacterial samples were treated with the methanolic fruit waste extract showing the ruptured cells.
We can compare all the images of SEM by observing the cells of bacteria (A, D) (Figure 1).
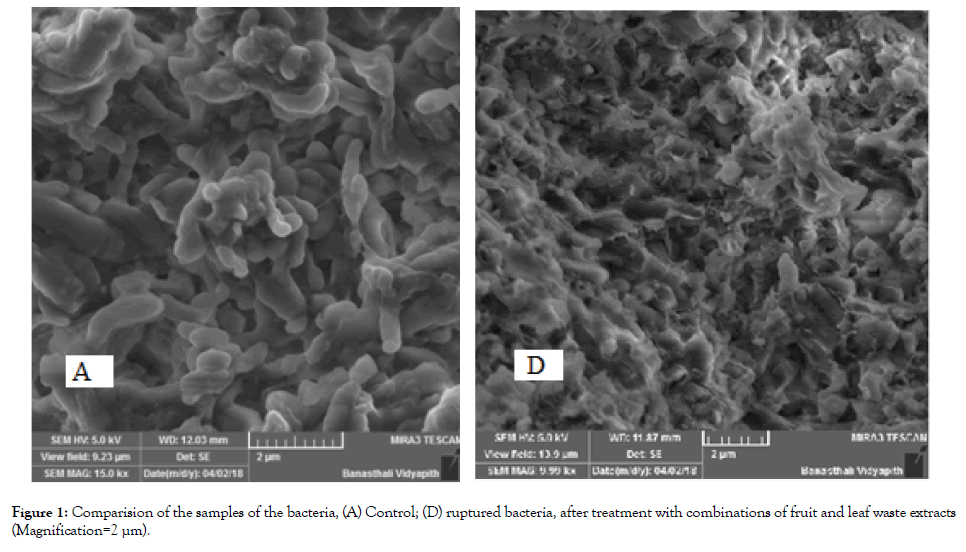
Figure 1. Comparision of the samples of the bacteria, (A) Control; (D) ruptured bacteria, after treatment with combinations of fruit and leaf waste extracts (Magnification=2 μm).
The scanning electron microscope can be useful to reveal morphological features of isolated organisms as well as for diagnosis, but difficulty with specimen preparation methods have in the past limited the use of SEM for routine microbiology.
Biological samples e.g. bacteria, DNA, proteins, contain significant amounts of water and exhibit low conductivity. In their natural state, these samples cannot be observed directly by conventional SEM because the surface and subsurface water quickly evaporates under the high vacuum conditions necessary for electron microscopy observation [11].
Conclusions
It is known that the by-products of some fruits represent an important source of sugars, minerals, organic acid, dietary fibre and phenolic that have a wide range of action, which includes antiviral, antibacterial, cardio protective and anti-mutagenic activities. Thus new aspects concerning the use of the wastes therapeutically are very attractive. The combination of fruit waste has shown the maximum zone of inhibition, this activity will help in disrupting the bacteria in natural way.
Therefore, this study will help us as a scope for future utilization of the waste for therapeutic purpose. The results also indicate that selective extraction from natural materials, by an appropriate solvent, is important for obtaining fractions with high antimicrobial activity. By antibacterial activity through peel and fruit waste, some of the antibacterial agents will help in preventing the oral pathogens in natural and economical methods.
REFERENCES
- Barros OB, Pernambuco RA, Tomita NE. Escovas dentais. Revis Fac Odont. 2011;4(1):32-34.
- Long SR, Dos Santos AS, Nascimento CMO. Avaliação da contaminação de escovas dentais por enterobactérias. Rev Odontol Univ Santo Amaro. 2000;5:21-25.
- Sammons RL, Kaur D, Neal P. Bacterial survival and biofilm formation on Saura-Calixto F and Goni I 2006 Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2004;94:442-444.
- Abraham NJ, Ciricione UK, Glass RT. Dentists and dental hygienists attitudes toward toothbrush replacement and maintenance. Clin Prev Dent. 1990;12(5):28-33.
- Taji S, Rogers A. The microbial contamination of toothbrushes: A pilot study. Aust Dent J. 1998;43(2):128-130.
- Leung AY, Foster S. Encyclopedia of common natural ingredients used in food, drugs, and cosmetics, 2nd ed., John Wiley and Sons (Wiley-Interscience), New York 1996.
- Lim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007;103:1003-1008.
- Mercy S, Banu M, Jenifer I. Application of different fruit peels formulations as a natural fertilizer for plant growth. Int J Sci Tech Res. 2014;3(1):300-307.
- Saito Y, Ohta H, Terasaki H, Katoh Y, Nagashima H, Jinno K, et al. Separation of polycyclic aromatic hydrocarbons with a C60 bonded silica phase in microcolumn liquid chromatography. J Sep Sci. 1995;18(9):569-572.
- Sharma DK. Bioprospecting for drug, research and functional foods for the prevention of diseases-Role of flavonoids in drug development. J Sci Ind Res. 2006;65:391-401.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 34th ed.: Nirali Prakashan 2006.
Citation: Singh P (2019) In vitro Study of Antibacterial Activity by Citrus aurantifolia Fruit Peel, Citrus limetta Fruit Peel and Citrus aurantifolia Leaves against Oral Pathogens. J Microb Biochem Technol 11:2. doi: 10.4172/1948-5948.1000411.
Copyright: © 2019 Singh P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

