Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- ResearchBible
- Cosmos IF
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 13, Issue 3
In Vitro Regeneration of Trichosanthes dioica Roxb. Plantlets through Callus Culture
Rashmi Komal*Received: 17-Dec-2020, Manuscript No. AGT-24-7567; Editor assigned: 22-Dec-2020, Pre QC No. AGT-24-7567 (PQ); Reviewed: 05-Jan-2021, QC No. AGT-24-7567; Revised: 16-Aug-2024, Manuscript No. AGT-24-7567 (R); Published: 13-Sep-2024, DOI: 10.35248/2168-9891.24.13.377
Abstract
An efficient protocol for plantlet regeneration from shoot tip and axenic node derived callus was developed for Trichosanthes dioica Roxb. The optimum callus production was observed in MS+2 mg/l BAP+4 mg/l 2, 4-D, after 45 days of culture. Proliferation of multiple shoots was obtained from the node derived callus cultures on MS+1.5 mg/l BAP+0.3 mg/l NAA, after 30 days of culture with an average shoot length of 4.88 cm ± 0.74 cm. The elongated shoots rooted within seven to eight days in half-strength MS medium supplemented with 1.0 mg/l IBA. The in vitro raised plantlets were sequentially acclimatized by transferring them in an inorganic MS salt solution, then to sterile distilled water and tap water before transferred to potted soil. The plantlets were successfully established in earthen pots filled with soil, sand and farmyard mixture in a ratio of 1:1:1, showing 70% survival rate.
Keywords
Callus formation; Multiple shoot regeneration; Organogenesis; Trichosanthes dioica Roxb
Introduction
Trichosanthes dioica Roxb. (pointed gourd) commonly known as parwal/patal in India, is one of the most important and expensive summer vegetable crops and holds a coveted position in the Indian market during the summer and rainy season. It is said to be the native of south east Asia and probably the northern and eastern states of India, especially, of Bengal, Assam and Bihar and has high economic value with export potential. It is mainly cultivated along the riverine belts of Bihar. Sudden flood and water logging destroys the fruits and plant/vines leading to shortage of planting materials. Micropropagation may help overcome these problems to a great extent. Moreover the plant’s dioecious nature and its vegetative mode of propagation make its reproduction and multiplication labour intensive. Genetic improvement of this crop has not been attempted adequately, hence, there is much work to be done from an agricultural point of view. Since, the crop is of immense economic importance, much work has to be done on its maintenance, conservation and in vitro propagation [1].
Though there are earlier reports on adventitious proliferation of shoots from the shoot tip and nodal explants of Trichosanthes dioica Roxb. still plantlet regeneration from the callus cultures has to be achieved from genetic variation and agricultural point of view. The availability of an efficient protocol for in vitro regeneration is a prerequisite to harness any biotechnological approach for genetic improvement of crop plants. Development of an efficient and reproducible protocol for differentiation of multiple shoots from the callus has a great potential in obtaining somaclonal variants and in vitro mutation breeding. Hence, the present study is an attempt to induce callus and regenerate plantlets from the callus cultures of shoot apices and axillary nodes of Trichosanthes dioica Roxb [2].
Materials and Methods
The explants such as axenic nodes, shoot tips, tender leaves and roots were collected from the field grown plants. The explants were washed in running tap water in plastic pots for 10 to 15 min, followed by surface sterilization with detergent and 1-2 drops of tween-20 (soap water) for 5 to 10 min. The explants were further transferred in an autoclaved plastic pot and treated with 0.1% (w/v) aqueous mercuric chloride solution and 2-3 drops of tween-20 and shaken for 8 min under laminar air-flow cabinet. Then explants were shaken in ethyl alcohol (70%) for 3 min. Further the explants were rinsed thrice with sterile double distilled water in an aseptic condition. Finally using sterile forceps, explants were transferred to sterile petri dishes and cut into small pieces (0.5 cm-1.0 cm) with sterile scalpel.
MS medium supplemented with 100 mg/l meso-inositol, 3% sucrose and 0.8% agar-agar was used as basal medium 16 different concentrations and combinations of BAP (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mg/l), Kn (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mg/l), 2, 4-D ( 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mg/l), NAA (0.1, 0.2, 0.3 and 0.5 mg/l), IAA (0.1, 0.2, 0.3 and 0.5 mg/l) and IBA (0.1, 0.2, 0.3 and 0.5 mg/l) were tested for callus induction and organogenesis. The pH of the medium was adjusted and maintained to 5.8 with the help of 0.1 N NaOH or 0.1 N HCl before adding agar (Hi-media, Mumbai) [3]. The cultures were incubated in the culture room maintained at 260 C ± 20 C with a relative humidity of about 55%-65% and 16 hour photo-period, 2000-2500 lux light intensity from fluorescent tubular lamps. The medium was sterilized by autoclaving at 121°C for 20 min at 15-psi pressure. The cultures were maintained by sub culturing at four weeks intervals to fresh medium with the same composition. After successful callus induction and proliferation these calluses were transferred to a cytokinin rich medium for shoot initiation. The cultures for callus induction were kept in the dark for a week prior to exposing them to 16 hrs photoperiod. After successful callus induction and proliferation these calluses were transferred to a cytokinin rich media for shoot initiation. Each proliferated and adventitious shoot was cut from the basal end and individual shoot was placed in half-strength MS medium supplemented with NAA (0.1, 0.3, 0.5, 1.0 and 1.5 mg/l), IAA (0.1, 0.3, 0.5, 1.0 and 1.5 mg/l) and IBA (0.1, 0.3, 0.5, 1.0 and 1.5 mg/l) for rooting. Sub cultures were done every 30 days. The rooted micro propagules were transferred to sterilized pots containing a mixture of sterilized soil, sand and farmyard mixture in the ratio of 1:1:1. These were then transferred to natural soil and environment [4].
Results and Discussion
Morphogenetic response of different explants viz., axenic nodes, shoot tips, leaves and roots of in vitro regenerated pointed gourd plants were observed on MS medium fortified with different concentrations of BAP, Kn, 2,4-D and NAA alone and BAP + 2,4-D in different combinations. Average effect of different concentrations (2.0, 2.5, 3.0, 3.5, 4.0, 4.5 mg/l) of growth regulators with MS medium on different explants of Trichosanthes dioica Roxb. on callus induction was observed (Table 1). This showed good callus growth in all the three explants except the root explants (Figures 1-3). Almost 80%-84% of shoot tip and nodal explants and about 58% of leaf explants showed callus induction in all media used (Table 2). The root explants showed some swelling but no callus formation was observed. Similar result related to the response of explants to growth regulators on callus induction was reported in Stevia rebaudiana through indirect organogenesis [5].
| Plant growth regulators (mg/l) | Callus induction ( % ) | |||
|---|---|---|---|---|
| Node | Shoot tip | Leaf | Root | |
| BAP | 84 | 80 | 58 | - |
| Kn | 80 | 76 | 50 | - |
| 2,4-D | 78 | 80 | 60 | - |
| NAA | 70 | 75 | 58 | - |
Table 1: Average effect of different concentrations (2.0, 2.5, 3.0, 3.5, 4.0, 4.5 mg/l) of growth regulators with MS medium on different explants of Trichosanthes dioica Roxb. on callus induction.
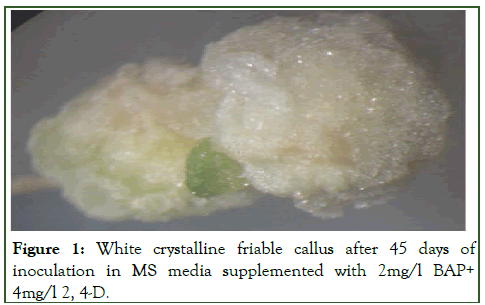
Figure 1: White crystalline friable callus after 45 days of inoculation in MS media supplemented with 2mg/l BAP+ 4mg/l 2, 4-D.

Figure 2: Off white to greenish white callus formed after 30 days of inoculation.

Figure 3: Origin of organogenesis seen in pale yellow callus sub cultured on MS media supplemented with 1.5 mg/l BAP + 0.3 mg/l NAA after 14 days of culture.
| Growth regulators mg/l | Morphogenic response of explants on callus induction | Amount of callus produced | ||
|---|---|---|---|---|
| Nodal explants | Shoot tip explants | Nodal explants | Shoot tip explants | |
| BAP | ||||
| 1.5 | Swelling of the explants | Swelling of the explants | - | - |
| 2 | White, crystalline | Creamy, soft, friable | ++ | ++ |
| 2.5 | White, crystalline | Creamy, soft, friable | +++ | +++ |
| 3 | White, crystalline | Creamy, soft, friable | ++ | + |
| 3.5 | White, crystalline | Creamy, soft, friable | ++ | + |
| 4 | White, crystalline | Swelling of the explants | - | - |
| 4.5 | - | - | - | - |
| Kn | ||||
| 1.5 | Swelling of the explants | Swelling of the explants | - | - |
| 2 | Swelling of the explants | Pale, soft, friable | - | ++ |
| 2.5 | Pale, compact | Creamy, soft, friable | + | ++ |
| 3 | White, crystalline | Creamy, compact | ++ | + |
| 3.5 | White, compact | Creamy, compact | ++ | + |
| 4 | White, compact | Swelling of the explants | + | - |
| 4.5 | - | - | - | - |
| BAP+2,4-D | ||||
| 2.0+2.0 | Creamy, soft, compact | Creamy, soft, friable | + | + |
| 2.0+2.5 | Creamy white, compact | Creamy, soft, friable | + | + |
| 2.0+3.0 | White, crystalline, friable | White, crystalline, friable | ++ | ++ |
| 2.0+3.5 | White, crystalline, friable | White, shiny crystalline, friable | +++ | +++ |
| 2.0+4.0 | White, crystalline, friable | White, shiny crystalline, friable | ++++ | +++ |
| 2.0+4.5 | Creamy, soft | Creamy, soft | ++ | + |
Note: -=No growth, +=Low growth, ++=Normal growth, +++=High growth, ++++=Optimum growth
Table 2: Effect of different concentrations and combinations of growth regulator on nature, colour, callus induction and callus proliferation using shoot tip and nodal explants of Trichosanthes dioica Roxb. after 45 days of culture.
In spite of the fact that BAP was found best for multiple shoot induction but higher concentration (3.0, 3.5, 4.0 mg/l) produced a large amount of callus which suppressed shoot elongation (Table 3). These findings are similar to those of Joshkutty et al., in Coccinia indica and in Trichosanthes dioica Roxb. Although visible callus induction started after 15 days of inoculation but the optimum callus formation was observed in MS+(2mg/l BAP+4mg/l 2,4-D) media after 45 days of inoculation. Contrary to the result obtained in the present investigation Saurabh et al., reported that MS medium with lower concentration of BA (0.5 mg/l) and 2, 4-D (0.5 mg/l) was the best medium for efficient callus induction in case of Trichosanthes dioica Roxb [6]. The callus formation and regeneration varied both with the type of explants and kind of supplements used.
The callus varied from shiny white, creamy white, pale yellow to greenish white in colour (Figures 4-6). It was crystalline and friable in nature and its friability retained even after several subcultures without any morphogenesis. The colour and nature of the callus also varied with the type of explants used and the concentration of different hormones. In some leaf explants roots were also generated along with callus which turned brown after 30 days of inoculation [7]. Gunasena and Senarath, also reported that leaf was better than nodal segment as explants for callus induction in case of stevia. Saurabh et al., observed that nodal explants were the best explants for callusing and direct embryogenesis. Genotypic dependent response in callus formation was also reported by Al-Hussaini et al.
| Plant growth regulators (mg/l) | Â Shoots regenerated (%) | No of shoots per culture | Â Shoot length (cm) | |||
|---|---|---|---|---|---|---|
| Node derived callus | Shoot tip derived callus | Node derived callus | Shoot tip derived callus | Node derived callus | Shoot tip derived callus | |
| BAP | ||||||
| 0.5 | 40 | 44 | 00.75 ± 0.35 | 00.64 ± 0.54 | 01.20 ± 0.28 | 01.88 ± 0.57 |
| 1 | 60 | 58 | 01.85 ± 1.00 | 01.40 ± 0.64 | 01.62 ± 0.50 | 03.42 ± 0.54 |
| 1.5 | 75 | 80.6 | 02.50 ± 0.73 |  03.52 ± 0.72 | 02.42 ± 0.59 | 04.42 ± 0.35 |
| 2 | 68 | 75 | 02.00 ± 0.62 | 02.22 ± 1.00 | 02.00 ± 0.44 | 02.44 ± 0.52 |
| 2.5 | 55 | 65 | 01.00 ± 0.62 | 01.57 ± 0.32 | 01.00 ± 0.20 | 01.52 ± 0.38 |
| 2,4-D | ||||||
| 1 | - | - | - | - | - | - |
| 1.5 | - | - | - | - | - | - |
| 2 | 20 | 33 | 02.00 ± 0.54 | 02.44 ± 0.34 | 02.50 ± 0.79 | 03.00 ± 0.42 |
| 2.5 | 10 | 22 | 01.00 ± 0.54 | 01.34 ± 0.56 | 02.00 ± 0.88 | 02.70 ± 0.39 |
| BAP+NAA | ||||||
| 1.5+0.1 | 74 | 78 | 03.33 ± 0.74 |  05.00 ± 0.66 | 02.12 ± 0.34 | 02.45 ± 0.44 |
| 1.5+0.2 | 80 | 86 | 05.44 ± 0.62 |  06.68 ± 0.34 | 02.34 ± 0.56 | 02.89 ± 0.56 |
| 1.5+0.3 | 84 | 90 | 05.78 ± 0.34 |  08.00 ± 0.64 | 03.22 ± 0.84 | 04.88 ± 0.74 |
| 1.5+0.5 | 80 | 85 | 04.86 ± 0.48 |  05.54 ± 0.22 | 03.46 ± 0.66 | 03.56 ± 0.24 |
| 1.5+1.0 | 78 | 80 | 03.66 ± 0.22 |  04.48 ± 0.24 | 02.24 ± 0.54 | 02.66 ± 0.64 |
| 1.5+1.5 | 60 | 69 | 02.22 ± 0.78 |  02.76 ± 0.54 | 01.45 ± 0.23 | 01.88 ± 0.44 |
Table 3: Effect of different combinations of growth regulators on shoot multiplication from shoot tip and axenic node derived callus on MS medium after four weeks.
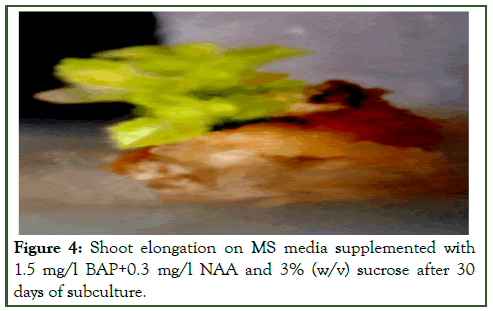
Figure 4: Shoot elongation on MS media supplemented with 1.5 mg/l BAP+0.3 mg/l NAA and 3% (w/v) sucrose after 30 days of subculture.
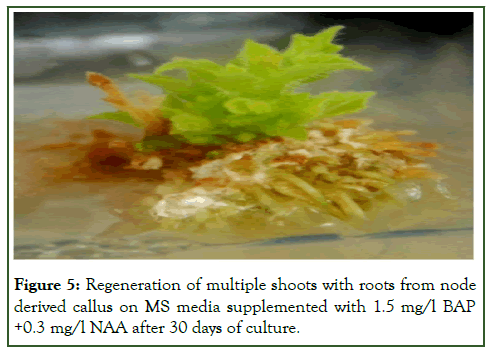
Figure 5: Regeneration of multiple shoots with roots from node derived callus on MS media supplemented with 1.5 mg/l BAP +0.3 mg/l NAA after 30 days of culture.

Figure 6: Professed rooting of in vitro obtained shoot on half strength MS media+1.0 mg/l IBA+3% (w/v) sucrose after four weeks of culture.
Well-developed callus was sub cultured on MS medium supplemented with different concentrations of BAP, Kn, 2, 4-D and NAA alone and on MS medium supplemented with different combinations of BAP and NAA. In earlier reports of Komal R., it is evident that a combination of BAP and NAA showed the highest percentage of shoot regeneration and maximum number of shoots per explant in Trichosanthes dioica Roxb. In the present study also similar data was observed. Sub cultures were done at every 45 days interval [8]. To avoid browning of the medium, frequent transfer of explants to a fresh medium has been earlier reported by Shutter et al., and Amjad and Srivastava in regeneration of strawberry and the same was found to be effective in the present investigation.
Efficient regeneration of multiple shoots was observed from shoot tip and nodal explants on MS medium fortified with 1.5 mg/l BAP. An average of 02.50 ± 0.73 and 03.52 ± 0.72 shoot buds were regenerated having a length of (0.2.42 ± 0.59 and 04.42 ± 0.35) cm from shoot tip and nodal explants respectively. The similar effect of BAP for regeneration of multiple shoots were also observed in different plants like Momordica charantia, Trichosanthes cucumerina L. var. cucumerina and in Trichosanthes dioica Roxb. BAP was found to be more potent in the induction of shoot buds as earlier observed by many workers [9]. Although shoot buds were formed in most of the combinations after 14 days of culture but proliferation of about 8 shoots was obtained from the callus cultures on MS medium fortified with (1.5 mg/l BAP+0.3 mg/l NAA) after 30 days of culture with an average length of 4.88 cm ± 0.74 cm. Results of the present investigation agrees with the investigations done in different plants like Momordica dioica, Cucurbita moschata, Trichosanthes dioica, Cucurbita foetidissima. Some reports show that BAP and Kn were required in optimal quantity for shoot proliferation in Mentha arvensis, Plumbago zeylanica.
BAP was more potent in induction of shoot buds as earlier observed by many workers. BAP plays a significant role in the regeneration of multiple shoots also reported in different plants like Trichosanthes cucumerina L. var. cucumerina, Trichosanthes dioica Roxb. and Momordica charantia [10].
Each proliferated and adventitious shoot was cut and sub cultured on half-strength MS media supplemented with IBA, IAA and NAA (Table 4). Either IAA or IBA at various concentrations are commonly used auxins to induce rooting from the explants both in culture and in vivo conditions. Varisai et al., were successful in inducing rooting of the shoot tip derived shoots on MS medium supplemented with IBA at various concentrations. In most of the cucurbits the root induction was achieved on either basal MS medium alone or with very low level of auxin as also observed in T. dioica. In the present study also halfstrength MS media supplemented with 1.0 mg/l IBA showed efficient rooting (29 ± 0.36 roots/shoot) and an average length of 8.48 ± 0.52 cm within seven to eight days (Figure 7). With a higher concentration of IBA (2.0 mg/l-3.5 mg/l) malformed and very thick roots were observed which were not suitable for field transfer. This finding is similar to the findings of Komal et al. In lower concentrations of IBA (0.3 mg/l-0.1 mg/l) direct rooting/ rooting with some callusing was observed in most of the cases. Number of roots per shoot and the percentage of shoots showing rhizogenesis varied with different concentrations of IAA, IBA and NAA. Within 30-35 days the plantlets were ready with strong professed roots to be hardened [11]. So, it was found that halfstrength MS supplemented with IBA proved to be better than IAA and NAA for rooting of pointed gourd. IBA has proved to be an effective rooting hormone and is continuously used by many workers. Contrary to findings of present investigation Saurabh et al., reported that 0.5 mg/L IAA was found more efficient for root development in pointed gourd.
| Auxin (mg/I) | Rooting (%) | Rooting period (days) | No. of roots/shoot | Root length (cm) |
|---|---|---|---|---|
| IAA | ||||
| 0.1 | - | - | - | - |
| 0.5 | 42.44 | 12-00 | 10 ± 1.14 | 3.78 ± 0.22 |
| 1 | 60.62 | 10-00 | 22 ± 1.14 | 6.42 ± 0.44 |
| 1.5 | 52.2 | 10-12 | 21 ± 0.88 | 4.46 ± 0.14 |
| IBA | ||||
| 0.1 | - | - | Â - | - |
| 0.5 | 46.6 | 08-10 | 06 ± 0.14 | 3.22 ± 0.24 |
| 1 | 76.34 | 06-07 | 29 ± 0.36 | 8.48 ± 0.52 |
| 1.5 | 58.24 | 10-00 | 18 ± 0.72 | 7.46 ± 0.24 |
| NAA | ||||
| 0.1 | - | - | - | - |
| 0.5 | 46.44 | 14-16 | 06 ± 0.24 | 2.16 ± 0.36 |
| 1 | 71.22 | 14-00 | 19 ± 0.48 | 3.36 ± 0.44 |
| 1.5 | 59 | 12-14 | 11 ± 0.16 | 2.00 ± 0.34 |
Table 4: Effect of different growth regulators on rhizogenesis of regenerated shoots on half strength MS medium after four weeks.

Figure 7: Plants with professed roots after 35 days of inoculation in half strength MS media fortified with 1.0 mg/l IBA.
The in vitro raised plantlets were sequentially acclimatized by transferring them in an inorganic MS salt solution, then to sterile distilled water and tap water before planting in potted soil. Within 30-35 days the plantlets were ready with strong professed roots to be hardened. The plantlets were successfully established in earthen pots containing a mixture of sterilized soil, sand and farmyard mixture in the ratio of 1:1:1 showing 60% survival rate (Figure 8). These were then transferred to natural soil and environment (Figure 9) [12].
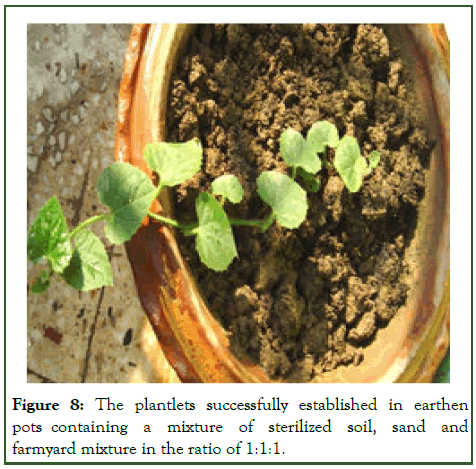
Figure 8: The plantlets successfully established in earthen pots containing a mixture of sterilized soil, sand and farmyard mixture in the ratio of 1:1:1.
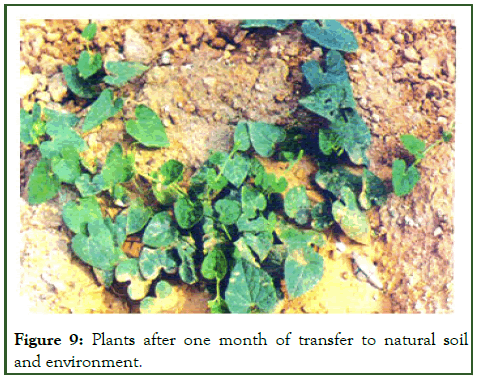
Figure 9: Plants after one month of transfer to natural soil and environment.
Conclusion
The present study reveals an efficient and reproducible protocol for multiple shoot regeneration from callus cultures in Trichosanthes dioica Roxb. an important medicinal and commercial vegetable plant. The plantlets obtained through callus cultures have a great potential in obtaining somaclonal variants and in vitro mutation breeding. There may be a difference in the ploidy level of these plants. These variations are still to be analyzed both qualitatively and quantitatively.
Acknowledgement
The author is thankful to the department of science and technology, SERC div. New Delhi (India) for providing financial support in the form of Women Scientist Scheme (WOS-A). I express my gratitude to Dr. A.K. Pandey, department of botany, Delhi university, Delhi for helping in manuscript preparation. I also give thanks to the head, Botany, Patna university, Patna for providing the lab facilities.
References
- Singh H, Kumar S, Singh BD. In vitro conservation of pointed gourd (Trichosanthes dioica) germplasm through slow-growth shoot cultures: Effect of flurprimidol and triiodobenzoic acid. Sci Hortic. 2015;182:41-46.
- Xu KD, Chang YX, Zhang J, Wang PL, Wu JX, Li YY, et al. A lower pH value benefits regeneration of Trichosanthes kirilowii by somatic embryogenesis, involving Rhizoid Tubers (RTBs), a novel structure. Sci Rep. 2015;5(1):8823.
[Crossref] [Google Scholar] [PubMed]
- Pillai GS, Martin G, Raghu AV, Lyric PS, Balachandran I, Ravindran PN. Optimizing culture conditions for in vitro propagation of Trichosanthes cucumerina L.: An important medicinal plant. J Herbs Spices Med Plants. 2008;14(1-2):13-28.
- Mali AM, Chavan NS. In vitro rapid regeneration through direct organogenesis and ex-vitro establishment of Cucumis trigonus Roxb.-an underutilized pharmaceutically important cucurbit. Ind Crops Prod. 2016;83:48-54.
- Anjusha S, Gangaprasad A. Callus culture and in vitro production of anthraquinone in Gynochthodes umbellata (L.) Razafim. and B. Bremer (Rubiaceae). Ind Crops Prod. 2017;95:608-614.
- Prajapati RV, Hakim MM, Patel IC. In vitro multiplication and phytochemical analysis of in vivo and in vitro plant parts of Pueraria tuberosa (Roxb. Willd DC). Egypt J Agric Res. 2023;101(1):224-235.
- Ramírez-Mosqueda MA, Cruz-Cruz CA, Atlahua-Temoxtle J, Bello-Bello JJ. In vitro conservation and regeneration of Laelia anceps Lindl. S Afr J Bot. 2019;121:219-223.
- Arciniega-Carreon IY, Oliver-Salvador C, Ramorez-Sotelo MG, Salas CE. Efficient in vitro plant regeneration from internode explants of Ibervillea sonorae: An antidiabetic medicinal plant. HortScience. 2017;52(7):1000-1005.
- El-Gedawey HI, Abido AI, Gaber MK. Impact of kinetin and benzyladenine on growth performance of croton in vitro. Alex Sci Exch J. 2020;41(7-9):381-391.
- Al-Qurainy F, Nadeem M, Khan S, Alansi S, Tarroum M, Al-Ameri AA, et al. Rapid plant regeneration, validation of genetic integrity by ISSR markers and conservation of Reseda pentagyna an endemic plant growing in Saudi Arabia. Saudi J Biol Sci. 2018;25(1):111-116.
[Crossref] [Google Scholar] [PubMed]
- Thakur M, Sharma V, Luharch R. Propagation of plum (Prunus salicina L.) cultivar Frontier in vitro through control of Shoot Tip Necrosis (STN) and validation of genetic integrity using ISSR markers. Plant Physiol Rep. 2021;26:238-246.
- Upadhyay A, Shahzad A, Ahmad Z. In vitro propagation and assessment of genetic uniformity along with chemical characterization in Hildegardia populifolia (Roxb.) Schott and Endl.: A critically endangered medicinal tree. In Vitro Cell Dev Biol Plant. 2020;56:803-816.
Citation: Komal R (2024) In Vitro Regeneration of Trichosanthes dioica Roxb. Plantlets through Callus Culture. Agrotechnology. 13:377.
Copyright: © 2024 Komal R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.