Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 6
Emerging Antifungal Resistance in Falco Species: A Novel Model for Human Medicine
Sibi Das1*, Sethi Das Christu2, Christudas Silvanose3, Ambili Binoy3, Panagiotis Azmanis3, Antonio Di Somma3 and Jibin V Gladston42Department of Medicine, Aster CMI Hospital, Bengaluru, India
3Department of Biology, Dubai Falcon Hospital, Dubai, United Arab Emirates
4Department of Pediatrics, District Hospital, Rajasthan, India
Received: 24-Mar-2023, Manuscript No. BLM-23-20238; Editor assigned: 27-Mar-2023, Pre QC No. BLM-23-20238 (PQ); Reviewed: 10-Apr-2023, QC No. BLM-23-20238; Revised: 29-May-2023, Manuscript No. BLM-23-20238 (R); Published: 05-Jun-2023, DOI: 10.35248/0974-8369.23.15.586
Abstract
Antifungal resistance is a growing concern in the medical community, as fungal infections are becoming increasingly difficult to treat. In this study, Falco species were used as novel models for studying antifungal resistance since aspergillosis, a fungal disease is common in falcons. The most isolated fungi in this study were A. fumigatus, A. flavus, A. niger, and A. terreus, all of which can cause aspergillosis in falcons. Isavuconazole, posaconazole, and voriconazole had the lowest MICs among the drugs tested, suggesting that they may be effective treatment options. However, this study showed that 34% of the isolates were resistant to itraconazole, which is an increase from 21% in 2006. There is no resistance to voriconazole found in 2006 and 2011, but a 9% resistance rate was noted in 2022. Similarly, there is no resistance to posaconazole and isavuconazole was noticed in 2011, but the resistance of 4.7% and 5.8%, respectively was noticed in 2022. Amphotericin B, which showed a 51% resistance rate in 2006, became even more resistant with an 80% rate in 2011, leading to its discontinuation from the treatment of falcons against aspergillosis. This study highlights a significant rise in antifungal resistance, which is a challenging problem in both falcon and human medicine.
Keywords
Posaconazole, Isavuconazole, Itraconazole, Voriconazole, A. fumigatus
Introduction
The emergence of resistant fungal pathogens posed a significant threat to antifungal treatments, particularly for immunocompromised patients and individuals with chronic conditions. To better understand antifungal resistance during treatment and develop new strategies for combating fungal infections, there was increasing interest in studying animal models. Falcons (Falco species) were particularly susceptible to fungal infections, including aspergillosis, which was a common cause of morbidity and mortality among falcons [1-4]. Recent studies had reported the emergence of antifungal resistance in falcons, making them an interesting model for studying antifungal resistance mechanisms [5].
Antifungal resistance in falcons was an increasingly concerning issue in avian medicine. Aspergillosis was caused by Aspergillus species, a fungal pathogen that could colonize the respiratory system and cause respiratory distress, chronic infections, and even death in severe cases [6]. Overuse or inappropriate use of antifungal drugs in captive birds, as well as the spread of resistant fungal strains between birds in falconry or rehabilitation centers, could lead to antifungal resistance in falcons [7]. The study of antifungal resistance in falcons was essential for several reasons. Firstly, falcons were a valuable animal model for studying fungal infections and host pathogen interactions. Secondly, the emergence of antifungal resistance in falcons could have implications for managing fungal infections in other animal species, including humans. Lastly, studying antifungal resistance in falcons could offer insights into resistance during treatment, which could identify new therapeutic targets for both animal and human medicine. Fungal diseases had emerged in association with post COVID-19, as the COVID-19 virus weakened the immune system, making individuals more susceptible to fungal infections. Thus, it was crucial to develop new and effective antifungal treatments to address this growing concern.
This manuscript investigated the emerging antifungal resistance in falcons, with a particular focus on triazole resistance, and its potential implications for human medicine. Thus, compared the findings with existing data on human antifungal resistance to identify potential areas of overlap and divergence. By studying the incidence and prevalence of aspergillosis in falcons, which was higher than any other species, advanced diagnosis and treatment procedures had been developed in falcon medicine, including in vivo and in vitro MIC antifungal studies. The project had the potential to provide important insights into the mechanisms of antifungal resistance and to identify novel therapeutic targets that could be applied to both animal and human medicine. By examining a non-traditional model such as falcons, we could also gain a new perspective on the evolution and spread of antifungal resistance and contribute to the development of innovative and effective strategies for managing fungal infections in both animals and humans.
Materials and Methods
Biopsy samples were collected during the endoscopy of air sacs of eighty-six falcons which include Peregrine falcons (Falco peregrinus), saker falcons (Falco cherrug), Gyr (Falco rusticolus), and hybrid falcons such as Gyr x Peregrine (F. rusticolus x F. peregrinus) and Gyr x Saker (F. rusticolus x F. cherrug), affected with lower respiratory tract fungal infection. Samples were cultured in Sabouraud’s Chloramphenicol Agar (SCA) and incubated at 37°C for 3-5 days. Fungi were identified by culture appearance and morphological characteristics under the microscope using lactophenol aniline blue stain preparation. Antifungal studies were done in RPMI media using antifungal MIC E-test strips (bioMerieux, France) and the plates were incubated at 37°C in an incubator for 48 hours. The Minimum Inhibitory Concentration (MIC) was recorded as the lowest concentration of the antifungal agent that inhibited fungal growth. The first phase of the study was done in 2006 with 100 isolates of Aspergillus species, the second phase of the study was done in 2011 with 117 isolates of Aspergillus species, and the current study, the third phase was done in 2023 with 86 fungal isolates to compare the evolution of antifungal resistance in the past 15 years.
Results
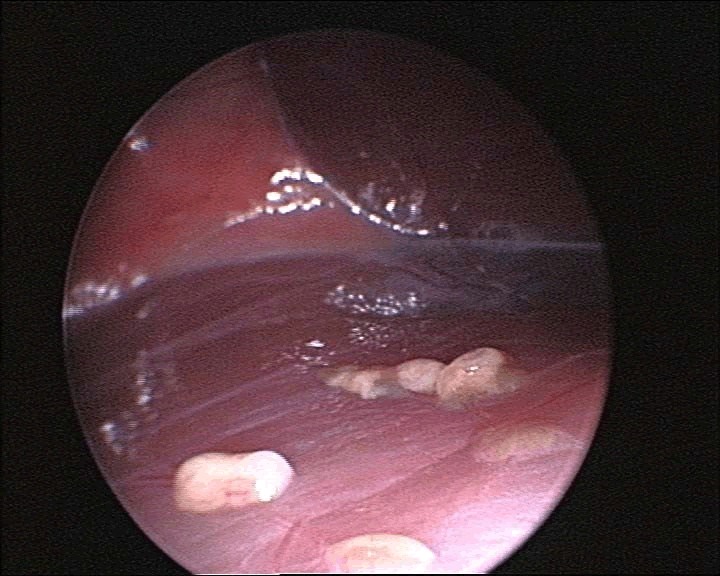
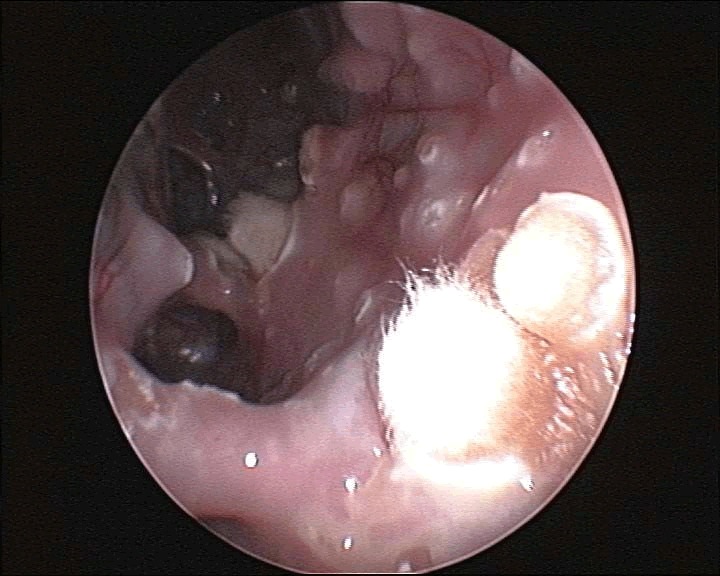
Aspergillosis was confirmed through endoscopic examinations, cytological examinations, and mycology cultures. During an endoscopy, characteristic signs of aspergillosis may include the presence of nodules or growths on the lining of the respiratory tract (Figure 1). These growths can appear as whitish yellow patches or raised bumps (Figure 2).
Figure 1: Endoscopic view of multiple aspergillomas showing multiple nodular raised growths in the air sac of a gyrfalcon.
Figure 2: Endoscopic view of multiple aspergillomas with few sporulating aspergillomas and raised bumps in the air sac of a gyrfalcon.
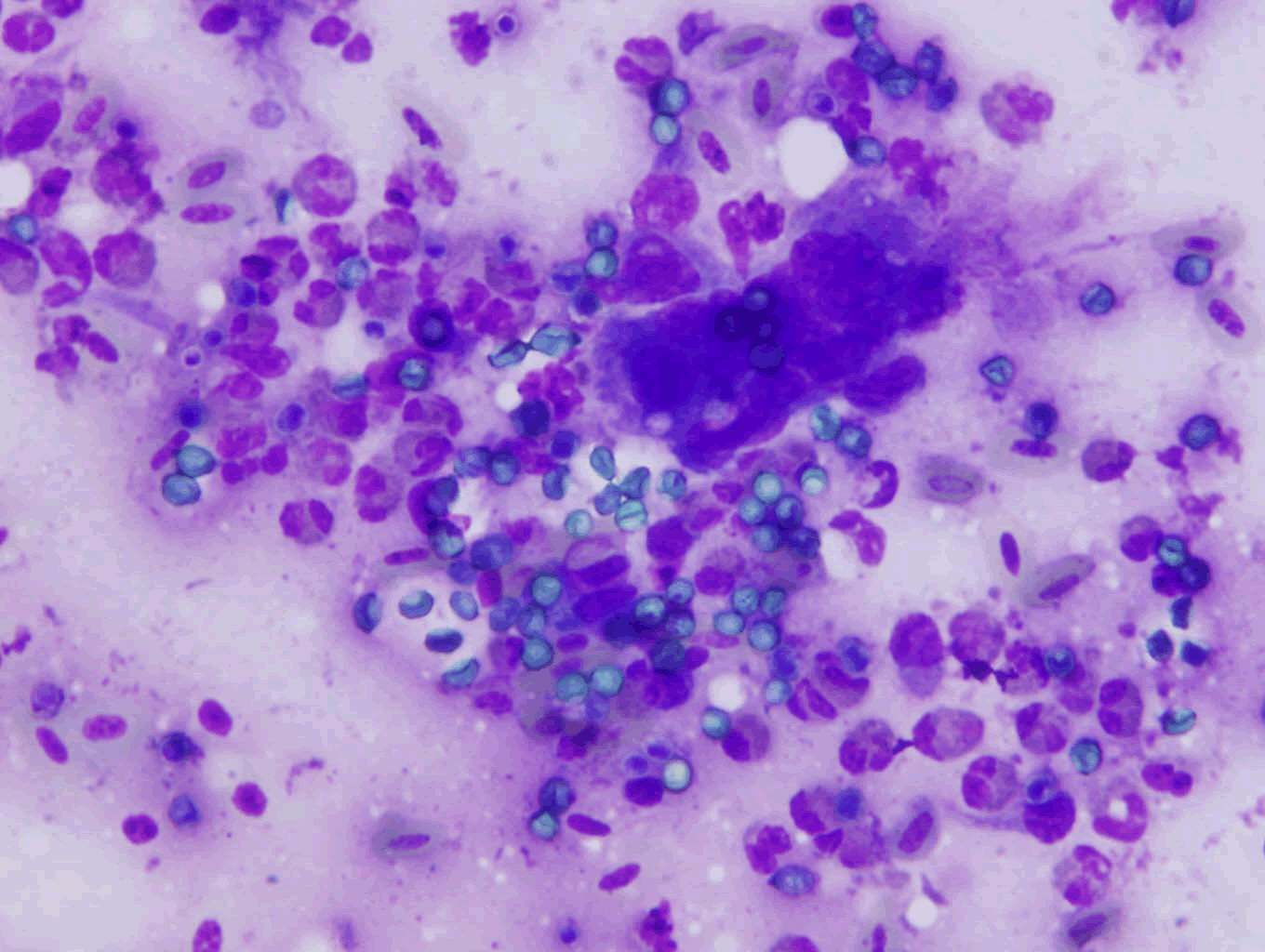
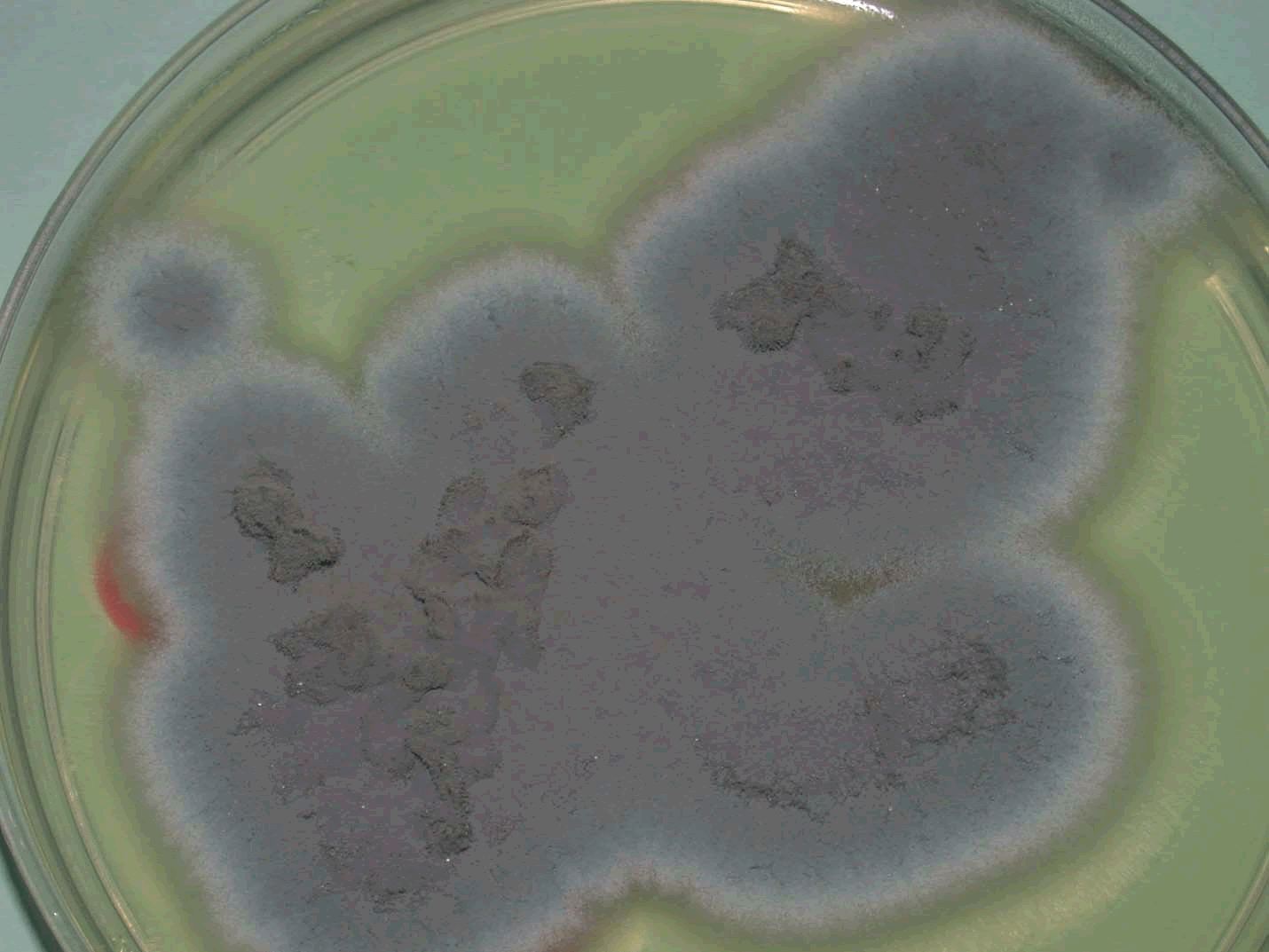
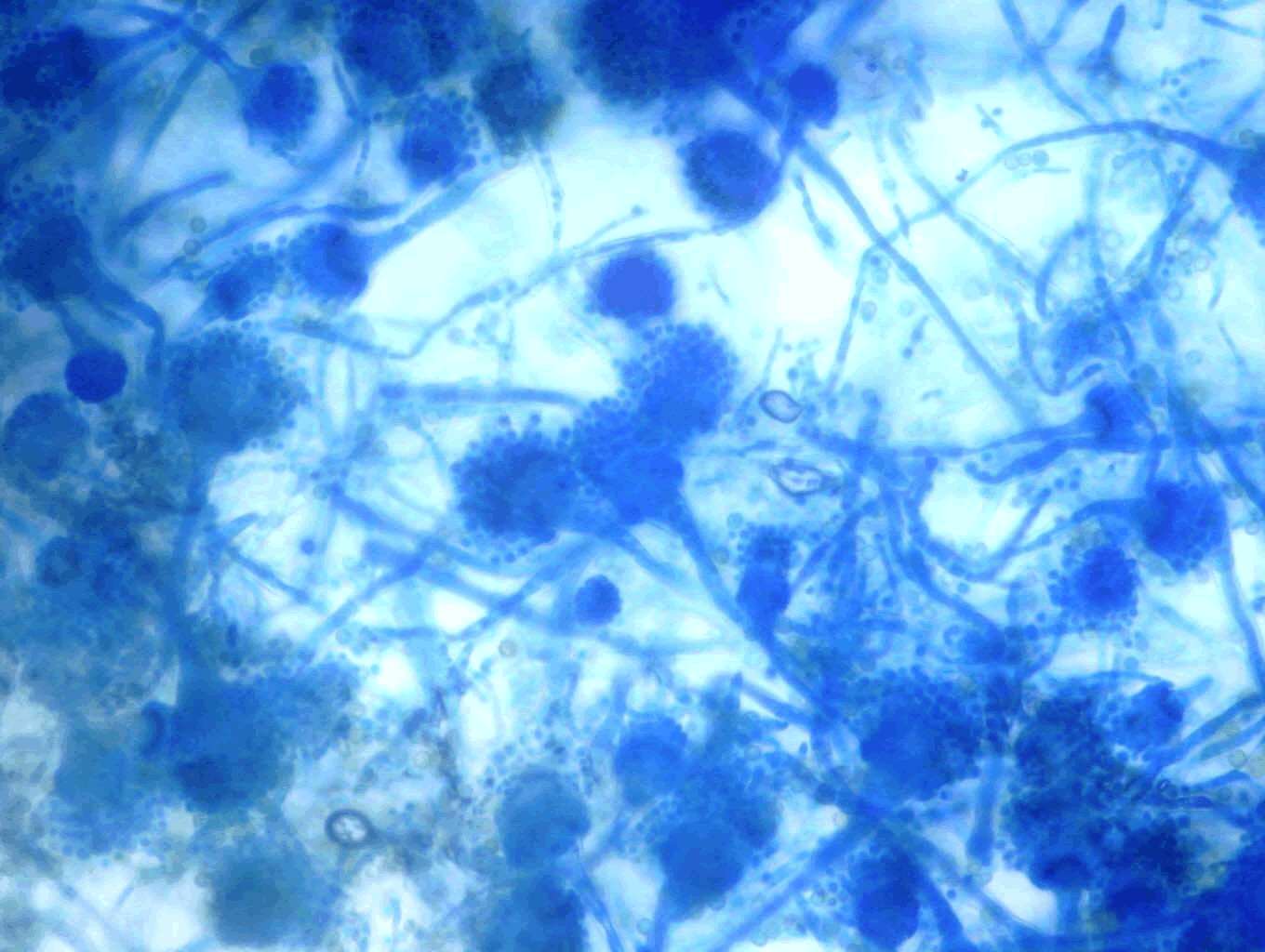
The diagnosis of aspergillosis through cytology includes the appearance of fungal elements and cellular changes depending on the site of infection (Figure 3). Confirmation of aspergillosis was through fungus culture (Figure 4) and microscopic examination of the culture under lactophenol blue stain preparation for the morphology of conidiophores and hyphae (Figure 5).
Figure 3: Cytology imprint smear of the biopsy sample collected from the air sac of a falcon with aspergillosis. Note the giant cell formation and fungal spores in an inflammatory cell background.
Figure 4: Culture appearance of Aspergillus fumigatus isolated from the air sac of Gyr falcon with aspergillosis showing bluish colonies after 72 hours of incubation.
Figure 5: Microscopic appearance of Aspergillus species conidiophores, conidiospores and septate hyphae.
The Minimum Inhibitory Concentration (MIC) value represents the lowest concentration of a drug required to inhibit the isolate, and the lowest MIC value indicates greater effectiveness against the fungal isolate. Table 1 reports the median MIC values and range of MIC values for each antifungal agent tested against each fungal species obtained from the air sac of falcons.
Table 1: MIC of fungal isolates from the air sac of falcons with ≤ 1 µg/ml.
| Fungi isolated (n)* | Posaconazole | Isavuconazole | Voriconazole | Itraconazole |
|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | |
| A. fumigatus (36) | 0.3 (0.094-1) | 0.19 (0.032-1) | 0.19 (0.032-1) | 1 (0.25-1) |
| A. flavus (13) | 0.75 (00.125-1) | 0.38 (0.25-1) | 0.025 (0.032-0.75) | 0.75 (0.38-1) |
| A. niger (18) | 0.38 (0.125-1) | 0.25 (0.125-0.75) | 0.25 (0.125-1) | 0.75 (0.2-1) |
| A. terreus (14) | 0.38 (0.19-0.75) | 0.38 (0.064-0.75) | 0.5 (0.094-0.75) | 0.75 (0.25-1) |
| A. nidulans (2) | 0.19 (0.19-1) | 0.064 (0.02-0.125) | 0.094 (0.047-0.125) | 0.5 (0.38-1) |
| A. versicolor (1) | 0.25 (0.25) | 0.023 (0.023) | 0.064 (0.064) | 0.25 (0.25) |
| Mucor species (2) | 0.75 (0.75) | 0.875 (0.25-1) | 0 | 0.625 (0.25-1) |
Note: *n= total number of isolates
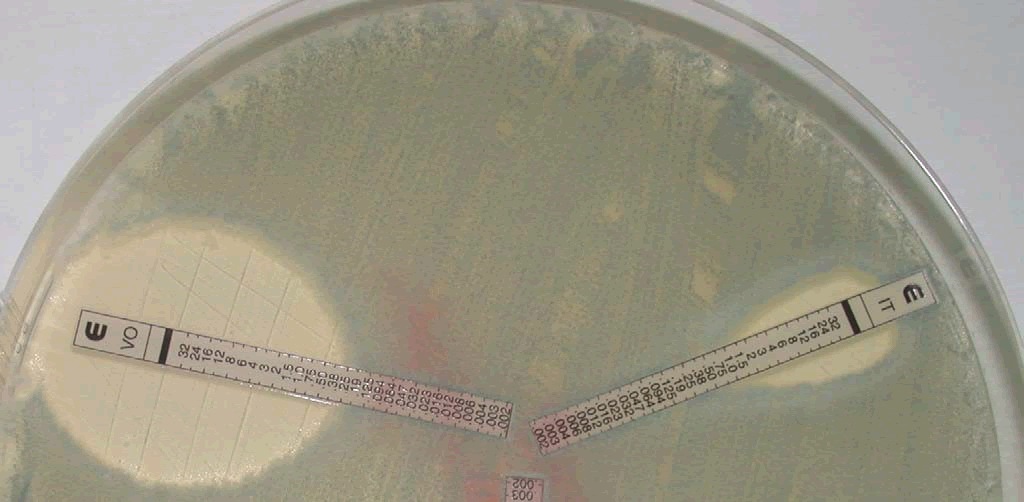
Figure 6: E-test reading of A. fumigatus, an isolate from the air sac of a gyrfalcon showing voriconazole with 0.38 µg/ml and itraconazole with 1 µg/ml.
The results of the study showed that posaconazole and isavuconazole were effective against A. niger, with median MIC values of 0.38 µg/ml and 0.25 µg/ml, respectively. Voriconazole also demonstrated good activity against A. niger, with a median MIC value of 0.25 µg/ml, while itraconazole showed a higher median MIC value of 0.75 µg/ml. A. terreus was most susceptible to isavuconazole and posaconazole, with a median MIC value of 0.38 µg/ml. Voriconazole showed similar activity against A. terreus, with a median MIC value of 0.5 µg/ml, while itraconazole had a higher median MIC value of 0.75 µg/ml. A. nidulans and A. versicolor showed good antifungal activity against all antifungals, but they were not commonly isolated. The two isolates of Mucor species were sensitive to posaconazole and itraconazole, with median MIC values of 0.75 µg/ml and 0.625 µg/ml, respectively. Isavuconazole showed a higher MIC value of 0.875 µg/ml, while voriconazole was found to be resistant against Mucor species.
Table 2 shows the mic of antifungal drugs against fungal isolates from the air sac of falcons with >1 µg/ml and it falls under the resistant level in birds. A. fumigatus isolated from 3 cases was resistant to posaconazole and isavuconazole with a median MIC of 1.5 µg/ml, 4 cases were resistant to voriconazole with a median MIC of 3 µg/ml, and 15 isolates were resistant to itraconazole with a median MIC of 3 µg/ml. The resistance of A. flavus includes one isolate to voriconazole and 3 isolates to itraconazole with MIC of 1.5 µg/ml. Each isolate of A. niger was resistant to isavuconazole and voriconazole with a MIC of 1.5 µg/ml, while 8 isolates were resistant to itraconazole with a median MIC of 4 µg/ml. A. terreus resistance was noticed in one case to posaconazole with 1.5 µg/ml, isavuconazole and voriconazole with 32 µg/ml; while 3 isolates of A. terreus were resistant to itraconazole with a median MIC was 1.5 µg/ml. Mucor species were isolated from two cases that were resistant to voriconazole with a median MIC >32 µg/ml.
Table 2: MIC of fungal isolates from the air sac of falcons with >1 µg/ml
| Fungi isolated | Posaconazole | (n)* | Isavuconazole | (n)* | Voriconazole | (n)* | Itraconazole | (n)* |
|---|---|---|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median | |||||
| A. fumigatus | 1.5 (1.5-4) | 3 | 1.5 (1.5-32) | 3 | 3 (1.5-32) | 4 | 3 (1.5-32) | 15 |
| A. flavus | 0 | - | 0 | 1.5 (1.5) | 1 | 1.5 (1.5-2) | 3 | |
| A. niger | 0 | 1.5 (1.5) | 1 | 1.5 (1.5) | 1 | 4 (2-32) | 8 | |
| A. terreus | 1.5 (1.5) | 1 | 32 (32) | 1 | 32(32) | 1 | 1.5 (1.5-2) | 3 |
| A. nidulans | 0 | - | 0 | - | 0 | - | 0 | |
| A. versicolor | 0 | - | 0 | - | 0 | - | 0 | |
| Mucor species | 0 | - | 0 | 32 (32) | 2 | - | 0 | |
| Total | 4 | 5 | 8 | 29 |
Note: *n=number of isolates.
Table 3 shows a comparison of antifungal resistance of Aspergillus species isolated in 2006, 2011, and 2022. These results may have important implications for the treatment and management of fungal infections in birds of prey, as well as for understanding the impact of antifungal use on the emergence of resistance in wildlife and human pathogens.
Table 3: Comparison of antifungal resistance of Aspergillus species isolated from the air sac of falcons in 2006, 2011, and 2022.
| Antifungals | 2006 (A)* | 2006 (B)* | 2011 | 2022 |
|---|---|---|---|---|
| Aspergillus sp., (resistant %) | Aspergillus sp., (resistant %) | Aspergillus sp., (resistant %) | Aspergillus sp., (resistant %) | |
| Posaconazole | - | - | 117, (00%) | 86, (4.7%) |
| Isavuconazole | - | - | - | 86, (5.8%) |
| Voriconazole | 45, (00%) | 16, (00%) | 117, (00%) | 86, (09%) |
| Itraconazole | 45, (21%) | 05, (20%) | 117, (06%) | 86, (34%) |
| Amphotericin B | 45, (51%) | 06, (100%) | 117, (80%) | - |
Note: *A-Before treatment, B-After treatment.
Discussion
MIC testing is a process of determining the lowest concentration of an antimicrobial drug that can inhibit the growth of a specific microorganism. However, the isolates in this study showed MICs higher than 1 µg/ml, indicating resistance to antifungal drugs. This highlights the need to monitor antifungal resistance in birds and develop new treatment strategies to address this issue.
This study aimed to assess the MICs of various antifungal drugs against fungal isolates obtained from the air sacs of falcons. The first phase of the study was conducted in 2006, the second phase in 2011, and the current study is the third phase, which looks back to understand the evolution of antifungal resistance over the past 15 years.
The phase 1 study in 2006 reported the MICs of fungi isolated from the air sacs of falcons before and after antifungal treatment. Before treatment, 95% of the isolates, including A. fumigatus, A. flavus, A. niger, and A. terreus, were susceptible to voriconazole at MICs up to 0.38 μg/ml, and all the isolates were susceptible at MICs up to 1 μg/ml. Before treatment, 21% of the isolates, including A. fumigatus (27.6%), A. flavus (16.6%), A. niger (100%), and A. terreus (23%), were resistant (MIC ≥ 1 μg/ml) to itraconazole. Furthermore, 51% of the isolates, including A. fumigatus (31%), A. flavus (78%), A. niger (14%), and A. terreus (77%), had MICs of over 1 μg/mL to amphotericin B. After treatment, their MICs increased significantly (8).
However, the study found no significant differences between the MICs of voriconazole and itraconazole for the different Aspergillus species before and after treatment with these antifungal agents. The findings suggest that voriconazole is highly effective against Aspergillus species in falcons, with 100% of the isolates being susceptible, while itraconazole was less effective, with 21% of the isolates showing resistance. In contrast, amphotericin B was less effective, with 51% of the isolates being resistant, and after treatment, the resistance of amphotericin B increased significantly (8).
Amphotericin B is an old antifungal drug that has been used in both human and veterinary medicine to treat systemic fungal infections. However, many reports in the literature on falcon medicine have shown that Aspergillus species can develop resistance to amphotericin B, which was a commonly used antifungal drug in the past. This has raised concerns about the drug's effectiveness in managing fungal infections in falcons, especially aspergillosis. Additionally, the drug is known to have potential adverse effects, such as renal toxicity, hypokalemia, and infusion related reactions. In falcons, amphotericin B use can also result in hepatotoxicity and nephrotoxicity [8]. Furthermore, reports have indicated that amphotericin B resistance is emerging in falcons, particularly in cases of chronic and recurrent aspergillosis. Studies have reported the emergence of amphotericin B-resistant Aspergillus fumigatus in captive falcons, leading to treatment failure and poor clinical outcomes [9]. Therefore, amphotericin B is no longer used in falcon medicine for the treatment of aspergillosis.
The phase 2 study was conducted in 2011 with 117 Aspergillus species isolates. All isolates were found to be sensitive to voriconazole and posaconazole, while 6% of the isolates were resistant to itraconazole, and 80% of the isolates were resistant to Amphotericin B. Furthermore, 86% of the isolates were resistant to ketoconazole, with a MIC>1 µg/ml. All isolates were resistant to 5-flucytosine with a MIC ≥ 2 µg/ml, caspofungin with a MIC ≥ 16 µg/ml, and fluconazole with a MIC ≥ 256 µg/ml [10]. The phase 2 study suggests that while voriconazole and posaconazole are highly effective, other commonly used antifungal agents, such as amphotericin B and ketoconazole, are less effective [11].
In the current study (2022), it was found that the resistance to itraconazole had increased by 13% compared to the resistance rate in 2006. Additionally, the resistance rate of voriconazole, which was 0% in 2006 and 2011, rose to 9% in 2023. The resistance rates for Posaconazole and isavuconazole, which were both 0% in 2011, increased to 4.7% and 5.8%, respectively, in 2023. Amphotericin B was discontinued in the treatment of falcons against aspergillosis due to its high resistance rate of 80% in 2011.
Itraconazole resistance has been reported in both humans and animals and may result from mutations in the CYP51 a gene responsible for the drug target [12]. However, a noticeable change in a decrease to itraconazole resistance was found in 2011 and it was due to the wide use of voriconazole in falcons and thus results in automatically less use of itraconazole (13-15). In vivo clinical studies have shown that voriconazole is an effective and safe treatment for aspergillosis in falcons [13]. Voriconazole has also been found to be the most active drug in many in vitro studies, with lower MIC values compared to other drugs [14]. However, reports of resistance to voriconazole in some avian isolates of Aspergillus species are a growing concern in the management of aspergillosis in birds, including falcons [15]. Voriconazole may not be an appropriate choice for the treatment of Mucor mycosis, as it has been found to be resistant to Mucor species.
Fluconazole is an antifungal medication commonly used in the treatment of human medicine, but its efficacy in treating avian aspergillosis is limited. There are several reasons why fluconazole may fail in the treatment of falcon aspergillosis. Firstly, Aspergillus species in birds are often resistant to fluconazole, and other antifungal medications such as itraconazole and voriconazole are preferred. Additionally, avian aspergillosis is often a systemic disease, meaning that the infection has spread beyond the respiratory tract and into other organs.
Posaconazole is an antifungal medication that belongs to the class of triazole drugs. It is a broad-spectrum antifungal agent that has activity against a wide range of fungal pathogens, including Aspergillus species, which can cause aspergillosis in birds, including falcons. Posaconazole has been shown to be effective in the treatment of avian aspergillosis, including in falcons. Recent studies found that a small percentage of the Aspergillus isolates were resistant to posaconazole, with MICs that were higher than the clinical breakpoints for this medication and a study noted that the falcon had been treated with multiple antifungal medications over an extended period, which may have contributed to the development of resistance.
Isavuconazole is a relatively new antifungal medication that has shown promise in the treatment of aspergillosis in birds, including falcons. Isavuconazole belongs to the class of triazole antifungals, which work by inhibiting the synthesis of ergosterol, a critical component of fungal cell membranes. A few studies have reported the use of isavuconazole for the treatment of aspergillosis in falcons. While the use of isavuconazole in falcons and other avian species is still relatively limited. MIC studies found that isavuconazole had the lowest MICs among the antifungal drugs tested, indicating that it may be a promising treatment option for aspergillosis in falcons. These results are consistent with previous studies that have shown isavuconazole to be effective against Aspergillus infections in humans and animals. However, this study also identified some isolates with MICs higher than 1 µg/ml, indicating resistance to antifungal drugs, particularly in A. fumigatus. Antifungal resistance is a growing problem in both human and veterinary medicine, and the emergence of resistant strains of Aspergillus species is a significant challenge to the effective treatment of aspergillosis.
Comparing the MIC values obtained in this study with those reported in the literature for human fungal infections, found that the MIC values for most of the isolates were within the susceptible range for human infections. The median MIC values of voriconazole and itraconazole for A. fumigatus isolates in this study were 0.19 µg/ml and 1 µg/ml, respectively, which are lower than the clinical breakpoints for susceptibility in humans, 1 µg/ml and 2 µg/ml, respectively. However, we observed some differences in MIC values between this study and those reported in the literature for human infections. For instance, the median MIC value of posaconazole for A. fumigatus isolates in this study was 0.3 µg/ml, which is slightly higher than the clinical breakpoint for susceptibility in humans, 0.125 µg/ml. Similarly, the median MIC value of isavuconazole for A. flavus isolates in this study was 0.38 µg/ml, which is higher than the clinical breakpoint for susceptibility in humans, 0.03 µg/ml.
Overall, these results suggest that the antifungal agents tested in this study could be effective in treating fungal infections in falcons caused by the isolates tested. However, caution should be exercised when extrapolating MIC values from veterinary to human medicine, as there can be differences in the pharmacokinetics and pharmacodynamics of antifungal agents between different species. Additionally, it is important to consider other factors such as clinical efficacy and safety when selecting antifungal agents for the treatment of fungal infections in animals. The mic-resistant value to voriconazole is >2 µg/ml in humans but in birds >1 µg/ml is considered as resistant. The efficacy of antifungal drugs may be reduced in cases where Aspergillus species are present in the air sac of falcons with high Aspergillus galactomannan antigen levels. In conclusion, our study provides valuable information on the susceptibility of various fungal isolates from the air sac of falcons to antifungal agents which are also commonly used in human medicine.
Conclusion
In conclusion, this study analyzed the Minimum Inhibitory Concentrations (MICs) of antifungal drugs against different fungal isolates from the air sac of falcons. The most isolated fungi were A. fumigatus, A. flavus, A. niger, and A. terreus, which can cause various infections in both humans and animals, including aspergillosis, which is a major concern in falcons. The study found that isavuconazole, posaconazole, and voriconazole had the lowest MICs among the antifungal drugs tested, suggesting that they could be effective treatment options for aspergillosis in falcons. However, itraconazole, which was widely used to treat aspergillosis in falcons, showed higher MIC values with emerging resistance. The high MICs for some isolates, especially A. fumigatus, suggest a risk of antifungal resistance, and thus MIC studies must be followed together with treatment. This study showed a significant increase in resistance to antifungals, with 34% of the isolates being resistant to itraconazole, and resistance rates for posaconazole and isavuconazole rising to 4.7% and 5.8%, respectively, in 2023. Amphotericin B showed 51% resistance in 2006, which rose to 80% in 2011, leading to its discontinuation from treatment and MIC study of falcons.
Overall, this study highlights the significant challenge of emerging antifungal resistance, which is not only a problem in falcon medicine but also in human medicine, as fungal diseases are emerging in association with post COVID-19. The COVID-19 virus can weaken the immune system, making individuals more susceptible to fungal infections. It is essential to consider these emerging trends and continually monitor the resistance patterns of antifungal drugs to ensure effective treatment of fungal infections in both humans and animals. Additionally, careful consideration of other factors, such as clinical efficacy and safety, is crucial when selecting antifungal agents for the treatment of fungal infections in animals. Lastly, it is important to exercise caution when extrapolating MIC values from veterinary to human medicine, as there may be differences in the pharmacokinetics and pharmacodynamics of antifungal agents between different species.
Acknowledgement
The authors would like to thank bioMerieux, France, and Al Hayat pharmaceuticals, UAE for their cooperation in this study.
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding Source
N/A.
Statement of Ethics
N/A.
References
- Maione A, La Pietra A, de Alteriis E, Mileo A, de Falco M, Guida M, et al. Effect of myrtenol and its synergistic interactions with antimicrobial drugs in the inhibition of single and mixed biofilms of Candida auris and Klebsiella pneumoniae. 2022;10(9):1773.
[Crossref] [Google Scholar] [PubMed]
- Testore GP, Falco F, Sarrecchia C, Sordillo P, Bontempo G, Andreoni M. Two-year surveillance on fluconazole susceptibility of Candida spp isolates in a general and university hospital in Rome. Diagn Microbiol Infect Dis. 2001;41(1-2):23-27.
[Crossref] [Google Scholar] [PubMed]
- Arne P, Risco-Castillo V, Jouvion G, Le Barzic C, Guillot J. Aspergillosis in wild birds. J Fungi. 2021;7(3):241.
[Crossref] [Google Scholar] [PubMed]
- Bernal-Martinez L, Gomez-Lopez A, Castelli MV, Mesa-Arango AC, Zaragoza O, Rodriguez-Tudela JL, et al. Susceptibility profile of clinical isolates of non-Cryptococcus neoformans/non-Cryptococcus gattii Cryptococcus species and literature review. Med Mycol. 2010;48(1):90-96.
[Crossref] [Google Scholar] [PubMed]
- Melo AM, Stevens DA, Tell LA, Verissimo C, Sabino R, Xavier MO. Aspergillosis, avian species and the one health perspective: The possible importance of birds in azole resistance. 2020;8(12):2037.
[Crossref] [Google Scholar] [PubMed]
- Maione A, La Pietra A, Siciliano A, Mileo A, de Falco M, de Alteriis E, et al. The arylamidine T-2307 as a novel treatment for the prevention and eradication of Candida tropicalis Int J Mol Sci. 2022;23(24):16042.
[Crossref] [Google Scholar] [PubMed]
- Silva A, Igrejas G, Gaivao I, Aires A, Klibi N, Enes Dapkevicius MD, et al. Valorization of winemaking by-products as a novel source of antibacterial properties: New strategies to fight antibiotic resistance. 2021;26(8):2331.
[Crossref] [Google Scholar] [PubMed]
- Zaccardelli M, Sorrentino R, Caputo M, Scotti R, de Falco E, Pane C. Stepwise-selected Bacillus amyloliquefaciens and subtilis strains from composted aromatic plant waste able to control soil-borne diseases. Agriculture. 2020 3;10(2):30.
- Martinez-Rossi NM, Peres NT, Bitencourt TA, Martins MP, Rossi A. State-of-the-art dermatophyte infections: Epidemiology aspects, pathophysiology, and resistance mechanisms. J Fungi. 2021;7(8):629.
[Crossref] [Google Scholar] [PubMed]
- Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcusgattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16-48.
[Crossref] [Google Scholar] [PubMed]
- Singh RK, Ruiz-May E, Rajput VD, Minkina T, Gomez-Peraza RL, Verma KK, et al. Viewpoint of chitosan application in grapevine for abiotic stress/disease management towards more resilient viticulture practices. 2022;12(9):1369.
- Badalamenti N, Russi S, Bruno M, Maresca V, Vaglica A, Ilardi V, et al. Dihydrophenanthrenes from a Sicilian accession of Himantoglossum robertianum (Loisel.) P. Delforge showed antioxidant, antimicrobial, and antiproliferative activities. 2021;10(12):2776.
[Crossref] [Google Scholar] [PubMed]
- Pall E, Niculae M, Brudasca GF, Ravilov RK, Sandru CD, Cerbu C, et al. Assessment and antibiotic resistance profiling in Vibrio species isolated from wild birds captured in danube delta biosphere reserve, Romania. 2021;10(3):333.
[Crossref] [Google Scholar] [PubMed]
- Hermanns R, Cremers NA, Leeming JP, van der Werf ET. Sweet relief: Determining the antimicrobial activity of medical grade honey against vaginal isolates of Candida albicans. J Fungi. 2019;5(3):85.
[Crossref] [Google Scholar] [PubMed]
- Silva V, Falco V, Dias MI, Barros L, Silva A, Capita R, et al. Evaluation of the phenolic profile of Castanea sativa By-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants. 2020;9(1):87.
[Crossref] [Google Scholar] [PubMed]
Citation: Das S, Christu SD, Silvanose C, Binoy A, Azmanis P, Somma AD, et al. (2023) Emerging Antifungal Resistance in Falco Species: A Novel Model for Human Medicine. Bio Med. 15:586.
Copyright: © 2023 Das S, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.