Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 9
Efficacy of Essential oil of Two Melaleuca Species in the Treatment of Infectious Diseases
Aqsa Qurban1,2*, Ayesha Ameen1 and Huda Ishfaq32Institute of Biochemistry and Biotechnology, University of the Punjab, Lahore, Pakistan
3University of Central Punjab, Lahore, Pakistan
Received: 03-Aug-2020 Published: 25-Sep-2020, DOI: 10.35248/2157-7471.20.11.516
Abstract
Purpose of this study was to evaluate antibacterial activity of oil extract obtained from parts (leaves) of Melaleuca species against bacterial strains to treat infectious diseases such as Urinary Tract Infection. In general, extracts obtained by extraction method showed antibacterial activity against different tested microorganisms. Agar well diffusion method was used to evaluate antibacterial activity against ten pathogens which include 7 different strains of E. coli, 2 strains of Klebsiella pneumoni and 1 strain of Entercoccus faecalis. According to the results of this study, Essential oils of Melaleuca plant showed the good antibacterial action of the bacterial strains due to the measurement of clear zone of inhibition but antibiotic susceptibility assay was more appreciable. Antibiotics are used as positive control and ethanol as negative control along with it. In conclusion, Extracts of Melaleuca species found to be containing chemical compounds useful in the treatment of many infectious diseases such as urinary tract infection, acne and many other skin diseases.
Keywords
Melaleuca Species; Infectious diseases; Antibacterial activity; Antibiotics, Essential Oils; Zone of inhibition
Introduction
Natural Products have always been a source of valuable modern medicines. A big variety of medicinal plants have been explored for their specificity of essential oils in the past few decades. Essential oils are mainly volatilic compounds in complex form, which are synthesized naturally from different parts of the plant during the process of secondary metabolism. Essential oils are becoming beneficial in the field of biomedicine as they effectively destroy a big range of bacterial, fungal and viral pathogens [1]. Medicinal plants proved as a good source for development of new drugs for the treatment of infectious diseases [2]. It has been evaluated that about 70,000 plants in whole world and in Pakistan 400-600 plants are medicinally important to destroy different pathogens [3]. They have reported promising effects in a wide range of infectious skin diseases such as Urinary tract infection, wounds and Acne disease. Many diseases can be cured more safely and cost effectively by them as compared to synthetic medicines. This is the reason that majority of rural individuals, people of lower socio-economic status, 80% of population in developing countries, and 90% people of Kenya use these traditional herbal medicines [4,5].
The genus Melaleuca consists of about 260 species and occurs mostly in Australia but is also found in South-East Asia, the United States and the Caribbean. Long ago, Melaleuca genus was introduced in Punjab, Pakistan. Among different Species of Melaleuca, Melaleuca bracteata and Melaleuca leucadendron are well known plants, which also possess some medicated properties [6]. Some alternative and complementary drugs such as tea tree oils have become increasingly popular in, recent decades. These essential oils has been used in Australia for almost 100 years but is now available worldwide, in the form of neat oil and as an active component in an array of products. Historically, the main uses of tea tree oil have on the antiseptic and anti-inflammatory actions against pathogens. Their study reported the recent developments in understandings of the antimicrobial and anti-inflammatory activities of the essential oil and its components, as well as clinical activities [7].
Melaleuca leucadendron commonly known as “Cajuput Oil Tree” or “Tea Tree” or “White Tree”. Melaleuca leucadendron is usually white large tree but also pinky or cream. Sometimes, it is more than 20m (70ft) tall. It has weeping branches and its leaves and young branches are covered with short and white hairs. The leaves of the plant are used to distill “Cajuput oil” or “tea tree oil” which has medicinal uses. The Indochinese used this as a well killer as well as for rheumatism and joint pains. In Malaysia, this oil is used in the treatment of cholera and colic. In Indonesia, the oil is used for cramps, earache, burns, colic, headache, headache, skin diseases, toothache and wounds, externally. Internally, it is used to induce sweating as an antispasmodic and as a stimulant. In Philippines, the leaves (Oil Extract) are used to treat asthma disease.
Melaleuca bracteata is commonly known as “Bottle Brush” or “Black Tea tree” or “River Tea Tree” and is endemic to Northern Australia. The plants have small narrow leaves (1.5cm long) and are spirally arranged around the stem and crowded together. The aim of the essential oil of the plant is to reduce bacteria and inflammation. Methyl Eugenol is present in its leaves which are used in insecticides and cosmetics [8]. It is effective to treat from mild to moderate skin diseases [9].
Both Species are commonly known as “Tea Tree” and have specificity in their activities. Their essential oil shows broad spectrum of antimicrobial activity in vitro, on the other hand, its efficacy in vivo relatively remains unsubstantiated. Tea tree oil first showed the antiviral activity by using mosaic virus and tobacco plant [10]. Their oil is also effective to skin problems in preventing different infections associated with skin microorganisms. They also have antiprotozoal activity & antitumoral activity and antioxidant activity [11,12]. Another approach to the treatment and prevention of infection diseases is probiotics. Probiotics have been proved as an alternative therapy for the treatment and prevention of infectious diseases. Probiotics are made from non-pathogenic organisms which have a beneficial effect on the digestive and other systems of the body by conferring resistance to infection or removing infectious agents. Basically, both bacteria and yeast have been used as probiotics [13]. The mechanism of the action of probiotics have been summarized by four possibilities; 1) antagonism by production of inhibitory substances; 2) For nutrients, competition with the pathogen; 3) immunomodulation of the host; 4)destruction of toxins [14]. Antibacterial are the compounds that can kill and restrict the growth of microbes e.g., antibiotics. They could be derived naturally or synthetically. But synthetic antibiotics not only have side effects but also develop resistance against antibiotics [15]. Which make the treatment of many infectious diseases difficult as they could not be easily treated with such antibiotics and require advance type of antibiotics associated with high risks of side effects? This problem can be resolved with the use of various plants derived chemical constituents that have great potential to be used as antibacterial while reducing the risk of side effects [16].
At present, a variety of antibiotics are available for treating various bacterial pathogens. However, increased multidrug resistance going to led the increased severity of many diseases caused by different bacterial pathogens in addition, low immunity in host’s cells and the ability of pathogen to develop biofilm-associated drug resistance which has further increased the number of bacterial infections which are life-threatening for humans [17]. Thus, bacterial infections made major causative agent for human death, even today. In addition, the use of several antibacterial agents at higher doses may also cause toxicity in humans. This has prompted researches to explore alternative new key molecules against bacterial strains [18]. In this way, essential oils of plants and their major chemical components are strong candidates as antibacterial agents.
Among a variety of Human infections, one of the most common is urinary tract infection (UTI) which needs urgent and continuous treatment. The pathogens most commonly causing UTIs are E. coli and other Enterobacteriacae bacteria such as Klebsiella [19]. It is reported that about 250 million people are suffering from UTI’s annually. In addition, 20% to 50% of adult women are involved to be parts of UTI. Plants have effective roles in UTIs treatment and have been used for the purpose for a long time ago. This could be indicated that 17 families associated with 35 native medicinal plants were effective in the treatment of urinary tract infection [20].
But there is a lack of research on the identification of such therapeutic properties of herbs or medicinal plants and thus of chemical constituents. For example, about estimation the number of pharmacologically screened plants account for only <1% of the 250,000 higher plants [21]. So keeping in view, the purpose of present study is designed to investigate the antibacterial activity of Melaleuca leucadendron and Melaleuca bracteata extracts. The antibacterial activity is to be determined against the pathogens while using well diffusion method with seeded cultures.
Materials and Methods
Plant material
Melaleuca bracteata leaves samples were collected from GC University Botanical Garden, Lahore. Melaleuca leucadendron samples were collected from Jinnah Garden, Lahore in January, 2018. Authentication of leaves of both species of plants was done by Professor Dr. Khalid Nasir the herbarium, Botany Department, University of the Punjab, Lahore, Pakistan. Then they were first washed with water and then they were dried in the shade for some hours before the steam Distillation.
Plant extracts preparation
Hydrodistillation: The extraction of the plant extracts (essential oils) was carried out by hydro distillation in a Clevenger type apparatus. Oil extracts obtained were filtered and stored at -4 ̊C in the dark and in the presence of anhydrous sodium sulfate.
Assay for antibacterial activity
Microorganisms: The antibacterial activity was evaluated using microorganisms. Microbes used in the assay were E. coli (75), E. feacalis (72), K. pneumoni (54), K. pneumoni (83), E. coli (43), E. coli (21), E. coli (44), E. coli (71), E. coli (74) and E. coli (93) that were collected from the research laboratories of IBB (Institute of Biochemistry and Biotechnology).
Sub-culturing and storage of microorganisms: Microbes were subcultured on LB agar slants and stored at 4 ̊C. They were refreshed whenever used again on LB agar.
Antibiotic susceptibility assay: The Muller Hinton Media in molten state (at nearly 45℃) was seeded with 2% bacterial inoculum (1×10-5 CFU/ml) and allowed to solidify. The commercially available antibiotic discs were placed onto the inoculated agar with the help of sterile forceps and incubated for 24 hours. Bacterial susceptibility or resistance to the antibiotics has been checked by determining zones of inhibition. The antibiotics-standard Ciprofloxacin (5 μg), Tetracycline (10 μg), Rifampicin (5 μg), Cefepime, Norfloxacin (10 μg), Vanomycin (30 μg), Imipenem, Gentamicin (10 μg), PenicillinG (10 μg), Ampicillin (10 μg), Sulfamethoxazole with trimethoprim (25 μg), kanamycin (30 μg), Minocycline (30 μg), Chloramphenicol (30 μg), Tobramycin (10 μg) and Ampicilin (10 μg) were used in this assay.
Antibacterial/antimicrobial activity: The antimicrobial activity of oil extracts was determined via well diffusion assay. The basis of this assay is the spreading of antimicrobial agents in a solid medium.
Well diffusion method: MHA in molten state (at nearly 45℃) was seeded with 2% bacterial inoculum (1×10-5 CFU/ml) and allowed to solidify. Wells of 6 mm and height were bored in the inoculated agar plate using a sterile plastic tip and 20 μl of plant extracts and 30 μl of probiotic were added into the wells with the help of micropipette. The plates were wrapped with parafilm carefully and placed in an incubator at 37℃ for 24 hours. After 24 hours, there will be the zone of inhibition which indicates the activity of plant extracts and probiotic against specific bacterial strains. If there will be no zone of inhibition, then there will be the no activity of plant extracts and probiotic against specific bacterial strains. Antibiotic was used as positive control and ethanol is used as negative control.
Results
Samples of Melaleuca leucadendron (Cajuput tree) were obtained from Jinnah Garden, Lahore and Melaleuca bracteata (black tea tree) were obtained from Government college University Botanical Garden, Lahore. The antibacterial potential of these extracts against pathogenic strains was investigated.
Antibacterial activity
Antibiotic susceptibility assay: Susceptibility of 10 different strains of Urinary Tract infection e.g., (microbes used in the assay were E. coli (75), E. feacalis (72), K. pneumoni (54), K. pneumoni (83), E. coli (43), E. coli (21), E. coli (44), E. coli (71), E. coli (74) and E. coli (93) against different Antibiotics including Ciprofloxacin, Tetracycline, Rifampicin, Cefepime, Norfloxacin, Vanomycin, Imipenem, Gentamicin, Penicillin G, Ampicillin, Sulfamethoxazole with trimethoprim, kanamycin, Minocycline, Chloramphenicol, Tobramycin and Ampicilin were determined by using well diffusion method with inoculated bacterial culture. Klebsiella pneumoni (54) showed susceptibility to Chloramphenicol (C) with zone of inhibition of 34 mm and Gentamicin (GN) with zone of inhibition of 24mm, Escherichia coli (93) showed susceptibility to Ciprofloxacin (CIP) with zone of inhibition of 26 mm , Escherichia coli (71) showed resistant to Penicillin G (P) with no zone of inhibition and CN with zone of inhibition of 29mm, , Escherichia coli (44) showed susceptibility to Sulfamethoxazole with trimethoprim (SXT) with zone of inhibition of 31 mm and AMP with zone of inhibition of 9 mm, , Escherichia coli (21) showed susceptibility to Tetracycline (TE) with zone of inhibition of 32 mm and RD with zone of inhibition of 37 mm , , Escherichia coli (74) showed susceptibility to kanamycin (k) with zone of inhibition of 15 mm and MI with zone of inhibition of 16mm, Escherichia coli (75) showed susceptibility to Norfloxacin (NOR) with zone of inhibition of 30mm, Entercoccus faecalis (72) showed susceptibility to Vancomycin (V) with zone of inhibition of 18 mm , Klebsiella pneumoni (83) showed susceptibility to FEP with zone of inhibition of 24 mm and , Escherichia coli (43) showed susceptibility to Imipenem (IPM) with zone of inhibition of 10 mm (Table 1 and Figure 1).
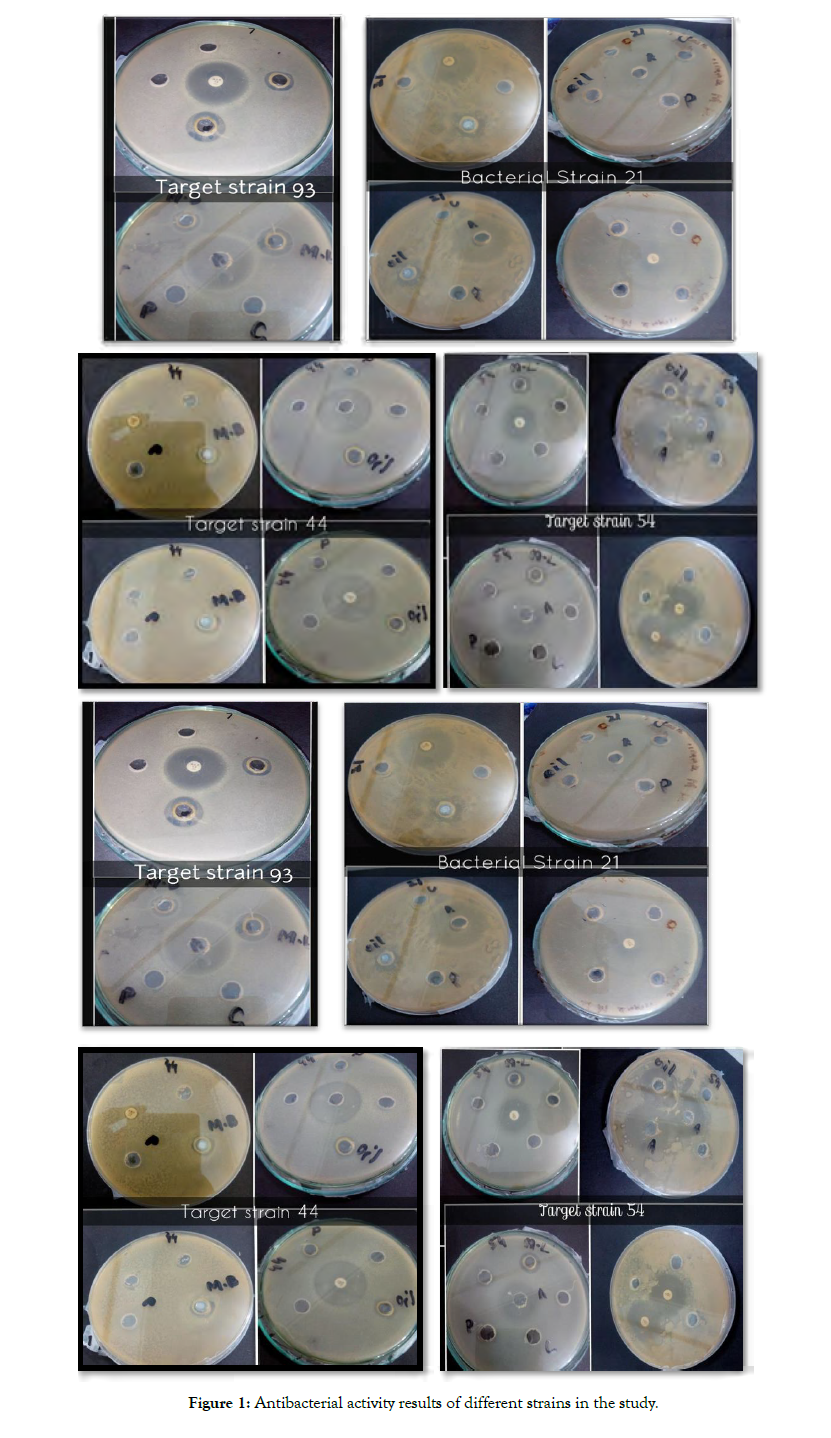
Figure 1: Antibacterial activity results of different strains in the study.
| S. No. | Targeted Strains |
Antibiotics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (mm) |
CIP (mm) |
P (mm) |
TE (mm) |
K (mm) |
SXT (mm) |
NOR (mm) |
VA (mm) |
FEP (mm) |
IPM (mm) |
GN (mm) |
MI (mm) |
TOB (mm) |
AMP (mm) |
RD (mm) |
||
| 1 | Escherichia coli (75) | - | - | - | - | - | - | 30 | - | - | - | - | - | - | - | - |
| 2 | Entercoccus faecalis (72) | - | - | - | - | - | - | - | 18 | - | - | - | - | - | - | - |
| 3 | Klebsiella pneumoni (54) | 34 | - | - | - | - | - | - | - | - | - | - | - | 35 | 31 | - |
| 4 | Klebsiella pneumoni (83) | - | - | - | - | - | - | - | - | 24 | - | - | - | - | - | - |
| 5 | Escherichia coli (43) | - | - | - | - | - | - | - | - | - | 10 | - | - | - | - | - |
| 6 | Escherichia coli (21) | - | - | - | 32 | - | - | - | - | - | - | - | - | - | - | 37 |
| 7 | Escherichia coli (44) | - | - | - | - | - | 31 | - | - | - | - | - | - | - | - | - |
| 8 | Escherichia coli (71) | - | - | NZ | - | - | - | - | - | - | - | 29 | - | - | - | - |
| 9 | Escherichia coli (74) | - | - | - | - | 15 | - | - | - | - | - | - | 16 | - | - | - |
| 10 | Escherichia coli (93) | - | 30 | - | - | - | - | - | - | - | - | - | - | - | - | - |
Ciprofloxacin (5 µg), Tetracycline (10 µg), Rifampicin (5 µg), Cefepime (10 µg), Norfloxacin (10 µg), Vanomycin (30 µg), Imipenem (30 µg), Gentamicin (10 µg), Penicillin G (10 µg), Ampicillin (10 µg), Sulfamethoxazole with trimethoprim (25 µg), kanamycin (30 µg), Minocycline (30 µg), Chloramphenicol (30 µg), Tobramycin (10 µg) and Ampicilin (10 µg).
Table 1: Antibiotic susceptibility assay against 10 bacterial strains of UTI by using disc diffusion method.
Well diffusion method: Antibacterial activity of M.B. and M.L. extracts and probiotics was investigated by well diffusion method against all seeded bacterial cultures of urinary tract infection (UTI) strains, while using Antibiotics i-e., Ciprofloxacin (5 μg), Tetracycline (10 μg), Rifampicin (5 μg), Cefepime (10 μg), Norfloxacin (10 μg), Vanomycin (30 μg), Imipenem (30 μg), Gentamicin (10 μg), Penicillin G (10 μg), Ampicillin (10 μg), Sulfamethoxazole with trimethoprim (25 μg), kanamycin (30 μg), Minocycline (30 μg), Chloramphenicol (30 μg), Tobramycin (10 μg) and Ampicilin (10 μg) as a positive control and ethanol as a negative control. Escherichia coli (75) showed susceptibility to M.L. with zone of inhibition of 17 mm and to M.B. with zone of inhibition of 14 mm. Entercoccus faecalis (72) showed susceptibility to M.L. extract with zone of inhibition 16 mm and to M.B. extract with zone of inhibition of 13 mm. Klebsiella pneumoni (54) showed susceptibility to M.L. extract with zone of inhibition of 16 mm and to M.B. extract with zone of inhibition of 18 mm. Klebsiella pneumoni (83) showed susceptibility to M.L. extract with zone of inhibition of 17 mm and to M.B. extract with zone of inhibition of 19 mm. Escherichia coli (43) showed susceptibility to M.L. extract with zone of inhibition 20 mm and to M.B. extract with zone of inhibition of 11 mm.
Escherichia coli (21) showed susceptibility to M.L. extract with zone of inhibition 17 mm and to M.B. extract with zone of inhibition of 15 mm. Escherichia coli (44) showed susceptibility to M.L. extract with zone of inhibition 24 mm and to M.B. extract with zone of inhibition of 14 mm. Escherichia coli (71) ) showed susceptibility to M.L. extract with zone of inhibition 15 mm and showed resistant to M.B. extract with no zone of inhibition. Escherichia coli (74) showed susceptibility to M.L. extract with zone of inhibition 18 mm and to M.B. extract with zone of inhibition of 9 mm. Escherichia coli (93) showed susceptibility to M.L. extract with zone of inhibition 18 mm and to M.B. extract with zone of inhibition of 9 mm. (Table 2 and Figure 1).
| S. No | Targeted Strain | Zone of Inhibition in (mm) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extracts | -ve control | +ve control (Antibiotic) | |||||||||||||||||
| M.L | M.B | Ethanol | C | CIP | P | TE | K | SXT | NOR | VA | FEP | IPM | GN | MI | TOB | AMP | RD | ||
| 1 | Escherichia coli (75) | 17 | 14 | NZ | - | - | - | - | - | - | 30 | - | - | - | - | - | - | - | - |
| 2 | Entercoccus faecalis (72) | 16 | 13 | NZ | - | - | - | - | - | - | - | 18 | - | - | - | - | - | - | - |
| 3 | Klebsiella pneumoni (54) | 16 | 18 | NZ | 34 | - | - | - | - | - | - | - | - | - | - | - | 35 | 31 | - |
| 4 | Klebsiella pneumoni (83) | 17 | 19 | NZ | - | - | - | - | - | - | - | - | 24 | - | - | - | - | - | - |
| 5 | Escherichia coli (43) | 20 | 11 | NZ | - | - | - | - | - | - | - | - | - | 10 | - | - | - | - | - |
| 6 | Escherichia coli (21) | 17 | 15 | NZ | - | - | - | 32 | - | - | - | - | - | - | - | - | - | - | 37 |
| 7 | Escherichia coli (44) | 24 | 14 | NZ | - | - | - | - | - | 31 | - | - | - | - | - | - | - | - | - |
| 8 | Escherichia coli (71) | 15 | NZ | NZ | - | - | NZ | - | - | - | - | - | - | - | 29 | - | - | - | - |
| 9 | Escherichia coli (74) | 18 | 9 | NZ | - | - | - | - | 15 | - | - | - | - | - | - | 16 | - | - | - |
| 10 | Escherichia coli (93) | 18 | 15 | NZ | - | 30 | - | - | - | - | - | - | - | - | - | - | - | - | - |
M.L =Melaleuca leucadendron, M.B=Melaleuca bracteata, NZ =No zone of inhibition
Table 2: Antibacterial activity of plant extracts by well diffusion method against all seeded bacterial cultures of urinary tract infection (UTI) strains along with antibiotics (positive control) and ethanol (negative control).
Antibacterial activity of probiotics was investigated by well diffusion method against all seeded bacterial cultures of urinary tract infection (UTI) strains. Escherichia coli (44) showed susceptibility to probiotics with zone of inhibition of 24 mm. Escherichia coli (74) showed susceptibility to probiotics with zone of inhibition of 5 mm. All other strains showed resistance to probiotic with no zone of inhibition. (Table 3 and Figure 1).
| S. No. | Targeted Strains | Probiotic strains | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb Strain 1 | Pb Strain 2 | Pb Strain 3 | Pb Strain 4 | Pb Strain 5 | Pb Strain 6 | Pb Strain 7 | Pb Strain 8 | Pb Strain 9 | Pb Strain 10 | ||||||||
| 1 | Escherichia coli (75) | NZ | - | - | - | - | - | - | - | - | - | ||||||
| 2 | Entercoccus faecalis (72) | - | - | - | - | - | - | - | - | - | NZ | ||||||
| 3 | Klebsiella pneumoni (54) | - | - | NZ | - | - | - | - | - | - | - | ||||||
| 4 | Klebsiella pneumoni (83) | - | - | - | - | - | NZ | - | - | - | - | ||||||
| 5 | Escherichia coli (43) | - | - | - | - | - | - | - | - | NZ | - | ||||||
| 6 | Escherichia coli (21) | - | - | - | - | - | - | NZ | - | - | - | ||||||
| 7 | Escherichia coli (44) | - | 24mm | - | - | - | - | - | - | - | - | ||||||
| 8 | Escherichia coli (71) | - | - | - | - | - | - | - | NZ | - | - | ||||||
| 9 | Escherichia coli (74) | - | - | - | 5mm | - | - | - | - | - | - | ||||||
| 10 | Escherichia coli (93) | - | - | - | - | - | - | NZ | - | - | - | ||||||
NZ=No Zone of Inhibition, Pb S1=Guava obtained probiotic strain, Pb S2=Tomato obtained probiotic strain, Pb S3=Dates obtained probiotic strain, Pb S4=Chilly obtained probiotic strain, Pb S5=Cucumber obtained probiotic strain, Pb S6=Brinjal obtained probiotic strain, Pb S7=Strawberry obtained probiotic strain, Pb S8=Pea obtained probiotic strain, Pb S9=Sweet potato obtained probiotic strain, Pb S10=Yellow cheese obtained probiotic strain.
Table 3: Antibacterial activity of probiotics by well diffusion method against all seeded bacterial cultures of urinary tract infection (UTI) strains.
Discussion
Natural therapies such as medicinal plants are considered as alternatives to synthetic drugs which are increasingly used to evaluate antimicrobial activity of different pathogens which are responsible to infectious diseases. The aim of this study was to focalize in essential oils usable against 7 different strains of E. coli, 2 strains of K. pneumoni and 1 strain of Entercoccus faecalis and to compare their antimicrobial activity against bacterial strains, which are responsible for infectious diseases such as urinary tract infection (UTI). Indeed, Phenols and aldehydes containing oil extracts are of potential importance in disease treatment due to their antimicrobial actions against various harmful pathogenic bacterial strains and also those essential oils containing terpene alcohols. Essential oils containing terpene ether or oxide have less activity. But the selected essential oils have good antibacterial activity against various bacterial strains.
Antibacterial activity of plant extracts was performed by agar well diffusion method against 10 different bacterial strains including E. coli (75), E. feacalis (72), K. pneumoni (54), K. pneumoni (83), E. coli (43), E. coli (21), E. coli (44), E. coli (71), E. coli (74) and E. coli (93) which showed the higher inhibitory activity. It was concluded that extracts containing phenols and aldehydes had more potential antibacterial activities than extracts containing terpene ether. All bacterial strains showed any zone of inhibition or susceptibility against these plants extracts except E. coli (71) showed no susceptibility against M. bracteata plant extract (Figure 1). The susceptibility of antibiotics and probiotics was also checked against such bacterial strains. But no probiotics showed any zone of inhibition or susceptibility against microbial strains except the tomato obtained probiotic strain (PbS2) and chilly obtained probiotic strain (PbS4) which exhibited little susceptibility against E. coli (44) and E. coli (74), in agar well diffusion method (Figure 1). This might due to the reason that E. coli (44) and E. coli (74) are more susceptible to the extracts as compare to other bacteria. Mainly antibiotics are used as a positive control but in this study, all bacterial strains showed susceptibility to the antibiotics except E. coli (71) showed no susceptibility against penicillin (Figure 1). Infact; this strain showed susceptibility to plant extracts (M.B. & M.L). The results were contrary to the previous study conducted by Singh BR, et al. [22]. Where different fractions of plant extracts of tea tree were tested against diverse range of micro-organisms giving a wide spectrum of antimicrobial potential and that study showed limitations of tea tree oil as only one fifth of clinical strains of bacteria were sensitive to it.
Similarly, Hammer KA, et al. [23] evaluated the differences in MIC value by the different strains tested by comparing plant extracts of Melaleuca sp. (Tea tree oil), manuka oil and cinnamon oil. The study didn’t do this evaluation. However, the tea tree oil against S. mutans didn’t obtain good results because tea tree oil presented the worst performance. Similarly, Daniela FV, et al. [24] also demonstrated the antibacterial properties of M. leucadendron, in vitro, against strains such as Staphylococcus aureus. Similarly, the results were also different from that of the Punjabi et al. [25] who evaluated the antibacterial activity of leaves extracts of M. leucadendron against different bacterial strains and gained positive results. However, the current research results were like that of Mohd Sayeed Akhtar et al. [26] who found M. bracteata extracts to be active against various microbes including E. coli, Salmonella typhimrium, Listeria monocytogenes, and Vibrio vulnificus which are responsible to foodborne diseases.
Conclusion
There are a lot of questions related to the research that demands further research but currently we can conclude that leaf extracts of M. bracteata and M. leucadendron have shown appreciable antibacterial activity against different pathogenic bacterial strains. In the present study, Melaleuca leucadendron shown higher antibacterial activity against bacterial strains as compared to M. bracteata; but overall they both were proved as good antibacterial agents against infectious diseases.
Acknowledgement
I am greatly thankful to Dr. Asma Manzoor, Lecturer at the Institute of Biochemistry and biotechnology Punjab University, Lahore and to Dr. Zahida Parveen, Senior Scientist of Pakistan Council of Scientific and Industrial Research (PCSIR), Lahore for providing me, all the facilities for this research work.
REFERENCES
- Swamy MK, Sinniah UR. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin An aromatic medicinal plant of industrial importance. Molecules. 2015;20(5):8521–8547.
- Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complementary Alter Med. 2016.
- Blumenthal M, Goldberg A, Brinckmann, J. Herbal Medicine: Expanded Commission E Monographs. Integrative Medicine Communications, Boston, USA. 2000.
- Hamayun M. Ethnobotanical profile of Utror and Gabral valleys, district Swat, Pakistan. Ethnobotanical leaflets. 2005;2005(1):1-9.
- Goleniowski ME, Bongiovanni GA, Palacio L, Nuñez CO, Cantero JJ. Medicinal plants from the “Sierra de Comechingones”, Argentina. J Ethnopharmacol. 2006;107(3):324-341.
- Tran DB, Dargusch P, Moss P, Hoang TV. An assessment of potential responses of Melaleuca genus to global climate change. Mitigation and Adaptation Strategies for Global Change. 2013;18(6):851-867.
- Carson CF, Cookson BD, Farrelly HD, Riley TV. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J Antimicrob Chemoter. 1995;35:421-424.
- Marcar N, Crawford D, Leppert P, Jovanovic T, Floyd R, Farrow R. Trees for saltland: A guide to selecting native species for Australia. Csiro Publishing; 1995..
- Concha JM, Moore LS, Holloway WJ. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podia Med Assoc. 1998;88:489-492.
- Carson CF, Cookson BD, Farrelly HD, Riley TV. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J Antimicrob Chemother. 1996;35:421–424
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifólia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81(3):321-326.
- Aravena-Román M, Inglis TJ, Henderson B, Riley TV, Chang BJ. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob Agents Chemother. 2012;56(2):1110-112.
- Filho-Lima JVM, Viera EC, Nicoli JR. Antagonistic effect of Lactobacillus acidophilus, Saccharomyces boulardii and Escherichia coli against experimental infections with Shigella flexneri and Salmonella enteritidis sub sp. typhimurium in gnotobiotic mice. J Appl Microbiol2000; 88:365–370.
- Borges A, Abreu AC, Ferreira C, Saavedra MJ, Simões LC, Simões M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J Food Sci Technol. 2015;52(8):4737-4748.
- Rahman K, Nisar M, Jan AU, Suliman M, Iqbal A, Ahmad A, et al. Antibacterial activity of important medicinal plants on human pathogenic bacteria. International J Agro Agri Res. 2015;6(06):106-111.
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crop Prod. 2014;62:250–264.
- Galvão LC, Furletti VF, Bersan SM, Da Cunha MG, Ruiz AL, Carvalho JE, et al. Antimicrobial activity of essential oils against Streptococcus mutans and their antiproliferative effects. Evid Based Complementary Altern Med. 2012.
- Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among US out patients from 2000 to 2010. Antimicrob Agents Chemother. 2012;56(4):2181-2183.
- Saderi H, Owlia P, Nadoushan MRJ, Zaeri F, Zandieh EA. A 3-year study of demographic characteristics of patients with urinary tract infection, microbial etiology, and susceptibility of isolated bacteria to antibiotics in Shaheed Mostafa Khomeini Hospital. Iran J Pathol 2006;1(3):99-104.
- Dixit,S Huma Ali. Antioxidant potential some medicinal plants of Central India. J Cancer Ther. 2010;1:87-90..
- Singh BR, Pal A, Pal K. Antioxidant activity of ethanolic and aqueous extract of Phyllanthus niruri – in-vitro. World J Pharm Pharm Sci. 2016;5: 1994-2000
- Hammer KA, Carson CF, Riley TV. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil). Am J Infect Control. 1996; 24:186–189.
- Falci SP, Teixeira MA, Chagas PF, Martinez BB, Loyola AB, Ferreira LM, et al. Antimicrobial activity of Melaleuca sp. oil against clinical isolates of antibiotics resistant Staphylococcus aureus. Acta cirurgica brasileira. 2015;30(7):491-496.
- Punjabi YS, Khilnani VL, Damle PN. The investigation of antibacterial activity of Cestrum nocturnum. Pharmacophore. 2015;1;1-6.
- Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid Based Complementary Alter Med. 2016.
Citation: Qurban A, Ameen A, Ishfaq H (2020) Study to Check Efficacy of Essential oil of Two Melaleuca Species in the Treatment of Infectious Diseases. J Plant Pathol Microbiol 11:516. doi: 10.35248/2157-7471.20.11.516
Copyright: © 2020 Qurban A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

