Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 6
Efficacy of Drug-Free Cationic Hybrid Liposomes Composed of L-α Dimyristoylphosphatidylcholine in Targeting Negatively Charged Cholangiocarcinoma Cell Membranes: An In Vitro and In Vivo Study
Hideaki Ichihara*, Hiromitsu Takaki, Muneaki Motomura and Yoko MatsumotoReceived: 23-Oct-2024, Manuscript No. JCM-24-27251; Editor assigned: 25-Oct-2024, Pre QC No. JCM-24-27251 (PQ); Reviewed: 08-Nov-2024, QC No. JCM-24-27251; Revised: 15-Nov-2024, Manuscript No. JCM-24-27251 (R); Published: 25-Nov-2024, DOI: 10.35248/2157-2518.2.15.460
Abstract
Cationic Hybrid Liposomes (CL) composed of 87 mol% Dimyristoylphosphatidylcholine (DMPC), 5 mol% Polyoxyethylene (21) Dodecyl Ether (C12(EO)21, also referred to as (HL21), and 8 mol% O,O’-Ditetradecanoyl-N-(α– Trimethylammonioacetyl) Diethanolamine Chloride (2C14ECl) were prepared by sonication. A clear solution of CL with a hydrodynamic diameter of 100 nm was maintained for four weeks. Cholangiocarcinoma cells exhibited a lower zeta potential than normal bile duct cells due to higher levels of negatively charged phosphatidylserine and Ganglioside 1 (GM1). Time-dependent immediate fusion of CL with cholangiocarcinoma cell membranes was confirmed using confocal microscopy. The 50% inhibitory concentration values of CL for the growth of human cholangiocarcinoma cells were markedly lower than those of DMPC and HL21 liposomes. CL-induced apoptosis of cholangiocarcinoma cells based on the propidium iodide assay using flow cytometry. The induction of apoptosis with the activation of caspase -3, -8, -9 and a decrease in the mitochondrial membrane in cholangiocarcinoma cells by CL were verified using flow cytometry. A remarkable reduction in tumor volume and weight in model mice of human cholangiocarcinoma intravenously treated with CL without drugs after the subcutaneous inoculation of cholangiocarcinoma cells was verified in vivo.
Keywords
Cationic hybrid liposomes; Chemotherapy; Cholangiocarcinoma; Membrane potential; Apoptosis; In vivo
Introduction
Bile ducts begin as a series of narrow ducts (intrahepatic bile ducts) running through the pancreas, gradually widening as they merge like branches of a tree and converge at the portal of the liver. This forms the common bile duct, which joins the cystic duct and passes through the pancreas to the duodenum, a part of the small intestine, along with the pancreatic duct that carries pancreatic juice. This junction is called the duodenal papilla. The bile duct inside the liver is called the intrahepatic bile duct, while the bile duct outside the liver is called the extrahepatic bile duct. Cholangiocarcinoma is one of the intractable cancers arising from the bile duct epithelium. The main symptoms include jaundice, right side pain and weight loss. Jaundice occurs when cancer narrows or blocks the bile ducts, causing bile containing the yellow pigment bilirubin to flow into the bloodstream. This can cause the skin and whites of the eyes to turn yellow, darken the urine and make the skin itchy. Stool may become whitish due to reduced bilirubin excretion into the duodenum. Although these symptoms rarely appear in the early stages and the incidence is low, the 5-year survival rate is second only to that of pancreatic cancer, which is a refractory cancer. In addition, it is difficult to completely cure cancer with the current pharmacotherapies, highlighting the need for the development of safe and effective anticancer drugs with fewer side effects.
Materials and Methods
Cancer cells exhibit properties distinct from those of normal cells. For example, Phosphatidylserine (PS) is three to seven times more abundant on the surface of various cancer cell membranes than on normal cell membranes [1-2]. In addition, sialic acid is more frequently expressed on cancer cell membranes than in normal cell membranes and is used as a tumor marker (CA19-9). Therefore, cancer cells carry a negative charge [3-5]. Few reports exist on anticancer drugs that exploit such electrostatic interactions, except for cationic liposomes.
CL, containing cationic lipid, have been produced by using the sonication method of a mixture of L-α Dimyristoylphosphatidyl- choline (DMPC), 5 mol% C12(EO)21 and 8 mol% 2C14ECl in a buffer solution [6]. Remarkably, CL with cationic lipids have shown inhibitory effects on the growth of Human Renal Cell Carcinoma (OS-RC-2) cells and induced apoptosis both in vitro and in vivo [6]. However, the antitumor mechanism of CL, which targets negatively charged cell membranes, has not yet been elucidated.
In this study, we investigated the inhibitory effects of CL composed of 87 mol% DMPC, 5 mol% C12(EO)21 and 8 mol% 2C14ECl, on the growth of Human Cholangiocarcinoma (HuCCT1) cells by targeting negatively charged cell membranes in vitro and in vivo.
Experimental section
Preparation of cationic hybrid liposomes: CL was prepared by sonication (VS-N300; VELVO, Tokyo, Japan) of a mixture containing DMPC (Purity>99%; NOF Co. Ltd., Tokyo, Japan), C12(EO)21 (Nikko Chemicals Co. Ltd., Tokyo, Japan) and 2C14ECl (DC-6-14; Sogo Pharmaceutical Co. Ltd., Tokyo, Japan) using a bath type sonicater in 5% glucose solution at 45ºC with 300 W. The mixture was filtered with a 0.20 μm cellulose acetate filter (Advantec, Tokyo, Japan) and stored at 37°C.
Dynamic light scattering measurements: The diameter of the CL was measured using a light scattering spectrometer (Otsuka Electronic, Osaka, Japan) with a He-Ne laser (633 nm) at a 90° scattering angle. The hydrodynamic diameter (dhy) was calculated using the Stokes-Einstein formula (dhy=κT/3πηD), where κ is the Boltzmann constant, T is the absolute temperature, η is the viscosity and D is the diffusion coefficient.
Cell culture: HuCCT-1 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Cholangiocarcinoma and normal bile duct cells were maintained in RPMI-1640 medium (Gibco, Gaithersburg, MD, USA) supplemented with 100 U/mL penicillin, 50 μg/mL streptomycin and 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA). The cells were cultured in a 5% CO2 humidified incubator at 37°C.
Fluorescence analysis of phosphatidylserine in plasma membranes of tumor cells: The level of PS in the plasma membranes of cholangiocarcinoma and normal bile duct cells was analyzed using Annexin-V binding assay [7]. After pre-incubating the cells (2.0 × 104 viable cells/mL) in a humidified atmosphere with 5% CO2 at 37ºC for 96 h, the tumor cells were processed using an Annexin-V-FLUOS Staining Kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. The stained cells were analyzed using a flow cytometer with a 488 nm single excitation wavelength and a 15 mW air-cooled Ar laser. The relative amount of PS in the outer plasma membranes was quantitatively measured from the annexin V signal intensity, detected by the FL1 sensor at a wavelength of 515-530 nm.
Fluorescence analysis of ganglioside 1 in plasma membranes of tumor cells: The amount of GM1 in the plasma membranes of cholangiocarcinoma and normal bile duct cells was observed using a confocal laser microscope with the fluorescent reagent Cholera Toxin Subunit B (CTB) conjugated to Alexa Fluor 647 (Invitrogen, Carlsbad, CA, USA) [8]. After pre-incubating the cells (2.0 × 104 viable cells/mL) in a humidified atmosphere with 5% CO2 at 37ºC for 24 h, the cells were washed with phosphate buffered saline (PBS (-)) and incubated in the culture media for 60 min. Subsequently, the cells were washed with PBS (-), incubated in culture media containing 30 mg/ml CTB for 30 min on ice and observed using a confocal laser microscope with a He-Ne laser (Excitation/detection: 633 nm/650-670 nm).
Zeta potential of Cholangiocarcinoma and normal bile duct cells: The zeta potential of cholangiocarcinoma and normal bile duct cells was measured using a zeta potential spectrophotometer (ELS-8000; Otsuka Electronics Co. Ltd., Osaka, Japan) [9,10]. All measurements were performed at a pH of 7 to simulate physiological conditions. The samples were diluted 1,000-fold in 0.1 M NaCl solution or deionized water for the zeta potential measurement and washed 5 min prior to measurement. The measurements were repeated three times.
Fusion and accumulation of CL into the cell membrane: The fusion and accumulation of CL, including a fluorescent probe, 1- palmitoyl-2-(12-((7-nitro-2-1,3-benzoxadiazol-4-yl) amino) dodecanoyl) -sn-glycero-3-phosphocholine (NBDPC, Avanti Polar Lipids, Alabama, U.S.A.), into the membrane of cholangiocarcinoma cells were investigated using a confocal laser microscope (TCS-SP, Leica Microsystems, Berlin, Germany). Cells (2.0 × 105 cells/mL) were cultured in a 5% CO2 humidified incubator at 37°C for 24 h. The cells were treated with CL ((DMPC)=0.45 mM, (C12(EO)21)=0.027 mM,(2C14ECl)=0.044 mM, (NBDPC)=0.022 mM) containing fluorescence-labeled lipids for 4 h. Observed were made using a confocal laser microscope with a 488 nm Ar laser line (detection wavelengths of 505-555 nm).
Assessment of growth inhibition caused by CL: The 50% Inhibitory Concentration (IC50) for the growth of cholangiocarcinoma cells was determined using WST-8 (2-(2- methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H - tetrazolium, monosodium salt] assay (Cell Counting Kit-8, Dojindo Laboratories, Kumamoto, Japan). Cells (5.0 × 104 cells/mL) were seeded in 96-well plates and cultured in a humidified incubator with 5% CO2 at 37ºC for 24 h. Cells were further cultured for 48 h after adding DMPC (0.1-5 mM), HL, or CL (0.1-2 mM based on the DMPC concentration). WST-8 solution was added and the cells were incubated for 3h. Absorbance was measured at a wavelength of 450 nm using a spectrophotometer (Emax; Molecular Devices Co., California, USA). The inhibitory effects of CL on tumor cell growth were evaluated using Amean/Acontrol, where Amean and Acontrol denote the absorbance of water-soluble formazan in the presence and absence of CL, respectively.
Caspase fluorometric protease assay: The caspase activity was measured as the protease activity of the caspases using the cell- permeable substrates PhiPhiLux G1D2 (caspase-3), CaspaLux 8- L1D2 (caspase-8) and CaspaLux 9-M1D2 (caspase-9) (OncoImmunin, Inc., Gaithersburg, MD, USA) according to the manufacturer’s instructions. HCT116 cells (6.0 × 106 cells) were treated with CL ((DMPC)=11.5 mM, (C12(EO)21)=0.66 mM and (2C14ECl)=1.06 mM) for 1-48 h. The cells were centrifuged at 3,000 rpm for 5 min and resuspended in 50 μL of chilled cell lysis buffer. The cell lysates were incubated with reaction buffer (50 μL) and the respective caspase substrate (75 μL) at 37ºC for 1 h. After washing twice with 1 mL of ice-cold PBS (-), the cells were resuspended in 1 mL PBS (-). The stained cells were observed using a confocal laser microscope with excitation at 488 nm using a 15 mW Ar laser (detection wavelength of 505-555 nm).
Assessment of antitumor activity in vivo: The animals were handled in accordance with the guidelines for animal experimentation under Japanese law. This study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Sojo University. The protocol was approved by the Committee on Ethics of Animal Experiments at Sojo University. All animal experiments were approved by the Committee on Animal Research at the Sojo University. BALB/c-R/J mice were generously provided by Prof. Okada (Kumamoto University, Japan) [11]. Cholangiocarcinoma cells (5.0 × 106 cells) suspended in Matrigel (BD Co., NJ, USA) were subcutaneously injected into the dorsal region of the mice. Five mice were used in each group. The tumor volume reached 100-300 mm3 on day 7 after the inoculation of cholangiocarcinoma cells and CL was intravenously administered once daily for 14 days on day 7. Tumor volume was measured using Vernier calipers and calculated using the equation V=0.5 × a2 × b, where a and b denote the smallest and longest superficial diameters, respectively [12]. The tumor volume reduction rate was calculated using the following equation: (1-(median tumor volume of treated group/median tumor volume of control group) × 100).
Statistical analysis
Results are presented as the mean Standard Deviation (S.D). Data were statistically analyzed using Student’s t-test. A p-value of less than 0.05 was considered to represent a statistically significant difference.
Results and Discussion
Physical properties of CL
The physical properties of CL composed of 87 mol% DMPC, 5 mol% C12(EO)21 and 8 mol% 2C14ECl were examined. The CL diameter was measured using dynamic light scattering and electron microscopy. The mean dhy of the CL was 100 nm, which remained stable for more than one month at 37ºC. In contrast, the DMPC liposomes were unstable and precipitated after 28 days. The absorbance of HL21 (95 mol% DMPC/5 mol % C12(EO)21) gradually increased (>400 nm) over time. These results suggest that a CL with a diameter of 100 nm is appropriate for in vivo and clinical applications to avoid the reticuloendothelial system when intravenously injected.
Membrane potential properties of cholangiocarcinoma cells: Surface exposure to PS occurs on the outer membrane leaflets of cancer cells. The level of PS in the outer plasma membrane of tumor cells is higher than that in normal cells [1-2]. The surface density of negatively charged PS in the outer membrane of cancer cells was evaluated to determine the surface negative charge density of various cancer cell types. PS in the outer membrane was stained with Annexin V-FITC and detected using flow cytometry. The results are shown in Figure 1A. The relative fluorescence intensity of PS in cholangiocarcinoma cell membranes was two times higher than that in normal bile duct cells. Cancer cell membranes have a substantially higher content of GM1, including sialic acid residues, than that in normal ones [3-5]. Next, we measured GM1 staining with fluorescent CTB in cholangiocarcinoma cell membranes using a flow cytometer, as shown in Figure 1B. The relative fluorescence intensity of GM1 in cholangiocarcinoma cells was double that in normal bile duct cells. Cancer cells have a lower membrane potential than that of normal cells (Figure 1), [12]. The zeta potential of cholangiocarcinoma cells was evaluated to assess electrostatic interactions between cholangiocarcinoma cell membranes. The results are shown in Figure 2. The zeta potential of cholangiocarcinoma cells was much lower than that of normal bile duct cells. These results indicate that CL selectively targets the negatively charged membranes of cholangiocarcinoma cells.
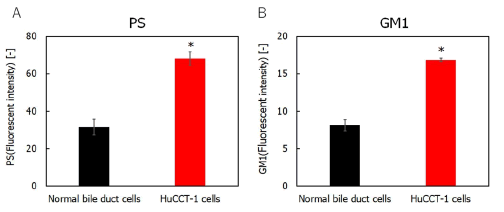
Figure 1: A) Relative fluorescence intensity of phosphatidylserine in cholangiocarcinoma and normal bile duct cells; B) Relative fluorescence intensity of ganglioside for cholangiocarcinoma cells and normal bile duct cells. Note: *p<0.05 (vs. normal bile duct cells).
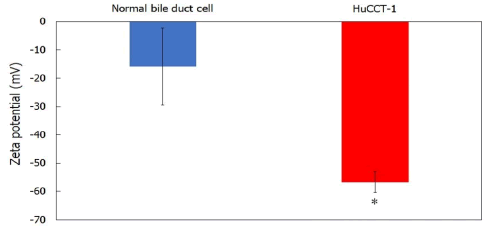
Figure 2: Zeta potentials of cholangiocarcinoma and normal bile duct cells. Note: *p<0.05 (vs. normal bile duct cells).
Fusion of CL to cell membranes of cholangiocarcinoma: We examined the fusion and accumulation of CL, including NBDPC as a fluorescent probe, in the cholangiocarcinoma cell membranes using a confocal laser scanning microscope. The results are shown in Figure 3. Remarkable accumulation of CL, including NBDPC, in the cholangiocarcinoma cell membrane was observed after 3 h, whereas lower accumulation of HL21 was observed. However, little accumulation of DMPC liposomes was observed. These results suggest that CL can selectively fuse and accumulate in cholangiocarcinoma cell membranes.
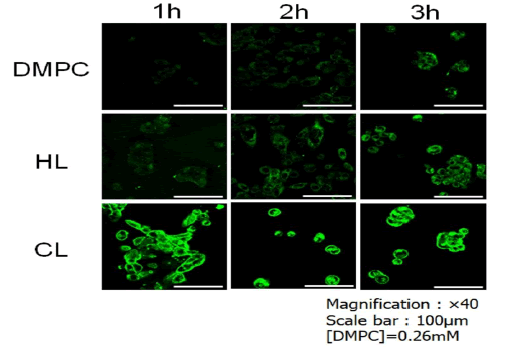
Figure 3: CL, including NBDPC, selectively fused to and accumulated in the plasma membrane of cholangiocarcinoma cells for 3 h.
Efficacy of treatment of CL on the growth of cholangiocarcinoma cells: We examined the IC50 of CL on the growth of human cholangiocarcinoma cells using a WST-8 assay in vitro. The results are shown in Figure 4. The IC50 values of CL against cholangiocarcinoma cells were much lower than those of DMPC liposomes and HL21. Notably, a high efficacy of CL treatment in human cholangiocarcinoma cells was observed.
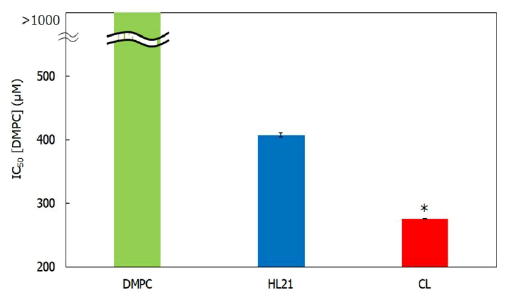
Figure 4: Inhibitory effects of CL on human cholangiocarcinoma cell growth. Note: *p<0.05 (vs. DMPC, HL21); Reaction time: 48 h
Induction of apoptosis for cholangiocarcinoma cells treated with CL: To investigate the apoptotic pathways of CL in cholangiocarcinoma cells, the apoptotic DNA rates in cholangiocarcinoma cells treated with CL were measured using flow cytometry. The results are shown in Figure 5. A high rate of apoptotic DNA was observed after treatment with CL, whereas the apoptosis induced by HL21 and DMPC was reduced. These results indicate that CL induces apoptosis in cholangiocarcinoma cells.
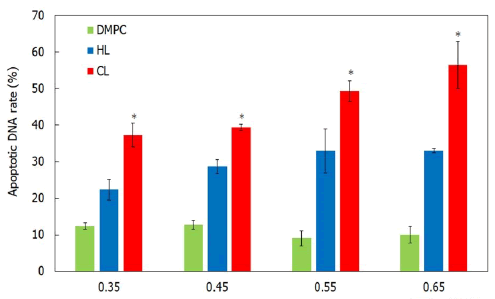
Figure 5: Rate of apoptotic DNA in cholangiocarcinoma cells treated with CL. Note: *p<0.05 (vs. DMPC, HL21); Reaction time: 48 h.
Pathway of induction of apoptosis in cholangiocarcinoma cells by CL: Caspase activation is essential during the execution of apoptosis. To investigate the apoptotic pathways of CL-induced cholangiocarcinoma cells, the activation of caspase -8, -9 and -3 was examined using a cell-permeable fluorescence substrate. The results are shown in Figure 6A. The caspase -3, -8 and -9 activities were observed in cholangiocarcinoma cells after treatment with CL. These results suggest that the activation of caspase -3, -8 and -9 is implicated in the apoptosis induced by CL in cholangiocarcinoma cells.
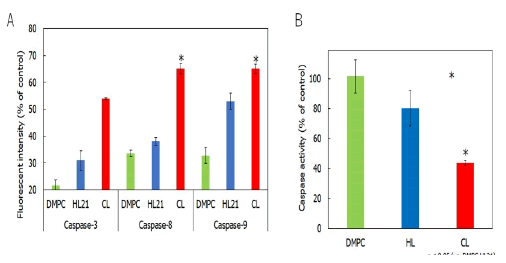
Figure 6: A) Activation of caspase -3, -8 and -9 in human cholangiocarcinoma cells after CL treatment; B) Mitochondrial transmembrane potential disruption of mitochondria in cholangiocarcinoma cells treated with CL. Note: In A, *p<0.005 (vs. Control, DMPC, HL21); In B, *p<0.05 (vs. DMPC, HL21); Reaction time: 48 h.
Furthermore, we examined the mitochondrial pathway of apoptotic signal transduction in CL-treated cells using flow cytometry. The results are shown in Figure 6B. Interestingly, the mitochondrial transmembrane potential decreased after treatment with CL, indicating that the mitochondrial pathway is also involved in CL-induced apoptosis in cholangiocarcinoma cells (Figure 6).
Therapeutic effects of CL on subcutaneous xenograft mice with cholangiocarcinoma in vivo
We examined the therapeutic effects of intravenous CL treatment on tumor volume in a subcutaneous xenograft mouse model of cholangiocarcinoma. The results are shown in Figure 7A. A 72% reduction (p<0.05) in tumor volume was observed in the subcutaneous xenograft mice treated with intravenous CL. We also investigated the therapeutic effects of intravenous CL on tumor weight in the same model. The results are shown in Figure 7B. Model mice treated with CL exhibited a considerable reduction in tumor weight (p<0.05) compared with the control group. Notably, a remarkable reduction in both tumor volume and weight was observed in the subcutaneous xenograft mice treated with intravenous CL, without additional drugs (Figure 7).
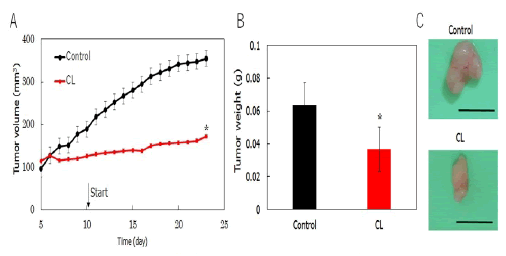
Figure 7: A) Tumor volume changes in a subcutaneous xenograft mouse model of cholangiocarcinoma treated with CL; B) Reduction in tumor weight in a subcutaneous xenograft mouse model of cholangiocarcinoma treated with CL. Note: *p<0.05 (vs. Control).
Conclusion
We demonstrated the therapeutic effects of CL in a mouse model of human cholangiocarcinoma. The noteworthy aspects of this study are as follows: a) A clear solution of CL with a hydrodynamic diameter of 100 nm was maintained over four weeks. b) Cholangiocarcinoma cells have a lower membrane potential than that of normal bile duct cells, as the levels of negatively charged PS and GM1 in cholangiocarcinoma cells were higher than those in normal bile duct cells. c) Immediate fusion of CL, including NBDPC, into cholangiocarcinoma cell membranes was confirmed using confocal laser microscopy. d) The IC50 value of CL for the growth of cholangiocarcinoma cells was remarkably lower than that of the HL21 and DMPC liposomes. e) CL-induced apoptosis of cholangiocarcinoma cells based on the propidium iodide assay using flow cytometry. f) Activation of caspases and a decrease in the mitochondrial membrane for apoptosis of cholangiocarcinoma cells induced by CL were verified by flow cytometric analysis. g) Therapeutic effects of CL were observed in subcutaneous xenograft mouse models of cholangiocarcinoma in vivo. The results of this study may be advantageous for the treatment of patients with cholangiocarcinoma in future clinical applications.
References
- Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062-3066.
[Google Scholar] [PubMed]
- Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132-6140.
[Google Scholar] [PubMed]
- Murayama T, Zuber C, Seelentag WK, Li WP, Kemmner W, Heitz PU, et al. Colon carcinoma glycoproteins carrying alpha 2,6-linked sialic acid reactive with Sambucus nigra agglutinin are not constitutively expressed in normal human colon mucosa and are distinct from sialyl-Tn antigen. Int J Cancer. 1997;70:575-581.
[Crossref] [Google Scholar] [PubMed]
- Sebzda T, Saleh Y, Gburek J, Warwas M, Andrzejak R, et al. Total and lipid-bound plasma sialic acid as diagnostic markers in colorectal cancer patients: correlation with cathepsin B expression in progression to Dukes stage. J Exp Ther Oncol. 2006;5:223-229.
[Google Scholar] [PubMed]
- Kwak DH, Ryu JS, Kim CH, Ko K, Ma JY, Hwang KA, et al. Relationship between ganglioside expression and anti-cancer effects of the monoclonal antibody against epithelial cell adhesion molecule in colon cancer. Exp Mol Med. 2011;43:693-701.
[Crossref] [Google Scholar] [PubMed]
- Umebayashi M, Makizono T, Ichihara H, Matsumoto Y, Ueoka R. Inhibitory effects of cationic hybrid liposomes on the growth of human renal cell carcinoma. Anticancer Res. 2010;30:327-337.
[Google Scholar] [PubMed]
- Schröder-Borm H, Bakalova R, Andra J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579:6128-6134.
[Crossref] [Google Scholar] [PubMed]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929-942.
[Crossref] [Google Scholar] [PubMed]
- Matsumoto Y, Cao E, Ueoka R. Growth inhibition by novel liposomes including trehalose surfactant against hepatocarcinoma cells along with apoptosis. Anticancer Res. 2013;33:4727-4740.
[Google Scholar] [PubMed]
- Hondroulis E, Zhang R, Zhang C, Chen C, Ino K, Matsue T, et al. Immuno nanoparticles integrated electrical control of targeted cancer cell development using whole cell bioelectronic device. Theranostics. 2014;4:919-930.
[Crossref] [Google Scholar] [PubMed]
- Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, et al. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011;539748:1-6.
[Crossref] [Google Scholar] [PubMed]
- Shimoda S, Ichihara H, Matsumoto Y, Ueoka R. Chemotherapy with hybrid liposomes for human breast tumors along with apoptosis in vivo. Int J Pharm. 2009;372:162-168.
[Crossref] [Google Scholar] [PubMed]
Citation: Ichihara H, Takaki H, Motomura M, Matsumoto M (2024). Efficacy of Drug-Free Cationic Hybrid Liposomes Composed of L-α Dimyristoylphosphatidylcholine in Targeting Negatively Charged Cholangiocarcinoma Cell Membranes: An In Vitro and In Vivo Study. J Carcinog Mutagen. 15:460.
Copyright: © 2024 Ichihara H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


