Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 14, Issue 2
Efficacy of CurvicTM to Boost Immunity in the Quarantine Patients of COVID-19: An Open Labelled Study
Yogesh Dound1*, Yogita Gaikwad2, Shrikant Suryavanshi3, Rajesh Sehgal4 and Pranav Patel52Department of Medicine, Niphad Sub District Hospital, Nashik, India
3Department of Psychology, Consulting Physician, Maharashtra, India
4Department of Research and Development, Pharma Instinct Pvt. Ltd, Noida, India
5Department of Life Science, Curactive Life Sciences Pvt. Ltd, Vadodara, India
Received: 01-Feb-2022, Manuscript No. JMBT-22-15599; Editor assigned: 03-Feb-2022, Pre QC No. JMBT-22-15599(PQ); Reviewed: 17-Feb-2022, QC No. JMBT-22-15599; Revised: 21-Feb-2022, Manuscript No. JMBT-22-15599(R); Published: 28-Feb-2022, DOI: 10.35248/ 1948-5948.22.14.485
Abstract
Background: COVID-19 outbreak is a global threat, with lack of vaccines and antiviral medicine exaggerating the issue further. CDC recommends maintenance of personal hygiene as a modality to reduce chances of infection. Yet, another way is by improving the immunity. Viral infections are depended on body’s natural defenses. Authors of current paper have developed formulation containing natural ingredients viz. Curcumin, Vitamin C, Vitamin K2-7 L-Selenomethionine and Zinc based on results of in-silico studies for activity against SARS-CoV-2’s Mpro Protein.
Objectives: To evaluate role of CurvicTM to boost immunity in quarantined patients of COVID-19 and its tolerability.
Study design: In an open-labelled study, 30 patients quarantined due to COVID-19 were administered CurvicTM tablets twice daily for 14 days. They were analysed on basis of VAS score for cough and respiratory distress, SF-36 Questionnaire and laboratory investigations.
Results: Within 48 hours, temperature reduced considerably to afebrile level and remained afebrile till end of study. Average VAS score reduced from 6-7 at baseline to 0-1 within 14 days. The high levels of serum Interleukin-6 and Homocysteine at baseline dropped considerably within 14 days. Body pain experienced by 20 patients had reduced significantly and the energy levels along with health were improved in all the patients, thereby leading to improved quality of life in all patients.
Conclusion: CurvicTM has shown to be useful in improving the immunity of the quarantined patients of COVID-19, within 48 hours of starting treatment. CurvicTM was well tolerated without any side effects in any of the patients treated.
Keywords
COVID-19; Vitamin K2-7; Curcumin; Selenomethionine; Vitamin C; Interleukin-6
Introduction
The current COVID-19 outbreak is a global threat, WHO recognized the outbreak as a pandemic on 11 March 2020 [1,2]. As of May 31st 2020, COVID-19 has affected almost 213 countries with approximately 6,160,295 cases, with recoveries of about 2,738,284 cases and death toll of 371,006 [3].
The disturbing fact is that though it is considered as an international public health problem, there are no vaccine and no specific antiviral medicine to prevent or treat COVID-19 [4]. The affected individual demonstrates the sign and symptoms of fever, cough and difficulty breathing. The other complications are pneumonia and acute respiratory distress syndrome. CDC recommends maintenance of personal hygiene such as regular hand washing, covering mouth and nose when coughing and sneezing, and social distancing as a modality to reduce the chances of infection. Another way to prevent from getting the chances of infection is by improving the immunity. Flaring of most viral infections are depended on the body’s natural defences, in fact they are inversely related.
Those affected should receive care to relieve symptoms. People with serious illness need to be hospitalized. Most patients recover due to supportive care. Various vaccines and certain drug treatments options are under investigation. They are being tested through clinical trials. WHO is collaborating efforts to develop newer vaccines and specific antiviral medicines to prevent and treat COVID-19. There are several ongoing clinical trials that include both western and traditional medicines. Recently Remdesivir (GS- 5734), a nucleoside analogue prodrug was studied in a randomized double blind placebo controlled trial in hospitalized adults diagnosed with COVID-19 [5].
Traditional Indian medicine has shown promising results in the management of various illnesses, including viral infections. The detailed analysis and study has shed light on the mechanism of action and hence providing a lead to develop as antiviral drug [6-9].
Authors of the current paper have developed a formulation containing natural ingredients such as Curcumin, Vitamin C, Vitamin K2-7 L-Selenomethionine and Zinc. The authors have evaluated the activity of the ingredients from the formulation through the in-silico studies against SARS-CoV-2’s main peptide. Based on the results of these studies, the authors proposed the clinical study for the quarantined patients who have been suspected to be positive for COVID-19 for improving their immunity. Here, we report the results of this open labelled study.
Methodology
Objective
The primary objective of the study was to evaluate the role of CurvicTM to boost immunity in quarantined patients of COVID-19 along with its safety and tolerability.
Study design
Study method: This was an open labelled study to assess the role of CurvicTM to boost immunity in quarantined patients of COVID-19 along with its safety and tolerability. This study was conducted at Niphad Sub District Hospital, Nashik, Maharashtra, India. The study was approved by Navsanjeevani Hospital Ethics Committee and registered with CTRI. Written informed consent was obtained from all patients.
Study participants: Thirty eligible patients were men and non- pregnant women who were at least 18 years of age and met the eligibility criteria among the thirty five patients screened in the month of April-May 2020. An ICF was signed from these 30 patients at baseline before inclusion into the trial. Table 1 gives the demographic details of these subjects. The subjects had undergone a detailed history and complete physical examination along with the laboratory investigations. These include CBC, Lipid profile, Liver Profile, Urine Routine, Blood sugar, Homocystine and Pro inflammatory cytokine IL-6. Once quarantined, these patients received the care as per the standard practices prescribed by WHO and/or applicable regulatory guidelines for 14 days. The subjects were examined daily for symptoms which include cough, fever with or without chills and difficulty in breathing. Each subject received the test formulation daily till the end point. The End point was till the point the patient is kept in quarantine i.e., 14 days or as decided by the Principal Investigator. All the laboratory investigations were repeated at end point. The investigations were carried out in NABL accredited laboratory.
| Males | Females | |
|---|---|---|
| N | 16 | 14 |
| Age (yrs.) | 32.60 ± 12.8 | 32.79 ± 9.0 |
| BMI (kg/m2) | 22.50 ± 1.8 | 23.16 ± 2.2 |
| Blood Pressure (mmHg) | 82.33 ± 6.8 |
80.14 ± 5.0 |
Note: Values expressed in mean ± SD; SD: Standard Deviation.
Table 1: Demographic details of the subjects participating in the study.
Study outcome: The primary outcome included clinical status as decided by the Principal Investigator based on the physical examination, laboratory investigations and signs such as axillary temperature, respiratory rate, pulse, blood pressure and symptoms which include cough, fever with or without chills and difficulty in breathing. Changes in cough VAS score for cough and respiratory distress (Figure 1) were used as a surrogate marker for response to treatment which has a scale from 0-10 cm with 10 cm being the worst imaginable cough [10]. Short Form 36 item (SF-36) health status questionnaire (adjusted to 2 weeks) which is a well-tested and validated generic health status measure was filled from each patient at baseline and at the end of study [11].
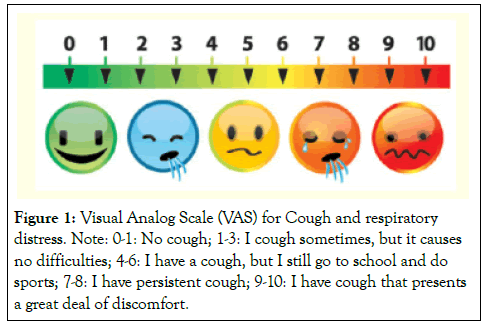
Figure 1:Visual Analog Scale (VAS) for Cough and respiratory distress. Note: 0-1: No cough; 1-3: I cough sometimes, but it causes no difficulties; 4-6: I have a cough, but I still go to school and do sports; 7-8: I have persistent cough; 9-10: I have cough that presents a great deal of discomfort.
Statistical analysis: Data was presented as descriptive statistics such as mean ± standard deviation. Various statistical tests were used for the statistical analysis which includes unpaired t test method. Categorical data were presented as frequency and percentage. Statistical Analysis was done by an independent statistician using GraphPad software.
Study intervention: The formulation tablets, i.e., CurvicTM were manufactured and supplied by Shreepad Shree Vallabh SSV Phytopharmaceutical in the form of tablets of each packed as 30 tablets per bottle. The tablets were supplied in bottles to patients at the time of the enrolment. Patient consumed each tablet orally twice a day immediately after meals for 14 days. The tablets compliance was judged by counting the number of tablets in the bottle left at the end of study. Patient was said to be compliant if he had consumed minimum 80% of the total dispensed tablets.
Results
All the 30 patients enrolled in the study completed their study. All the patients had complaints of cough, fever without chills and difficulty in breathing. These patients received the care as per the standard practices prescribed by WHO and/or applicable regulatory guidelines for 14 days.
Out of 30 patients, there were 16 males and 14 females. The average age of males was 32.60 ± 12.8 and that of females was 32.79 ± 9.0 (Mean ± SD). Upon enrolment the average temperature of male patients was 101.60 ± 0.5 0F, while that of female patients was 101.57 ± 0.5 0F (Mean ± SD). Within 48 hours of starting the CurvicTM tablets the fever reduced to 98.07 ± 0.7 0F and 97.86 ± 0.7 0F (Mean ± SD) in males and females respectively. This temperature range remained constant till the end of study. By 14th day, the temperature in males was 97.47 ± 0.5 0F and in females it was 97.43 ± 0.5 0F (Mean ± SD). Figure 2 describes the decrease in axillary temperature from baseline to 14 days.
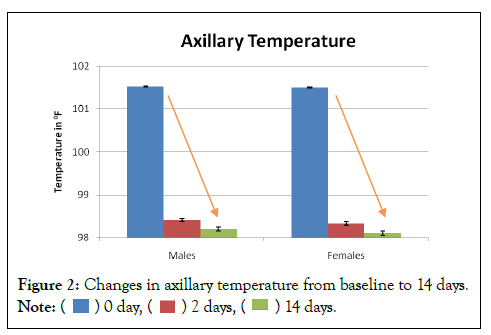
Figure 2: Changes in axillary temperature from baseline to 14 days.
The average VAS score for cough and respiratory distress of male patients during enrolment was 6.63 ± 0.6 while that of female patients was 6.79 ± 0.6 (Mean ± SD). Within 48 hours of starting the CurvicTM tablets the VAS score reduced to 3.31 ± 0.5 and 3.29 ± 0.5 in males and females respectively (Mean ± SD). After 14 days, the VAS score in male and female patients reduced to 0.69 ± 0.5 and 0.64 ± 0.5 (Mean ± SD) respectively. The decrease in the VAS score was found to be statistically significant (p<0.0001) in males as well as female patients. Figure 3 describes the decrease in VAS score from baseline to 14 days.
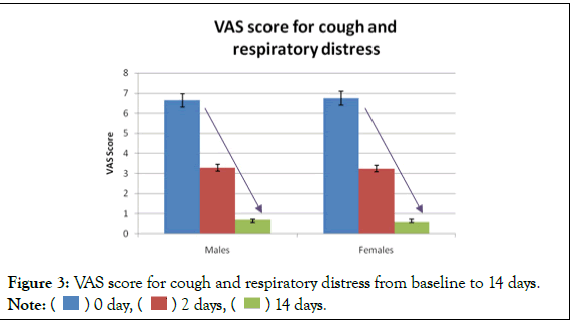
Figure 3: VAS score for cough and respiratory distress from baseline to 14 days.
43% of the patients experienced often daily cough several times per day without any impact of that on the quality of life. Around 12% of the patients have suffered from cough that interferes with daily activities. Among them 30% have reported sleep disturbance due to the issues with cough. After the treatment, all participants experienced no cough or cough occasionally but without disturbing daily activity. None of the participant had cough that interfered daily activities by the end of 14th day. More than 97% of Patientshave noticed some kind of improvement within 48 hours of treatment with CurvicTM.
All the participants completed the SF-36 questionnaire and the rate of completion for each dimension was over 95%. As studied from these filled questionnaires it was observed that the general health improved in all the patients. The Limitation of Activities was also reduced in all the patients, thereby improving their working capacity. The physical and the emotional health problems also reduced. The body pain was experienced by 20 out of 30 patients. Within 48 hours, there were significant reduction in the body pain experienced by these 20 patients. The energy levels and the general wellbeing along with health improved in all the patients. The Quality of Life also improved in all the patients.
The serum IL-6 and Homocysteine levels were very high at baseline (Table 2). The serum IL-6 levels were at 4937.79 ± 329.2 pg/ml (Mean ± SD). By the end of 14 days, the levels dropped considerably to 1507.29 ± 1095.8 pg/ml (Mean ± SD). The Homocysteine levels were 40.00 ± 14.2 μmol/L at baseline (Mean ± SD). By 14 days the levels decreased to 29.86 ± 12.7 μmol/L (Mean ± SD). There was no change in rest of the biochemical as well as organ function tests within 14 days of intake of CurvicTM .
| IL-6 (pg/ml) | Homocysteine (μmol/L) | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Baseline | 4883.87 ± 449.8 | 5000.00 ± 1128.5 | 46.87 ± 14.0 | 32.65 ± 10.5 |
| 14th day | 1802.32 ± 1085.3 | 1166.87 ± 1045.5 | 35.89 ± 13.2 | 23.41 ± 8.5 |
Note: Values expressed in mean ± SD; SD: Standard Deviation.
Table 2: Serum IL-6 and homocysteine levels from baseline to 14 days.
CurvicTM was well tolerated without any side effects in any of the patients treated, throughout the study duration. Among these patients there were some who were on oral medications for Hypertension and Type 2 diabetes mellitus. These patients continued with their regular medications along with CurvicTM. It was observed that there was no drug interaction of CurvicTM with any of these medications.
Discussion
Coronavirus is an envelope, positive-sense single-stranded RNA virus belonging to the Coronaviridae family. SARS-CoV-2 is a new strain that was discovered in 2019 and has not been previously identified in humans. This virus primarily spreads between people via respiratory droplets from coughing or sneezing [12,13]. With currently no specific vaccine or medicine being available and the research process for newer vaccines and medicines are taking longer time, the medicines based on natural sources are being considered by many researchers worldwide [6-9]. Authors of the current study have tried to achieve the same by considering the natural ingredients as the base for CurvicTM formulation.
Vitamin K2-7, one of the ingredients within the CurvicTM, belonging to Vitamin K group has shown to be effective in reducing pro inflammatory cytokines [14]. A recent preliminary research conducted by Schruggers et. al., has shown that low Vitamin K status may lead to worse outcomes for COVID-19 patients [15]. The authors of the current study have in-silico evaluated the activity of Vitamin K2-7 against SARS-CoV’s main peptide and COVID-19 Mpro and have observed that Vitamin K2-7 has a suggestive activity against SARS-CoV’s main peptide and COVID-19 Mpro, suggesting its potential role in prevention of COVID-19 [8]. The used formulation containing Vitamin K2-7 which usedby SSV, is more than 98.5% trans isomers and less than 0.2% cis-isomers.
Another ingredient used in CurvicTM is standardised proprietary blend of Curcumin, developed by SSV. Curcumin has exhibited various pharmacological activities attributed to its major metabolites like dimethoxycurcumin and tetrahydrocurcumin [16,17]. Recently Demethoxycurcumin was shown to have the best potential to act as COVID-19 Mpro inhibitor [18]. Authors of the current study for the first time showed that Tetrahydrocurcumin, one of the major compounds of the standardised proprietary blend of Curcumin, has promising antiviral effect by inhibiting SARS-CoV’s main peptide and COVID-19 Protein [8].
Yet another ingredient used within the CurvicTM is is L-Selenomethionine. Selenium deficiency is associated with increased viral virulence and has been suggested to play a role in “the emergence of novel viral diseases” [19]. Viral infections leading to lung pathology complications are more common in those who are selenium deficient probably due to impaired glutathione peroxidase production and increased lung tissue inflammatory response to viral infection [20]. Recently, researchers have linked Selenium supplementation for prevention of coronavirus infection [21].
The another vitamin and rather the most decorated vitamin used within CurvicTM is Vitamin C. Vitamin C acts as an antioxidant, which destroys free radicals and supports the body’s natural immune response. Vitamin C potentially protects against infection caused by SARS-CoVs by improving the immunity, function of phagocytes, transformation of T lymphocytes and production of interferon [22,23]. Vitamin C is also recommended for prevention of SARS-CoV-2 infections by the Chinese Center for Disease Control and Prevention and Chinese Nutrition Society. Zinc which is used in the Curvic TM formulation is an acting as a catalyst. It helps as an anti-oxidant as well as shown to be prophylaxis agent to be prevent and mitigate the potentially bas SARS-COV2 infection.
In the current study, the authors for the first time have proposed a formulation combining these ingredients as a prophylaxis against current outbreak of COVID-19 and to boost the immunity. The decrease in temperature within 48 hours and VAS score for cough and respiratory distress along with improvement in health status as judged by the globally validated SF-36 Questionnaire are suggestive of the efficacy of CurvicTM. IL-6 acts as a pro- inflammatory cytokine as well as an anti-inflammatory myokine. Inflammation is closely related to severity of COVID-19 and IL-6 is considered to be one of the important therapeutic targets [24]. It has also been shown that the serum levels of IL-6 predict the outcome in patients with COVID-19 [25]. In the current study the very high levels of IL-6 at baseline reduced by approximately 69% within 14 days. Homocysteine is a non-proteinogenic amino acid and elevated Homocysteine concentrations are shown to be associated with immune system activation and increased neopterin serum concentrations [26]. It has been suggested to determine the plasma homocysteine as a potential marker for severe disease in COVID-19 patients [27]. In the current study the high levels of Homocysteine at baseline reduced approximately by 25% within 14 days. This decrease is quite suggestive of improvement in the status of inflammation, thereby improving the immunity in these quarantined patients for COVID-19.
Conclusion
The authors of the current study have shown for the first time in humans that the average VAS score for cough and respiratory distress reducing from 6-7 at baseline to 0-1 by the end of 14 days after intake of CurvicTM formulation. At the end of study, all participants experienced no cough that interfered their daily activities. Within 48 hours of intake of CurvicTM formulation, the temperature reduced considerably and the temperature remained constant till the end of study. Body pain experienced by 20 patients had reduced significantly and the energy levels and the general wellbeing along with health were improved in all the patients, thereby leading to improved Quality of Life in all the patients. In addition to this, the authors of the current study for the first time have observed that there was significant decrease in very high serum levels of IL-6 and Homocysteine following intake of CurvicTM formulation. Hence this formulation is useful in improving the immunity of the quarantined patients for COVID-19. CurvicTM has shown to be useful in improving the immunity of the quarantined patients of COVID-19, within 48 hours of starting of treatment.
CurvicTM was well tolerated without any side effects in any of the patients treated, throughout the study duration.
Acknowledgement
Funding
This study was funded by Shreepad Shree Vallabh SSV Phytopharmaceuticals, Mumbai, India. We thank Mr. Chandrakant Wale, SRL Diagnostics, Nashik Road for the laboratory evaluation of biochemical and organ function tests and Niphad Sub District Hospital Staff.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published Compliance with Ethics Guidelines.
Disclosures
Dr. Yogesh Dound is Proprietor Shreepad Shree Vallabh, SSV Phytopharmaceuticals respectively.
Compliance with Ethics Guidelines
The study was approved by Navsanjeevani Hospital Ethics Committee and registered with Clinical Trial Registry of India: CTRI/2020/04/024659 (www.ctri.nic.in). The trial was conducted in accordance with the principles of the Declaration of Helsinki (1964), the International Conference on Harmonization-Good Clinical Practice guidelines and New Drugs and Clinical Trials Rules, 2019. Written informed consent was obtained from all patients.
REFERENCES
- Coronavirus disease 2019. World Health Organization. Retrieved 15 March 2020.
- WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020. World Health Organization. 11 March 2020. Retrieved 11 March 2020.
- Coronavirus Update (Live): Worldometer. Retrieved 31 May 2020.
- Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020;13:667-73.
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020;395(10236):1569-78.
- Lin LT, Hsu WC, Lin CC. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014;4(1):24-35.
- Wen CC, Kuo YH, Jan JT, Liang PH, Wang SY, Liu HG et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087-95.
- Dound YA, Chaudhary S, Chaudhary SS, Ahmad MM, Geesi MH, Alam P. Preventive strategies for management of COVID-19 using natural molecules.
[Google Scholar][Crossref][Indexed]
- Chen L, Hu C, Hood M, Zhang X, Zhang L, Kan J et al. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: A perspective from system biology analysis. Nutrients. 2020;12(4):1193.
- Brightling CE, Ward R, Wardlaw AJ, Pavord ID. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. European Respiratory Journal. 2000;15(4):682-686.
- Brazier JE, Harper R, Jones NM, O'cathain A, Thomas KJ, Usherwood T et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. British Medical Journal. 1992;305(6846):160-4.
- Coronavirus Disease 2019 (COVID-19). Centers for disease control and prevention. Retrieved 16 March 2020.
- Symptoms of Novel Coronavirus (2019-nCoV)". US Centers for Disease Control and Prevention. 10 February 2020. Retrieved 11 February 2020.
- Simes DC, Viegas CS, Araújo N, Marreiros C. Vitamin K as a powerful micronutrient in aging and age-related diseases: pros and cons from clinical studies. Int J Mol Sci. 2019;20(17):4150.
- Dofferhoff AS, Piscaer I, Schurgers LJ, Walk J, van den Ouweland JM, Hackeng TM et al. Reduced vitamin K status as a potentially modifiable prognostic risk factor in COVID-19.
- Paramasivam M, Poi R, Banerjee H, Bandyopadhyay A. High-performance thin layer chromatographic method for quantitative determination of curcuminoids in Curcuma longa germplasm. Food Chem. 2009;113(2):640-644.
- Zhongfa L, Chiu M, Wang J, Chen W, Yen W, Fan-Havard P et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. 2012;69(3):679-689.
- Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. 2020;2020:2020030226.
- Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. Journal Nutr. 2003;133(5):1463S-1467S.
- Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S et al. Selenium deficiency increases the pathology of an influenza virus infection. The FASEB Journal. 2001;15(8):1481-1483.
- Kieliszek M, Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Medical Hypotheses. 2020;143:109878.
- Hemila H. Vitamin C and SARS coronavirus. J Antimicrob Chemother. 2003;52(6):1049-1050.
- Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92(5):479-90.
- Gong J, Dong H, Xia Q, Huang Z, Wang D, Zhao Y et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv. 2020.
- Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370.
- Li T, Chen Y, Li J, Yang X, Zhang H, Qin X et al. Serum homocysteine concentration is significantly associated with inflammatory/immune factors. PLoS One. 2015;10(9):e0138099.
- Ponti G, Ruini C, Tomasi A. Homocysteine as a potential predictor of cardiovascular risk in patients with COVID-19. Medical Hypotheses. 2020;143:109859.
Citation: Dound Y, Patil S, Gaikwad Y, Suryavanshi S, Ghaisas M, Sehgal R, et al. (2022) Efficacy of CurvicTM to Boost Immunity in the Quarantine Patients of COVID-19. J Microb Biochem Technol. 14:485.
Copyright: © 2022 Dound Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

