Indexed In
- SafetyLit
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 27, Issue 3
Effects of Plant Functional Types Substrates on Soil Arthropod Community in Coastal Urban Wetlands
Ortiz-Ramírez Gloria1,3*, Hernández-Figueroa Elix1,3, Pinto-Pacheco Solimar1,3 and Cuevas Elvira2,32Department of Biology, College of Natural Sciences, University of Puerto Rico, San Juan, Puerto Rico
3Center for Applied Tropical Ecology and Conservation, College of Natural Sciences, University of Puerto Rico, San Juan, Puerto Rico
Received: 10-Apr-2024, Manuscript No. JCZM-24-25522; Editor assigned: 12-Apr-2024, Pre QC No. JCZM-24-25522 (PQ); Reviewed: 02-May-2024, QC No. JCZM-24-25522; Revised: 09-May-2024, Manuscript No. JCZM-24-25522 (R); Published: 16-May-2024, DOI: 10.35248/2473-3350.24.27.620
Abstract
This research examined the effects of plant functional types, via associated litter quantity and quality, on soil arthropods community structure and composition in a tropical urban coastal wetland. Substrate samples were collected from four plant functional types—tree, shrub, grass, fern—during different hydroperiod conditions in 2020 and 2021 and were processed using lighted Tullgren–Berlese extractors. Carbon-to-nitrogen ratios (C:N ratio), along with carbon and nitrogen contents, were measured for each sample. The study demonstrated statistically significant associations between the mass of loose litter, the carbon (%C) and nitrogen (%N) content of the substrate, and the richness and abundance of soil arthropods. The mass of loose litter exerted a more pronounced influence on both richness and abundance. A succession of taxa-dependent interactions related to C:N ratio was quantified, demonstrating how common, rare, and dominant groups interact, thus illustrating the complex interplay among different soil arthropod taxa. Soil arthropod trophic guild densities peaked in both the equilibrium (C:N ratio between 20:1 and 30:1) and immobilization (C:N ratio>30:1) phases of decomposition, with a distinct separation from the mineralization phase (C:N ratio<20:1) highlighting the sensitivity of these communities to nitrogen availability and the crucial role of primary and secondary decomposers in ecosystem processes. This research enhances our understanding of the intricate ecological interconnections between plant litter attributes, environmental hydroperiods, and soil arthropod biodiversity, emphasizing the integral role of vegetation and water in shaping soil ecosystem dynamics. It offers valuable insights for ecosystem management and conservation strategies aimed at preserving biodiversity and ecosystem functionality in wetland environments.
Keywords
Urban wetland, Coastal wetlands, Puerto Rico, Hydroperiods, Vegetation functional types, Soil arthropods, Biodiversity
Introduction
Aboveground-belowground interactions are fundamental to ecosystem structure and function [1]. Plants supply detritus resources such as litter, while soil arthropods, via their trophic activities, mediate the breakdown of plant litter and contribute significantly to organic matter turnover and nutrient availability, which are essential for plant growth [2-4]. These complex bidirectional relationships between plant communities and soil arthropods enhance soil health, ecosystem services, and ecosystem resilience and stability [5,6]. The soil-litter system is characterized by the interdependent dynamics of litter inputs and arthropod responses, moderated by the spatial and temporal distribution of detrital resources and the diversity and abundance of arthropod functional groups, including micro-predators, litter transformers, and ecosystem engineers [7-13]. The nutritional content, physical structure, and decomposition stage of plant litter generate a diverse array of habitats and resources, catering to the specific needs and feeding strategies of various arthropod groups. This diversity influences the composition and structure of soil arthropod communities [10,12-16]. For example, in agroforestry plantations, soil arthropod diversity is greater in plots with thicker litter layers and enhanced nutrient content [17]. Diplopoda (millipedes) and Isopoda (woodlice) species show increased richness and abundance in response to food quality, as well as soil temperature and humidity [18].
Collembola (springtails) exhibit significant aggregation at small spatial scales, influenced by litter quality and quantity, and microclimatic conditions, potentially driven by pheromonal signaling that directs them to optimal micro-environments [10]. Moreover, as detritus decomposition proceeds, arthropod assemblages experience successional changes in the food web as a results of changes in resource quality and habitat microclimate conditions [12-16,19]. The “sleeping beauty paradox” states that dormant microbial communities need a “Prince Charming”, be it a microorganism, a physical process or an environmental factor, which “awakens them” by facilitating their contact with the nutrient pools [7], showed that colonization, succession, and subsequent decline of arthropod assemblages on detritus followed a predictable sequence: Initial of surface-dwelling species such as ants, beetles, and flies, transitioning to an intermediate stage dominated by soil-dwelling organisms like mites and springtails (collembolas), and ultimately evolving into a decline phase where the assemblage is predominantly comprised by more specialized species [20]. In wetland ecosystems the availability and spatial distribution of detrital resources are influenced by the hydroperiods and drying and wetting dynamics. During dry periods, terrestrial plant litter accumulates and undergoes partial in situ decomposition. Upon flooding, both loose and decomposed litter is redistributed, creating a mixture of fresh, comminuted and partly decomposed organic substrate. This process yields microhabitats and food resources unevenly distributed across time and space, thus modulating soil arthropods responses [9-19,21-23]. In the soils of floodplain forests, wetting and drying cycles trigger a rapid mineralization burst, leading to a high C:N ratio and significant mass and carbon losses from decomposing litter. These alterations subsequently modulate soil arthropods trophic assemblages by fostering the development of two interactive decomposition channels: bacterial or fungal-based food webs, sensu Coleman [13,24-26]. Bacteria utilize the labile components of plant litter for their growth, while the more recalcitrant resources (such as cellulose and lignin) are consumed by fungi. This succession in bacterial and fungal communities cascades up to higher trophic levels. In the bacterial-based food web, bacteria, protozoa, and nematodes predominate. Conversely, in the fungal-based food web, fungi and mesofauna (such as mites and springtails) are involved [12,14,27].
The complexity, heterogeneity, distribution, and accumulation of plant litter is recognized to significantly influence the overarching structure of their associated soil food webs and their effect on soil organic accumulation and nutrient dynamics [10-12]. However, there remains a research gap in Caribbean Coastal Wetlands (Table 1). The lack of prior research is particularly noteworthy given the critical importance of plant-soil interactions in developing management strategies for ecosystem conservation [28]. In critically endangered areas such as the Caribbean: the fourth primary biodiversity hotspot worldwide which represents one of the world’s most complex mosaics of marine freshwater and terrestrial habitats, this gap emphasizes the need for focused fundamental investigations in this unique ecological setting [29-31]. Their significance is further underscored within the framework of global and regional challenges, including climate change, sealevel rise, and anthropogenic impacts.
| Study reference | Wetland location | Key findings |
|---|---|---|
| Guo et. al., 2022 | Qinghai-Tibetan Plateau Peatland. | Water table decline significantly arthropod community structure by shifting plant communities and leaf nutrient profiles |
| Ward et. al., 2015 | Peatlands National Nature Reserve, Northern England | Litter quality and changes in vegetation composition play a significant role in regulating short-term litter decomposition and belowground communities. These influences are found to have a more pronounced effect than that of moderate warming. |
| Krab et, al,. 2013 | Abisko Subarctic Peat Bogs, North Sweden | The influence of litter quality on Collembolan populations is more significant than its indirect effects on microclimate. |
| García-Gómez et. al.,2014 | National Park Reefs of Cozumel Island in the South of Mexico | This study provides valuable insights into the seasonal dynamics of arthropod diversity in relation to the dominant mangrove species and climatic conditions. |
| Weilhoefer et. al., 2017 | Smith and Bybee Wetlands Natural Area in Portland, OR, USA | Influence of reed canary grass on the arthropod community is predominantly indirect, mediated through alterations in habitat structure and conditions, rather than by directly changing the food resources available to the arthropods. |
Table 1: Global studies on the effects of vegetation on soil arthropod communities in various wetland ecosystems.
A thorough understanding of arthropod responses to variations in litter quality, distribution, and accumulation is essential for elucidating the functioning of the wetland litter system and its impact on ecosystem dynamics [5,8,11-13,27], which encompass both aboveground and belowground processes [32-36].
The aim of this research is to determine the influence of plant functional types on soil arthropods community structure and composition. It addresses the central question; how does plant litter cover, through their associated litter quantity and quality, modulate the assemblages of soil arthropod groups across wetland hydroperiods? The hypothesis posits that the synchrony and synlocation of soil arthropod community structure and composition are influenced by specific plant functional types. This influence is mediated through the composition of plant residues, the quality and accumulation of litter, and the dynamics dictated by hydroperiod variations. This research endeavor seeks to fill the existing knowledge gap by providing insights into the nuanced interactions between plant litter characteristics and soil arthropod communities in a coastal urban wetland, thereby contributing to the effective management and conservation of wetland ecosystems under the current global environmental challenges.
Materials and Methods
Study area
The study took place in 2.2 ha (research area) within the Ciénaga Las Cucharillas Natural Reserve, a palustrine-estuarine coastal urban wetland on the northern coast of the Caribbean Island of Puerto Rico. The reserve is in the municipality of Cataño (18 26’25.27” N, 66 08’08.39” W). The wetland comprises the western side of the San Juan Bay (Figure 1) [37].

Figure 1: (A) Ciénaga las Cucharillas located on the northern coast of the Caribbean Island of Puerto Rico, at the western side of the San Juan Bay; (B) study area (2.2 ha); and (C) study plots 3, 5, 10, and 6.
Average monthly temperature ranges from 31°C to 25°C from May to October, and 22°C to 28°C from December to March. The area has a humid climate with an average annual precipitation of 1920 millimeters. The rainfall distribution is bimodal, with lower precipitation occurring from December to April-May and two peak periods from May to June, and September to November [37]. The study was carried out from 2020 to 2021 (Figure 2) [37]. In 2020, the wettest month was July, with monthly mean precipitation of 9.64 millimeters and 24 rainy days.

Figure 2: (A) The climate diagram illustrates monthly average air temperatures in C (left y axis,  and average total monthly precipitation in mm (right y-axis,
and average total monthly precipitation in mm (right y-axis,  from January 2017 to December 2021 (months are represented by letters) at Ciénaga Las Cucharillas Natural Reserve; (B) The graph presents the mean monthly precipitation and the total number of rainy days, using climatological data from January 2020 to November 2021, sourced from the Toa Baja Levittown, PR Meteorological Station (National Weather Service, 2023). Note:
from January 2017 to December 2021 (months are represented by letters) at Ciénaga Las Cucharillas Natural Reserve; (B) The graph presents the mean monthly precipitation and the total number of rainy days, using climatological data from January 2020 to November 2021, sourced from the Toa Baja Levittown, PR Meteorological Station (National Weather Service, 2023). Note:  Total rainy days.
Total rainy days.
The driest month was May, with monthly mean precipitation of 0.51 millimeters and 6 rainy days. In 2021, the wettest month was September, with monthly mean precipitation of 8.63 millimeters and 18 rainy days. The driest month was May, with monthly mean precipitation of 1.78 millimeters and 13 rainy days. Ciénaga Las Cucharillas Natural Reserve is representative of how coastal wetlands in the Tropics, especially in the Caribbean, have been hydrologically modified from colonial times to the present. The hydrological modifications include: (a) drainage channels for agricultural use from the 17th century until the mid-20th century [38,39]; (b) the construction of a flood control channel (La Malaria channel) in the late 1940s, bringing a direct flow of fresh water to the wetland from the upper and middle parts of the basin; and (c) restricted seawater exchange due to the dike effect of an outflow water pump structure at the mouth of the channel (Figure 3) [38], adapted. As a result, tidal interaction in this wetland occurs via deep subsurface flow [39]. Historical and present hydrological modifications bring about a mosaic of physicochemical conditions, habitats, and vegetation cover.

Figure 3: La Malaria flood control channel (represented by  ) positioned northwest of the delineated research area at (highlighted by
) positioned northwest of the delineated research area at (highlighted by  ) Cienaga LasCucharillas Natural Reserve (outlined with a
) Cienaga LasCucharillas Natural Reserve (outlined with a  ). The location of the outflow water pump structure is indicated by a yellow square at the channel’s downstream point of discharge.
). The location of the outflow water pump structure is indicated by a yellow square at the channel’s downstream point of discharge.
Data collection
Four study plots (3, 5, 6, and 10), each encompassing 100 square meters, were established in a research area within La Ciénaga las Cucharillas Natural Reserve (Figure 4).
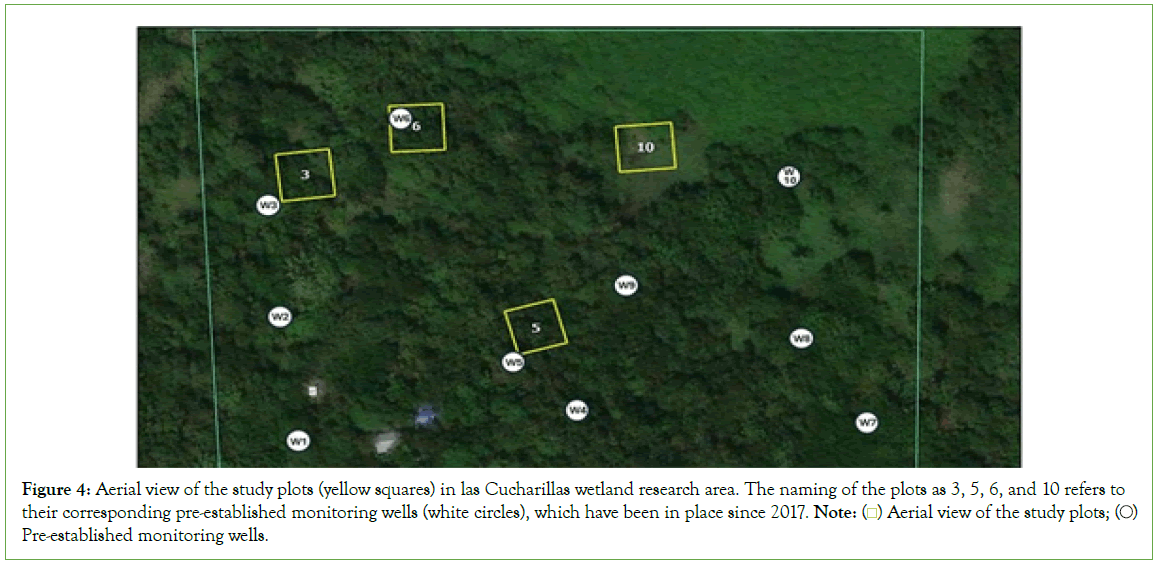
Figure 4: Aerial view of the study plots (yellow squares) in las Cucharillas wetland research area. The naming of the plots as 3, 5, 6, and 10 refers to their corresponding pre-established monitoring wells (white circles), which have been in place since 2017. Note:  Pre-established monitoring wells.
Pre-established monitoring wells.
The naming of the plots as 3, 5, 6, and 10 refers to their corresponding pre-established monitoring wells (Figure 4), which have been in place since 2017. This approach was chosen to maintain consistency with the long-term monitoring data available from these wells.
Five plant species were chosen based on their functional type and occurrence within the study plots [36-39]. (Hernández, 2022; Pérez-Harguindeguy, 2013; Box, 1981; Native Plant Trust, 2024): Dalbergia ecastaphyllum (L.) Taub (shrub), Echinochloa polystachya (Kunth) Hitchc. (grass), Poaceae family (grass), Acrostichum danaeifolium Langsd. and Fisch. (fern) and Laguncularia racemosa C.F.Gaertn (tree). D. ecastaphyllum was present in two plots, E. polystachya in one plot, while the remaining species were found in three plots each (Table 2). The chemical composition and structure of plant residues vary among different types, influencing their decomposition dynamics and nutrient cycling processes (Table 2) [40-48].
| Functional type | Definition | Taxa | General description | Chemical composition of litter |
|---|---|---|---|---|
| Fern | Small plants with prostrate, underground stems and large, erect, compound leaves called fronds. | A.danaeifolium | Non-flowering, vascular plants with large and robust fronds, from the ferns family (Pteridaceae) common in brackish swamps. | High concentrations of fiber, lignin, and tannins, further contributing to their slow decomposition. |
| Shrub | Woody plant ~5 m tall. with multiple stems arising at or near the base. | D. ecastaphyllum | Decumbent shrub. Grows in non-forested areas generally forming monospecific stands. | Low lignin, high in organic carbon (C) and nitrogen (N) largely because of the plant's ability to fix atmospheric nitrogen. |
| Grass | Narrow, flat, linear-leaved herb, growing from well- developed underground rootstocks. | Poaceae family | Annual, biennial, or perennial plants can be terrestrial or aquatic, with leaves that are evergreen or deciduous, usually much longer than they are wide. They typically grow, forming tufts or mats. | Grasses are characterized by high silica content and a relative scarcity of phosphorus (P), which enhances their structural rigidity and resistance to decomposition. |
| E. polystachya | Perennial grass with decumbent erects stems that could reach 2 m height. It is commonly found in flooding areas. | |||
| Tree | Woody plant usually >5 m tall, with a trunk supporting branches and leaves forming a characteristic crown. | L. racemosa | A representative species of mangrove trees; characterized by a solitary or clustered trunk, gray bark. Elliptic to oblong-shaped leaves. | High carbon-to-nitrogen (C/N) and lignin-tonitrogen (lignin/N) |
Table 2: General description of dominant plant functional types within the study plots (adapted from Hernández, 2021; Pérez-Harguindeguy, 2013; Box, 1981; Native Plant Trust, 2024).
Within each plot, three specimens of each functional type were chosen and three litter samples per plant were collected every sampling date. Each sample, measuring 7.62 cm in diameter and 5 cm in depth, was divided into two fractions: 1) loose litter, which is relatively undecomposed, and 2) a combination of old litter, ranging from partly to fully decomposed, and organic soil. Substrate samples were collected on five dates, each chosen to represent distinct hydroperiod conditions (Figure 5). Moderate Dry (June 18-25, 2020), Flood (October 23, 2020), Moist/Between Floods (March 19, 2021), and Wet (June 9, 2021). The hydroperiod classification in this study was primarily based on the phreatic level measurements recorded at sampling time. This approach was adopted due to the direct influence of phreatic level on soil microenvironment (reflecting soil antecedent patterns of drying/wetting cycles) [18,19,21]. Local precipitation data and the average tidal range for the 14 days prior to sampling date were also considered due to their impact on the wetland’s dry and wet cycles, as well as site’s phreatic level at the sampling time. For instance, during prolonged dry periods, bimodal high tide reaches the study site in 20 minutes, but this process can extend up to 2 hours during wet periods [36].

Figure 5: A) Schematic diagram of the wetland hydrodynamics showing phreatic level (m) on the date of sampling; B) Overview of local total rainy days, precipitation (cm), mean tidal daily range, and mean phreatic level (m) for the 14-day period leading up to and including the sampling date (National Weather Service, 2023). Note:  Ground level (0 m).
Ground level (0 m).
Hydroperiod conditions on sampling day were categorized as “moderate dry” and “moist” at mean phreatic levels of -0.56 m and -0.38 m ground level, respectively. “Wet” and “flood” conditions were identified at mean phreatic levels of -0.12 m and at or above the ground level (0 m). It is noteworthy that the moist sampling period, which took place on March 19, 2021, occurred between flooding events. Specifically, the site experienced flooding both a week before and after the sampling date, although the week of the sampling itself was dry. The sampling for this period was conducted immediately following the first flood event. The wet sampling period was characterized by cycles of prolonged drying followed by shorter periods of flooding, leading up to the sample collection (Figure 6). Additionally, the flood sampling date, which was October 23, 2020, coincided with the receding of floodwaters. Prior to this date, the wetland had been subjected to significant atmospheric events, including tropical storms Isaias and Laura, followed by a prolonged rainy period that lasted until the end of October 2020. This resulted in approximately three months of flooding at the site, spanning from August 2020 to October 2020 (National Weather Service, 2020).
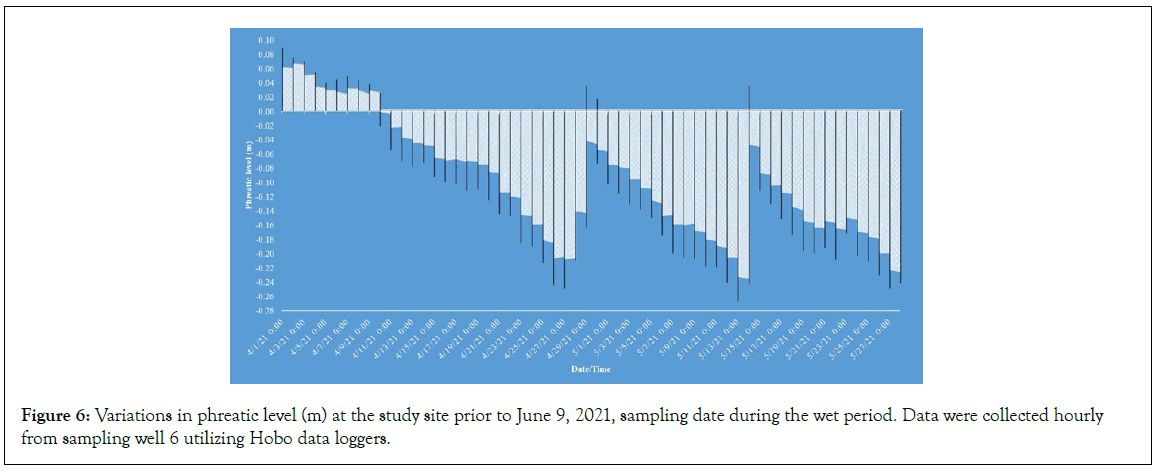
Figure 6: Variations in phreatic level (m) at the study site prior to June 9, 2021, sampling date during the wet period. Data were collected hourly from sampling well 6 utilizing Hobo data loggers.
Sampling was conducted from 7:00 am to 10:00 am to ensure uniform environmental conditions across plots, specifically regarding soil temperature, water content, and tidal influence. This timing aligns with the transition from high to low tide (Table 3).
| Sampling date | Sampling time* | Tide (m) | Tide description |
|---|---|---|---|
| June 18, 2020 | 7:00 | 0.22 | High |
| 10:00 | 0.05 | Low | |
| June 25, 2020 | 7:00 | 0.48 | High |
| 10:00 | 0.23 | Low | |
| October 23, 2020 | 7:00 | 0.31 | High |
| 10:00 | 0.14 | Low | |
| March 19, 2021 | 7:00 | 0.29 | High |
| 10:00 | 0.15 | Low | |
| June 9, 2021 | 07:00 | 0.22 | High |
| 10:00 | 0.10 | Low |
Note: *Sampling occurred in the morning, between 7:00 am to 10:00 am.
Table 3: Tide conditions at the time of sampling, detailing the tidal phase (low or high) corresponding to the sampling events. Data was obtained from the National Weather Service (2021).
The collected samples were transported to the laboratory, where their fresh weight was recorded before being placed, in lighted Tullgren-Berlese extractors for one week. The extracted arthropods were preserved in 70% ethanol solution placed under each extractor [14,33]. Collected soil arthropods were taxonomically identified tothe lowest category possible, either class, subclass, order or suborder, and family, and classified as adults or immatures. Collembola were not separated as adults or immatures because it is difficult to differentiate among developments stages [14,33,40]. Soil arthropods were also categorized within the soil food web framework based on the predominant feeding habit of the group (Table 4) [14,41-44].
| Trophic guild | Basal resource | Description | Ecological contribution |
|---|---|---|---|
| Detritivores (animal primary decomposers) | Detritus | They feed directly on organic matter | Litter transformation (comminution), decomposition, nutrient mobilization, soil formation, structural stabilization |
| Herbivores (phytophages) | Plant material | Living vascular plants shoots, sap, and roots | Nutrient cycling, soil aggregation, respiration |
| Microbivore (secondary descomposers) | Microflora | Fungi and organic matter, eating also the bacteria growing on it. | Biological control, nutrient cycling |
| Fungivores (mycophages) | Fungi | Fungi and lichen associated fungi | Biological control, nutrient cycling |
| Omnivores | Organic matter, plants, small arthropods | Plant material, fungi, detritus, and smaller soil organisms. | Organic matter redistribution, microbe dispersal, nutrient cycling, soil aggregation |
| Predators | Soil organisms | Smaller arthropods, nematodes, and various soil invertebrates | Nutrient cycling, soil aggregation, biological control |
Table 4: Basal resources and corresponding trophic guilds within the soil food web framework [9,14,43].
For each sample, organisms were identified and counted using an Amscope SF2TRA stereoscopic binocular microscope or a Nikon Eclipse 80i microscope. After extraction, the samples were oven- dried at 60°C for a period of seven days, and the dry weight of the sample fractions was determined. Loose litter samples were homogenized to a 5 µm size using a Retsch® grinding mill, while old litter and organic soil were ground using a mortar and pestle until they passed through a 20-mesh sieve.
A 5.00 mg subsample from each sample was taken for carbon-to- nitrogen (C:N) ratio, carbon (C) percentage, and nitrogen (N) percentage analysis using a Vario EL Cube organic elemental analyzer.
Data Analysis
Non-parametric statistical approaches, including the Wilcoxon/ Kruskal-Wallis test followed by the post-hoc Steel-Dwass test, were employed to detect variations in litter mass (g), as well as C%, N%, and the Carbon-to-Nitrogen (C:N) ratio in substrate fractions among plant functional types and hydroperiods. To further explore these relationships and quantify the effects of these variables on soil arthropods diversity and abundance, a General Linear Model (GLM) with a quasi-Poisson distribution was applied. The analysis was further refined by categorizing the C:N ratio into specific ranges, defined by mineralization and immobilization rates of organic material [45,46]. This categorization provided a systematic framework to assess the impact of the C:N ratio on soil arthropod communities (Table 5).
| C:N Ratio | Process | Description |
|---|---|---|
| Below 20:1 | Mineralization | Mineralization is occurring, indicating a relatively high availability of nitrogen for microbes. |
| 20:1 to 30:1 | Balance between mineralization and immobilization | Optimal range for microbial activity, indicating a balance between mineralization and immobilization. |
| Above 30:1 | Immobilization | Immobilization is likely to occur as microbes require nitrogen for their growth, leading to the sequestration of nitrogen in microbial biomass. |
Table 5: C:N ratio categories, defined by mineralization and immobilization rates of organic material [45,46].
Soil arthropod metrics, including Menhinick’s Index for diversity, alongwithrichness, abundance, and density (density beingquantified as the number of individuals per gram of soil), were determined [14,47]. A two-way non-parametric analysis was implemented to examine the influence of plant functional types and hydroperiods on these metrics. Contingency tables were employed to investigate the relationships between soil arthropod taxa and the combined effects of plant functional types and hydroperiods. Taxonomic classifications were designated as “dominant,” “common,” or “rare” based on their relative densities, facilitating comparisons of community compositions via the Bray-Curtis similarity index and Non-metric Multidimensional Scaling (NMDS). Dominant taxa were identified as those with a relative density of 10% or greater. Common taxa were those with a relative density between 1% and 10%, while rare taxa were defined by a relative density of less than 1% [49,50]. A PERMANOVA (Permutational Multivariate Analysis of Variance) and Multi-Response Permutation Procedure (MRPP) were conducted to elucidate the combined effects of vegetation types and hydroperiods on the community composition and structure of soil arthropods. These statistical analyses were performed using SAS JMP® Pro 16 and RStudio (R Core Team, 2023) statistical software. Statistical analyses in RStudio were completed using the following packages: ‘stats’, ‘vegan’, ‘gplots’, and ‘pheatmap’.
Results
Substrate quality and quantity across plant functional type and hydroperiod
Significant variations were identified in the C% and N%, as well as in the C:N ratios, through two comprehensive analyses. The first analysis investigated the influence of varying hydroperiods on these parameters within each distinct plant functional type (Figure 7). In contrast, the second analysis compared these metrics across plant functional types under differing hydroperiods (Table 6, Figure 8). These significant differences were observed in both loose litter and old litter-organic soil fractions. Results from the first analysis (Figure 7), centered on loose litter fractions, indicated that variations in N% content between flood and moderate dry periods were not statistically significant. However, when considered collectively, these conditions displayed significant contrast from those observed during moist and wet periods, which presented lower values. Under flood conditions, the loose litter fractions from grasses, shrubs, and ferns exhibit significantly elevated nitrogen levels, whereas shrubs and trees demonstrate a markedly higher carbon percent content across both substrate fractions, with all values statistically distinct from those observed in other hydroperiods. C:N ratios within the old litter-organic soil fractions are significantly lower than those observed in the loose litter fractions, a trend consistent across all vegetation types and hydroperiods. When comparing these metrics across plant functional types under differing hydroperiods (Table 6, Figure 8), it was found that within both substrate fractions, shrubs consistently exhibited the highest C% and N% in all conditions. In contrast, trees were characterized by having the highest carbon- to-nitrogen (C:N) ratios. These distinctions, which were statistically significant, differentiate shrubs and trees from other plant functional types and are related to the chemical composition of the residues from these specific types (Tables 2 and 7, Figure 9). During the moist and flood periods, there was a redistribution of both loose and decomposed litter. As a result, the loose litter collected under ferns and grasses was an amalgamation of fresh and aged organic materials originating from disparate plant sources (Figure 10). Conversely, the loose litter collected under shrub and tree types was homogeneous, consisting of materials from the same vegetation type, without a mixture of different plant sources. During the moist period, the composition under fern vegetation was 88% from trees, grasses, and shrubs, and 12% from ferns; under grass, the composition was 79% from trees and shrubs, and 21% from grasses. In the flood period, the litter composition under fern vegetation constituted 62% from trees, grasses, and shrubs, and 38% from ferns; while under grass, was 91% from trees, grasses, and shrubs, and 9% from grasses. Given the diverse quality of these residues, attributable to their distinct plant types (Table 2), their presence contributes to a habitat with heterogeneous resources beneath both fern and grass vegetation.
| Flood | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate quality parameters | Loose litter | Old litter-Organic Soil | ||||||||||||||
| Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | |||||||||
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| C:N ratio | 18.60 | 3.75 | 18.12 | 3.75 | 18.48 | 2.01 | 24.04 | 8.09 | 15.38 | 1.32 | 15.08 | 1.08 | 14.43 | 0.41 | 16.41 | 2.38 |
| N% (mg/g) | 2.44 | 0.57 | 2.49 | 0.48 | 2.78 | 0.2 | 2 | 0.80 | 2.71 | 0.44 | 2.87 | 0.36 | 3.26 | 0.03 | 2.54 | 0.39 |
| C%(mg/g) | 45.45 | 2.2 | 45.12 | 4.11 | 51.31 | 1.49 | 48.18 | 1.87 | 41.68 | 4.73 | 43.3 | 5.39 | 47.00 | 1.23 | 41.76 | 3.41 |
| Moderate dry | ||||||||||||||||
| Subtrate Quality Parameters | Loose litter | Old litter-Organic Soil | ||||||||||||||
| Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | |||||||||
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| C:N ratio | 17.98 | 7.45 | 19.57 | 5.70 | 20.44 | 2.04 | 27.82 | 6.92 | 15.92 | 1.95 | 15.71 | 0.95 | 14.91 | 0.14 | 17.24 | 7.48 |
| N% (mg/g) | 2.34 | 0.73 | 2.05 | 0.51 | 2.42 | 0.21 | 1.58 | 0.30 | 2.06 | 0.58 | 2.34 | 0.36 | 2.87 | 0.10 | 2.15 | 0.50 |
| C%(mg/g) | 42.03 | 3.16 | 40.20 | 5.96 | 49.41 | 0.61 | 43.87 | 3.17 | 32.77 | 8.04 | 36.80 | 5.16 | 42.85 | 1.76 | 36.99 | 5.20 |
| Moist | ||||||||||||||||
| Subtrate Quality Parameters | Loose litter | Old litter-Organic Soil | ||||||||||||||
| Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | |||||||||
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| C:N ratio | 25.67 | 8.10 | 19.73 | 4.99 | 18.82 | 2.39 | 23.10 | 9.31 | 14.76 | 1.00 | 14.97 | 1.14 | 15.72 | 2.68 | 16.23 | 1.93 |
| N% (mg/g) | 1.86 | 0.49 | 2.13 | 0.34 | 2.61 | 0.29 | 2.02 | 0.71 | 2.65 | 0.32 | 2.66 | 0.34 | 2.77 | 0.36 | 2.39 | 0.51 |
| C%(mg/g) | 47.79 | 3.49 | 42.02 | 3.39 | 49.17 | 4.14 | 46.59 | 3.11 | 39.11 | 3.43 | 39.78 | 4.61 | 43.62 | 2.24 | 38.71 | 5.33 |
| Wet | ||||||||||||||||
| Subtrate Quality Parameters | Loose litter | Old litter-Organic Soil | ||||||||||||||
| Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | |||||||||
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| C:N ratio | 23.15 | 12.47 | 23.18 | 6.01 | 22.77 | 7.08 | 31.31 | 10.61 | 16.48 | 1.86 | 14.42 | 1.16 | 15.21 | 0.37 | 16.80 | 1.75 |
| N% (mg/g) | 1.91 | 0.61 | 1.88 | 0.41 | 2.21 | 0.62 | 1.48 | 0.69 | 2.50 | 0.45 | 2.75 | 0.75 | 2.98 | 0.42 | 2.53 | 0.37 |
| C%(mg/g) | 44.16 | 1.72 | 43.57 | 1.88 | 50.28 | 2.86 | 46.48 | 2.10 | 41.26 | 6.28 | 39.64 | 9.39 | 45.41 | 7.12 | 42.48 | 3.82 |
Table 6: Mean and standard deviation of Carbon (C) and Nitrogen (N) percentage contents, as well as the C:N ratio, for different plant functional types across hydroperiods within loose litter and old litter-organic soil fractions.
| Litter mass (g) | Flood | Moderate Dry | ||||||||||||||
| Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | |||||||||
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| 0.07 | 0.07 | 0.06 | 0.04 | 0.07 | 0.02 | 0.10 | 0.06 | 0.09 | 0.06 | 0.10 | 0.04 | 0.2 | 0.05 | 0.19 | 0.11 | |
| Litter mass (g) | Moist | Wet | ||||||||||||||
| Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | |||||||||
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | |
| 0.04 | 0.04 | 0.05 | 0.03 | 0.08 | 0.04 | 0.05 | 0.05 | 0.05 | 0.03 | 0.03 | 0.01 | 0.09 | 0.03 | 0.11 | 0.09 | |
Table 7: Mean and standard deviation of litter mass (g), for different plant functional types across hydroperiods within loose litter and old litter-organic soil fractions.
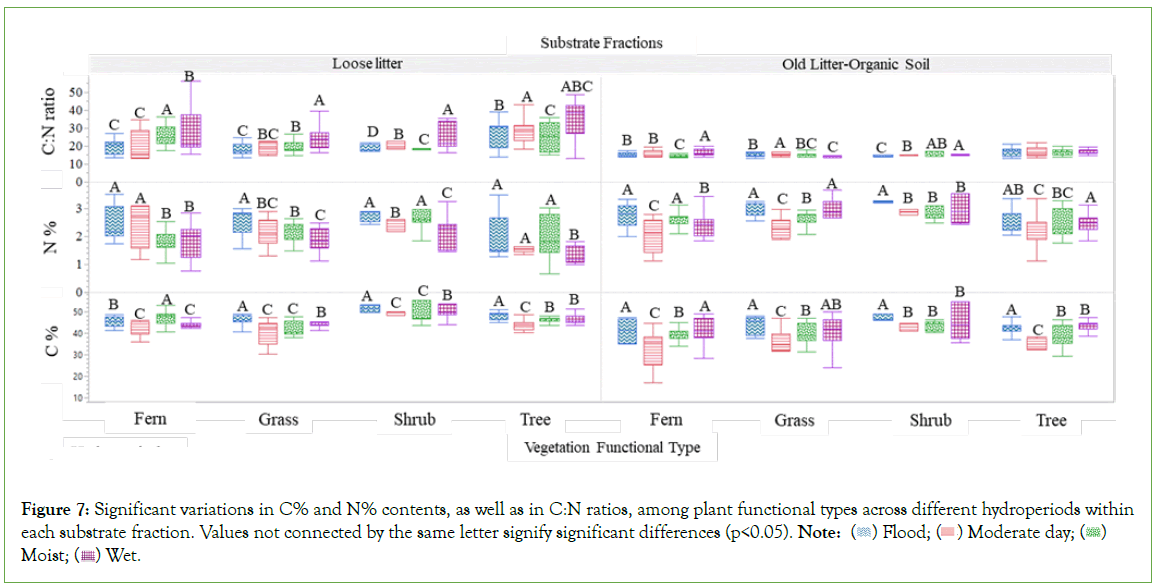
Figure 7: Significant variations in C% and N% contents, as well as in C:N ratios, among plant functional types across different hydroperiods within each substrate fraction. Values not connected by the same letter signify significant differences (p<0.05). Note:  Moist;
Moist;  Wet.
Wet.

Figure 8: Significant variations in C% and N%, as well as in C:N ratios, across different hydroperiods within each plant functional type, segmented by substrate fractions. Values not connected by the same letter indicate significant differences (p<0.05). Note:  Tree.
Tree.
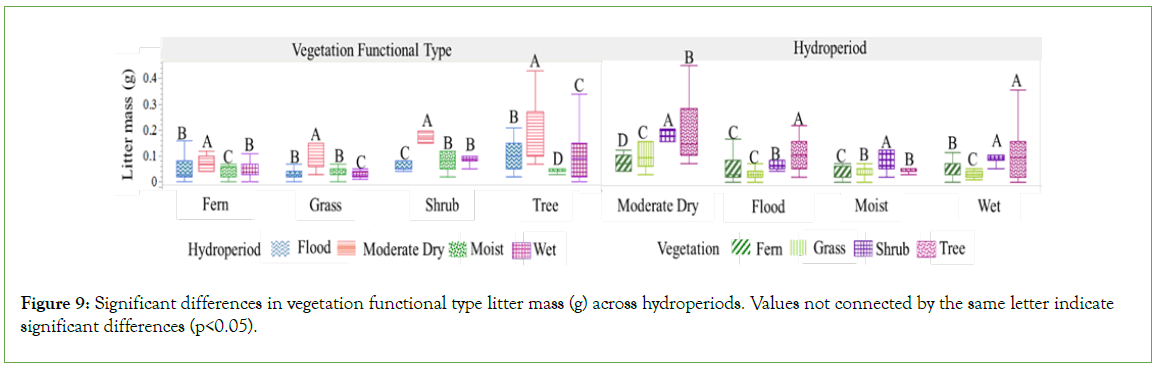
Figure 9: Significant differences in vegetation functional type litter mass (g) across hydroperiods. Values not connected by the same letter indicate significant differences (p<0.05).
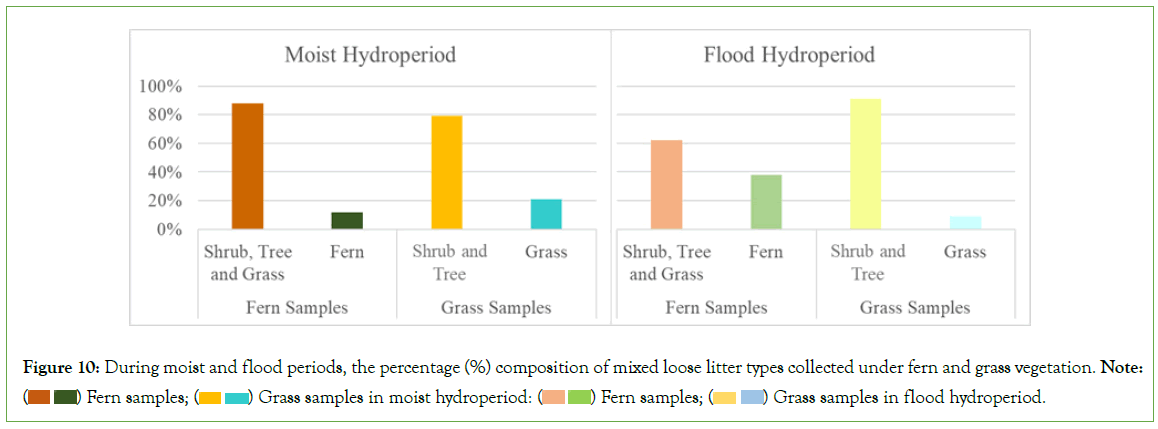
Figure 10: During moist and flood periods, the percentage (%) composition of mixed loose litter types collected under fern and grass vegetation. Note:  Grass samples in flood hydroperiod.
Grass samples in flood hydroperiod.
Soil arthropod community responses
Influence of loose litter mass, carbon and nitrogen content, and C:N ratio on soil arthropods communities: The application of Generalized Linear Models (GLM) has demonstrated statistically significant correlations (p-values<0.01) between the percentage of carbon (%C), nitrogen (%N), and loose litter mass with species richness and abundance (Table 8). The analysis revealed that while, %C has relatively smaller coefficients of 0.01 for richness. High tvalues of 13.70 for richness and 4.29 for abundance. Litter mass showed more substantial coefficients of 0.20 for richness and 0.93 for abundance, with corresponding t-values of 2.61 and 2.26, respectively. These results suggest that litter mass has a more pronounced quantitative impact on both richness and abundance. In contrast, nitrogen (%N) exhibited a complex influence on the ecological metrics. It negatively affected species richness, as indicated by a coefficient of -0.06 and a t-value of -7.80, suggesting a suppressive effect on diversity. However, nitrogen (%N) also showed a positive influence on species abundance, with a coefficient of 0.09 and a t-value of 1.97. While this t-value indicates statistical significance, it is comparatively lower than those observed for other variables, suggesting a more subtle effect.
| Quality Factors | Richness | Abundance | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate coefficient | Estimate Error | t value | p-value | Estimate coefficient | Estimate Error | t value | p-value | |
| %C | 0.01 | 0.001 | 13.70 | <0.01 | 0.02 | 0.005 | 4.29 | <0.01 |
| %N | -0.06 | 0.007 | -7.80 | <0.01 | 0.09 | 0.045 | 1.97 | <0.01 |
| Litter mass | 0.20 | 0.067 | 2.61 | <0.01 | 0.93 | 0.412 | 2.26 | <0.01 |
Table 8: Results from a Generalized Linear Model (GLM) analysis, utilizing a quasi-Poisson distribution to evaluate the influence of loose litter mass, carbon and nitrogen content, and C:N ratio on the richness and abundance of soil arthropods.
The impact of the C:N ratio on soil arthropod communities indicated that all trophic guild densities (ind/g) were significantly elevated in conditions where the C:N ratio ranged between 20:1 and 30:1, denoted as the equilibrium phase of the mineralizationimmobilization continuum, and during the immobilization phase, characterized by a C:N ratio greater than 30:1 (Figure 11). No statistical differences were observed between these two phases. However, both phases were statistically distinct from the mineralization phase, identified by a C:N ratio less than 20:1. At the mineralization phase, herbivores exhibited statistical differences compared to detritivores and microbivores, whereas microbivores differed significantly from predators (Figure 12).

Figure 11: Statistical differences in trophic guild densities (individuals per gram) among various decomposition phases, as delineated by C:N ratio ranges. Values not connected by the same letter indicate significant differences (p<0.05). Note: Decomposition phase Equilibrium mineralization-C:N ratio between 20:1 to 30:1;
Equilibrium mineralization-C:N ratio between 20:1 to 30:1;  Mineralization-C:N ratio <20:1.
Mineralization-C:N ratio <20:1.
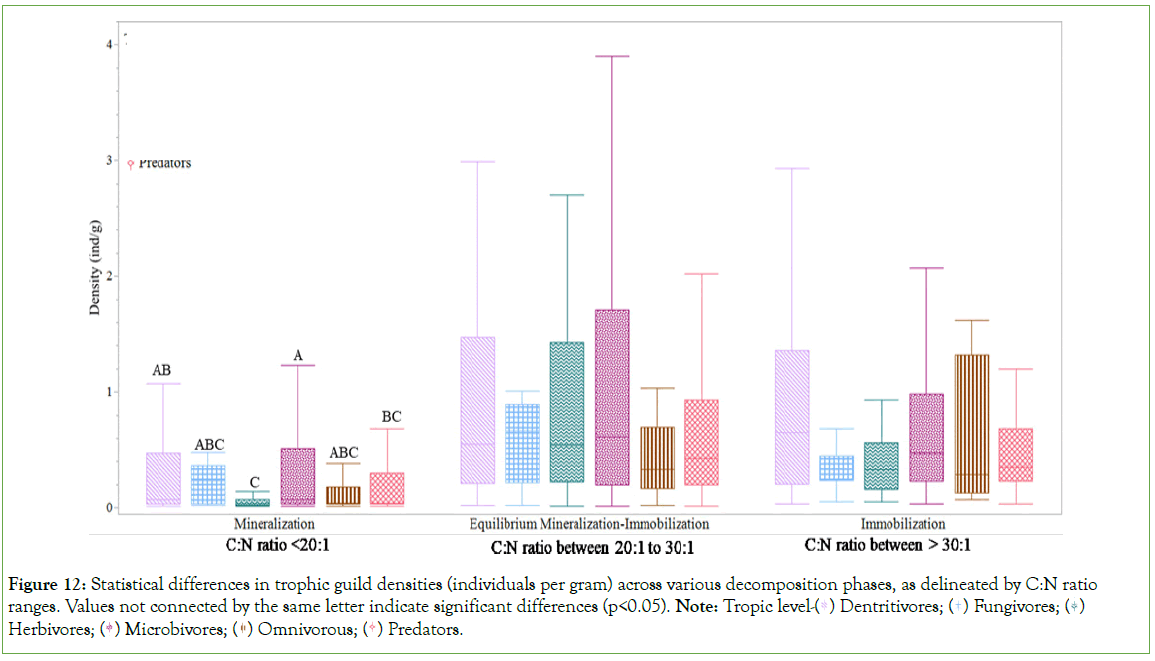
Figure 12: Statistical differences in trophic guild densities (individuals per gram) across various decomposition phases, as delineated by C:N ratio ranges. Values not connected by the same letter indicate significant differences (p<0.05). Note: Tropic level  Herbivores;
Herbivores;  Predators.
Predators.
Hydroperiod influences on soil arthropod diversity across vegetation types
The two-way non-parametric analysis demonstrated significant variations in the diversity matrices among plant functional types across different hydroperiods for both mesofauna and macrofauna, as delineated in Figure 13. Macrofauna densities were significantly elevated within the fern, grass, and tree vegetation types during flood and wet hydroperiods. Regarding mesofauna, significantly higher densities were quantified in association with grass and shrub types during the flood, wet, and moist hydroperiods, while the peak density within tree type was recorded during the flood period. Richness was significantly higher for fern, shrub, and tree functional types during the wet period, in contrast to grass, where higher richness was observed during the moderate dry period. The Menhinick’s Index revealed an enhanced diversity during moist hydroperiods for fern, shrub, and tree types, and during the moderate dry period for grass types. These patterns of richness and diversity were consistent across both mesofauna and macrofauna groups.

Figure 13: Statistical differences of macro and mesofauna density (ind/g), richness and Menhinick’s Index within vegetation functional types among hydroperiods. Values not connected by the same letter indicates significant differences (p<.05). Note: 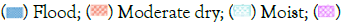 Wet.
Wet.
Significant variations in macro and mesofauna density (individuals per gram), richness, and diversity were observed across different vegetation functional types under varying hydroperiods, as detailed in Figure 14. During the moist and wet hydroperiods, macrofauna density associated with shrub vegetation was found to differ significantly from that observed within tree vegetation. Significant disparities in mesofauna density were observed between shrub and grass vegetation types compared to tree vegetation during the moderate dry period, whereas in wet periods, the density within tree vegetation was markedly different from that in other types. In wet periods, richness levels of macrofauna and mesofauna associated with tree vegetation were found to significantly exceed those observed in other vegetation types. In contrast, during the moderate dry period, the highest richness was observed in grass vegetation. Furthermore, shrub vegetation not only exhibited higher richness compared to other types during the moist and flood period but also recorded the highest values of the Menhinick’s Index.
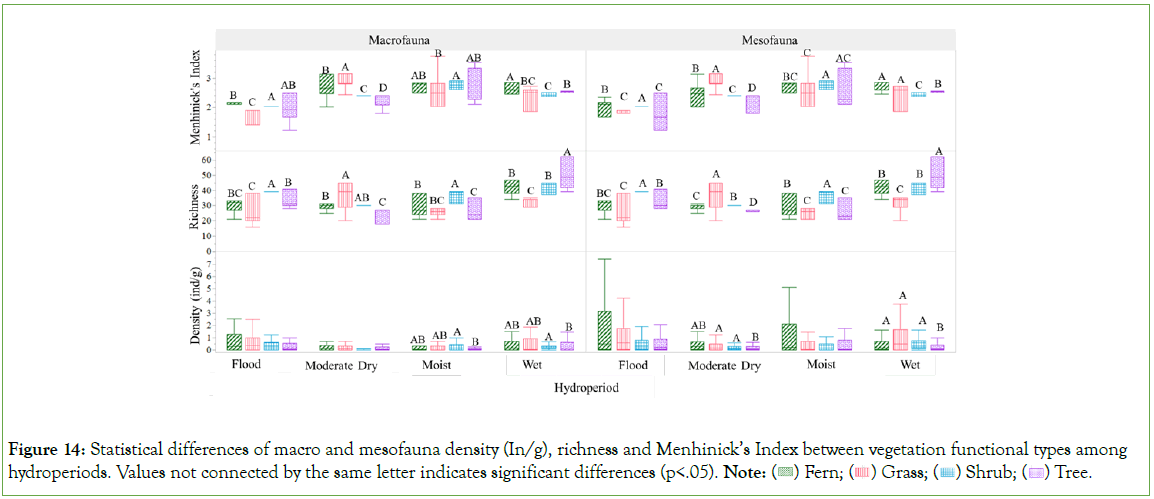
Figure 14: Statistical differences of macro and mesofauna density (In/g), richness and Menhinick’s Index between vegetation functional types among hydroperiods. Values not connected by the same letter indicates significant differences (p<.05). Note:  Tree.
Tree.
Relationships between soil arthropod taxa and the combined effects of plant functional types and hydroperiods
A total of 9,881 soil arthropods, encompassing 93 families across 20 taxonomic groups (Orders and Suborders), were identified (Figure 15). Of these, 43 were classified within the mesofauna1 group, while 50 were attributed to macrofauna.
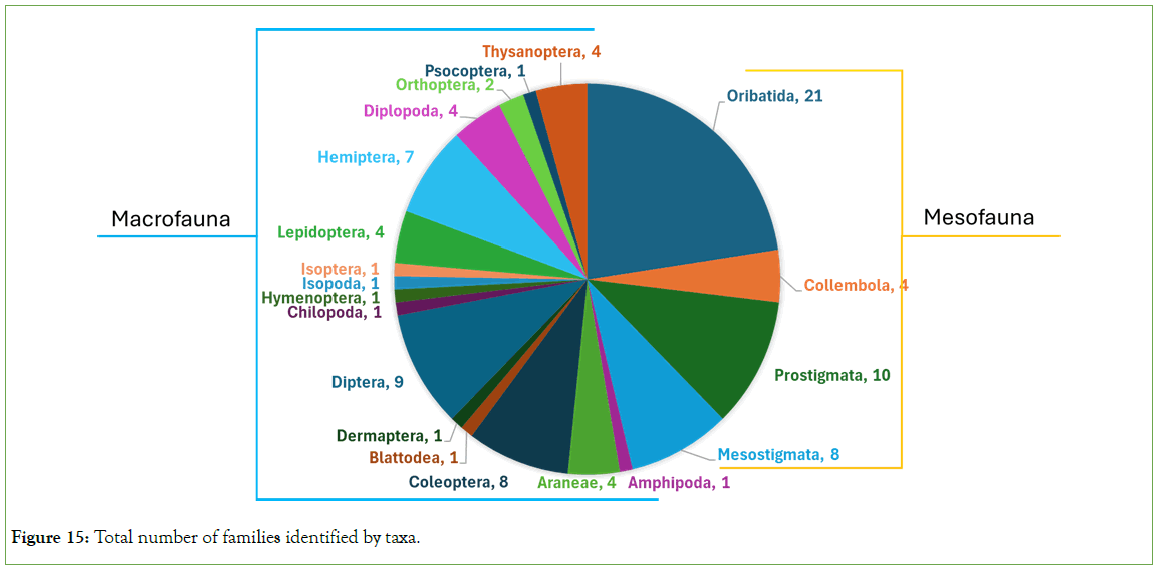
Figure 15: Total number of families identified by taxa.
Biodiversity variations, classified into dominant, common, and rare taxa, were quantified across various vegetation functional types subject to differing hydroperiods (Table 9). Among all hydroperiods, common and rare taxa relative density exceeded the dominant groups across all functional types. Notably, the moist period exhibited the greatest relative density of common taxa among all vegetation types, with observed percentages of 66.7% for ferns, 60.0% for grasses and shrubs, and 68.4% for trees. The highest relative densities for rare taxa groups were quantified under wet conditions, while the flood period was for dominant taxa, with rare taxa presenting values of 36.84% for ferns, 42.86%for grasses, 33.33%for shrubs, and 42.11%for trees, and dominant groups exhibited densities of 23.08% for ferns, 15.38% for grasses, 27.27% for shrubs, and 18.18% for trees.
| Flood | Moderate dry | Moist | Wet | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree | Fern | Grass | Shrub | Tree |
| Amphipoda | 0.3% | 1.2% | 1.8% | 1.3% | 0.1% | 0.3% | ||||||||||
| Araneae | 0.5% | 0.4% | 0.3% | 0.30% | 1.1% | 1.5% | 0.7% | 0.8% | 1.7% | 2.1% | 1.0% | 2.1% | 1.8% | 0.6% | 2.5% | 0.6% |
| Arthropleona | 14.5% | 20.0% | 14.3% | 30.4% | 3.2% | 2.6% | 14.0% | 3.3% | 9.7% | 39.1% | 6.4% | 7.9% | 25.8% | 40.2% | 15.6% | 22.1% |
| Blattodea | 0.2% | 40.0% | 0.3% | 0.2% | 0.3% | 0.6% | 0.1% | |||||||||
| Coleoptera | 6.3% | 4.2% | 3.6% | 8.7% | 1.5% | 4.6% | 4.3% | 3.1% | 5.6% | 1.9% | 5.9% | 5.0% | 4.9% | 3.9% | 4.7% | |
| Dermaptera | 0.1% | 0.2% | 0.4% | 0.7% | 0.5% | 0.6% | 0.3% | 0.4% | ||||||||
| Diptera | 10.6% | 4.3% | 4.8% | 6.4% | 4.2% | 4.0% | 1.3% | 4.3% | 3.5% | 2.1% | 2.2% | 3.2% | 8.9% | 3.1% | 3.2% | 7.1% |
| Geophilomorpha | 0.2% | 0.7% | 0.3% | 0.7% | 0.3% | 0.3% | 0.1% | |||||||||
| Hemiptera | 0.7% | 1.3% | 0.4% | 1.7% | 1.6% | 1.3% | 2.0% | 9.9% | 3.5% | 1.9% | 5.0% | 2.5% | 4.0% | 0.5% | 1.8% | |
| Hymenoptera | 0.7% | 2.2% | 13.4% | 1.1% | 12.9% | 5.5% | 1.3% | 1.0% | 2.6% | 3.2% | 2.2% | 4.4% | 1.2% | 0.6% | 0.7% | 0.6% |
| Isopoda | 3.4% | 0.2% | 3.3% | 1.5% | 4.5% | 0.3% | 13.8% | 7.4% | 4.2% | 0.6% | 5.4% | 1.8% | ||||
| Isopter | 0.2% | 1.9% | 0.3% | |||||||||||||
| Lepidoptera | 1.0% | 1.2% | 0.6% | 1.2% | 0.6% | 0.3% | 0.2% | 1.1% | ||||||||
| Mesostigmata | 2.0% | 3.6% | 8.7% | 6.30% | 13.1% | 7.9% | 12.0% | 5.8% | 5.7% | 2.7% | 6.1% | 1.5% | 7.3% | 5.9% | 10.9% | 7.7% |
| Oribatida | 59.0% | 56.6% | 46.9% | 43.4% | 51.7% | 64.7% | 61.3% | 55.4% | 48.3% | 32.7% | 59.3% | 50.3% | 30.7% | 31.6% | 41.3% | 39.3% |
| Orthoptera | 0.2% | |||||||||||||||
| Polyzoniida | 1.1% | 0.7% | 0.3% | 0.1% | 0.2% | |||||||||||
| Prostigmata | 4.3% | 5.3% | 4.2% | 1.80% | 2.1% | 3.1% | 2.7% | 6.8% | 4.2% | 4.3% | 2.2% | 4.1% | 6.2% | 2.8% | 7.5% | 7.8% |
| Psocoptera | 0.1% | 3.4% | 0.9% | 12.5% | 0.5% | 0.3% | 0.9% | 0.4% | 3.0% | 0.6% | ||||||
| Spirobolida | 0.3% | 0.4% | 0.9% | 0.20% | 0.2% | 0.7% | 0.5% | 0.2% | 1.3% | 1.8% | 0.4% | 0.3% | 0.2% | 0.2% | ||
| Symphypleona | 0.8% | 1.3% | 2.7% | 1.00% | 0.4% | 0.3% | 0.5% | 2.2% | 4.9% | 3.5% | 2.9% | |||||
| Thysanoptera | 0.1% | 0.3% | 0.8% | 0.4% | 0.3% | 2.4% | 1.0% | 1.2% | 0.8% | 1.8% | 1.0% | |||||
Dominant  |
23.08% | 15.38% | 27.27% | 18.18% | 18.75% | 5.56% | 25.00% | 11.76% | 5.56% | 13.33% | 13.33% | 5.26% | 10.53% | 14.29% | 20.00% | 10.53% |
Rare  |
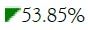 |
30.77% | 27.27% | 36.36% | 31.25% | 44.44% | 33.33% | 35.29% | 27.78% | 26.67% | 26.67% | 26.32% | 36.84% | 42.86% | 33.33% | 42.11% |
Common  |
23.08% | 53.85% | 45.45% | 45.45% | 50.00% | 50.00% | 41.67% | 52.94% | 66.67% | 60.00% | 60.00% | 68.42% | 52.63% | 28.57% | 46.67% | 47.37% |
| Richness | 13 | 13 | 11 | 11 | 16 | 18 | 12 | 17 | 18 | 15 | 15 | 19 | 19 | 14 | 15 | 19 |
| Abundance | 761 | 694 | 3.35 | 1309 | 526 | 547 | 150 | 399 | 424 | 373 | 312 | 340 | 926 | 674 | 571 | 1540 |
Table 9: Biodiversity variations, classified into dominant, common, and rare taxa, across various vegetation functional types subject to differing hydroperiods.
In the moist period, notable common taxa included Arthropleona and Hemiptera for both fern and tree vegetation types, Coleoptera and Prostigmata for grasses, and Arthropleona and Mesostigmata for shrubs. During wet conditions distinct rare taxa include Lepidoptera and Thysanopterafor ferns; Araneae, Hymenoptera and Isopoda for grasses; Hymenoptera and Hemiptera for shrubs, alongside Araneae, Hymenoptera and Psocoptera for trees. It is noteworthy that Oribatida maintained dominance across all hydroperiods within all vegetation functional types, while Arthropleona was predominant during both flood and wet periods (Figure 16)

Figure 16: Soil arthropods assemblages and trophic structure across various vegetation functional types subject to differing hydroperiods. Note:  Flood;
Flood;  Wet.
Wet.
Across all vegetation types and hydroperiods, seven taxonomic groups of soil arthropods were consistently present, establishing a similarity of approximately 32% among plants/hydroperiods (Figure 16). The remaining 68% of the taxa, which were not shared, contributed to assemblages’ variations between types across hydroperiods. These non- shared groups included Amphipoda (scuds or side-swimmers), Blattodea (cockroaches), Coleoptera (beetles), Dermaptera (earwigs), Geophilomorpha (soil centipedes), Hemiptera (true bugs), Isopoda (woodlice), Isoptera (termites), Lepidoptera (moths and butterflies), Orthoptera (grasshoppers and crickets), Polyzoniida (a group of millipedes), Psocoptera (barklice or booklice), Spirobolida (a group of millipedes), Symphypleona (a subgroup of springtails), and Thysanoptera (thrips). The Bray-Curtis Index and Non-metric Multidimensional Scaling (NMDS) analyses revealed that the variations in assemblages between different types across hydroperiods yield the following combinations with the highest similarity, as illustrated in Figures 17 and 18: Fern-flood and grass- flood (64%), moderate dry-fern and moderate dry-grass (74%), wet- fern and wet-grass (65%), wet-tree and wet-fern (65%), and moist- tree and moist-shrub (64%). Conversely, the combinations showing the least similarity include wet-tree and moderate dry-shrub (16%), and moderate dry-shrub and flood- tree (17%).
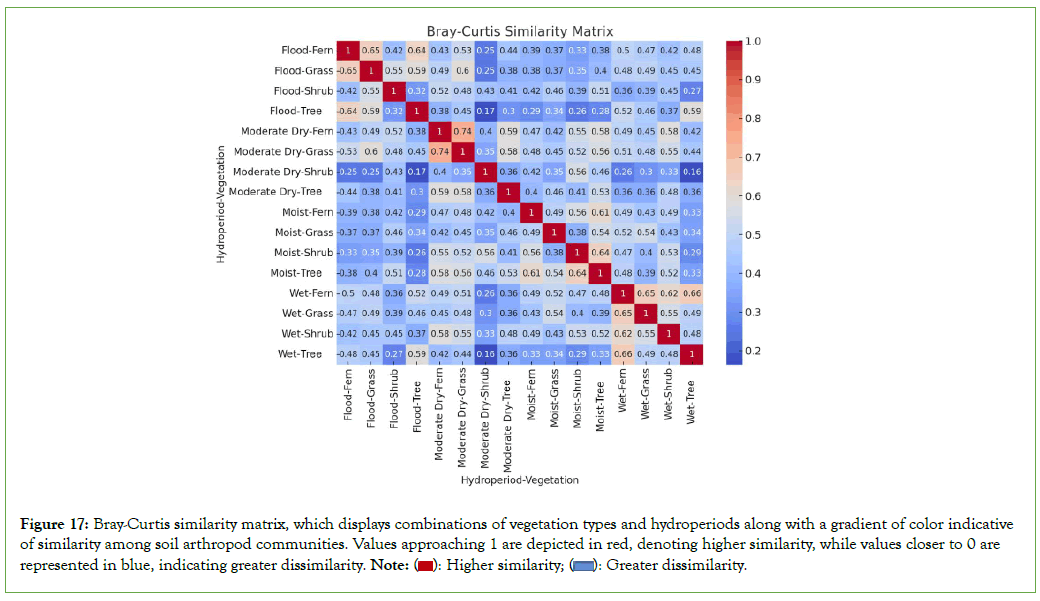
Figure 17: Bray-Curtis similarity matrix, which displays combinations of vegetation types and hydroperiods along with a gradient of color indicative
of similarity among soil arthropod communities. Values approaching 1 are depicted in red, denoting higher similarity, while values closer to 0 are
represented in blue, indicating greater dissimilarity. Note:  Greater dissimilarity.
Greater dissimilarity.
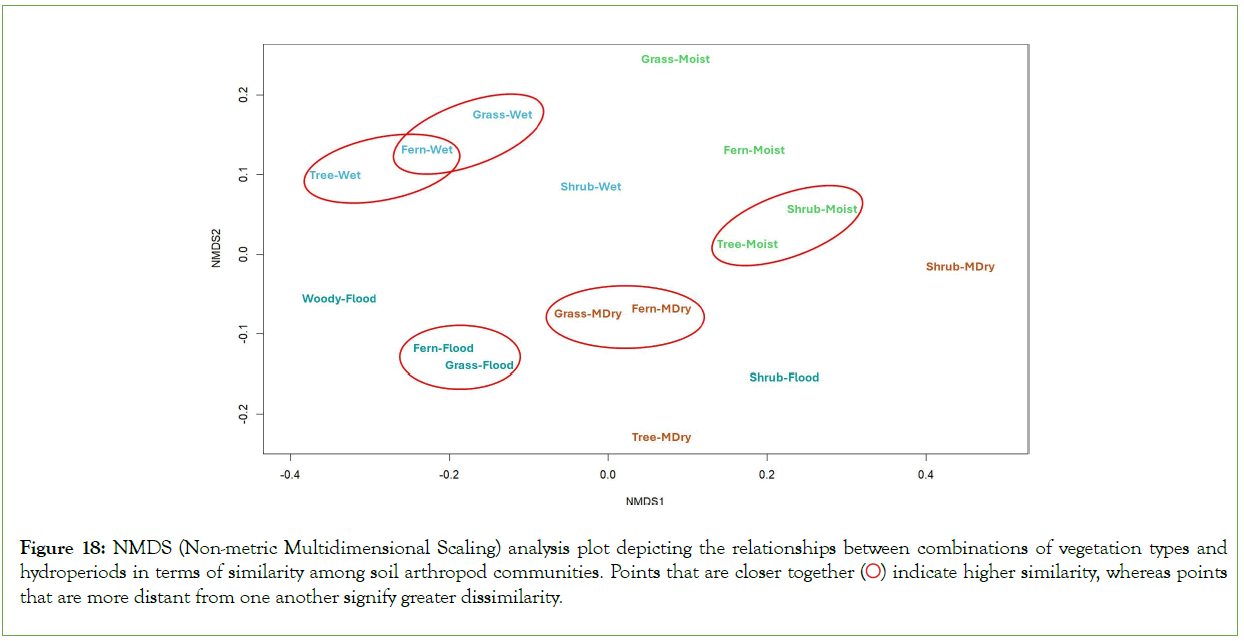
Figure 18: NMDS (Non-metric Multidimensional Scaling) analysis plot depicting the relationships between combinations of vegetation types and hydroperiods in terms of similarity among soil arthropod communities. Points that are closer together  indicate higher similarity, whereas points that are more distant from one another signify greater dissimilarity.
indicate higher similarity, whereas points that are more distant from one another signify greater dissimilarity.
Combined effects of vegetation types and hydroperiods on the community composition and structure of soil arthropods
The PERMANOVA and Multi-Response Permutation Procedure (MRPP) analyses reveal significant variations in soil arthropods assemblages and trophic structure attributable to differences among hydroperiods and vegetation (Tables 10 and 11, Figure 16). The significant p-values obtained from the PERMANOVA analysis (Hydroperiod: p=0.0; Vegetation: p=0.01) indicate that both factors distinctly influence the community composition. Hydroperiods account for 43.0% of the total observed variation, while vegetation functional type explains 22% of the variation. These results are echoed by the MRPP analysis, where the overall significance of delta (p=0.001) underscores notable variations in trophic level composition.
| Factors | Degrees of freedom | Sum of squares | R2 | F- statistics | Level of significance |
|---|---|---|---|---|---|
| Hydroperiod | 3 | 1.01 | 0.42 | 3.55 | 0.00 |
| Vegetation | 3 | 0.52 | 0.22 | 1.81 | 0.01 |
| Residual | 9 | 0.85 | 0.36 | ||
| Total | 15 | 2.38 | 1 |
Table 10: PERMANOVA analysis results, which reveals statistically significant differences in the community composition of soil arthropods. These differences are attributable to variations among hydroperiods and vegetation functional types.
| Vegetation Type | Hydroperiod | Tropic Level | Delta (A) | n |
|---|---|---|---|---|
| Fern | Moist | Microbivores | 4.36 | 152 |
| Flood | Detritivores | 3.44 | 24 | |
| Wet | Detritivores | 2.68 | 41 | |
| Moderate | Dry Herbivores | 2.52 | 19 | |
| Moist | Detritivores | 2.33 | 14 | |
| Moderate | Dry Omnivorous | 2 | 89 | |
| Moderate | Dry Detritivores | 1.83 | 18 | |
| Moist | Fungivores | 0.22 | 9 | |
| Wet | Fungivores | 0.17 | 12 | |
| Grass | Moist | Omnivorous | 2.67 | 6 |
| Wet | Microbivores | 2.12 | 125 | |
| Moderate Dry | Dry Detritivores | 1.64 | 13 | |
| Flood | Detritivores | 1.07 | 8 | |
| Flood | Detritivores | 0.93 | 20 | |
| Moist | Detritivores | 0.77 | 13 | |
| Moist | Herbivores | 0.75 | 25 | |
| Wet | Detritivores | 0.7 | 14 | |
| Flood | Fungivores | 0 | 13 | |
| Wet | Fungivores | 0 | 2 | |
| Shrub | Moderate | Dry Microbivores | 6.17 | 110 |
| Wet | Omnivorous | 5.29 | 17 | |
| Wet | Detritivores | 2.77 | 18 | |
| Flood | Detritivores | 2.72 | 26 | |
| Moist | Detritivores | 2.69 | 16 | |
| Moderate | Dry Detritivores | 1.4 | 5 | |
| Flood | Flood | 1 | 7 | |
| Wet | Herbivores | 0 | 17 | |
| Tree | Moist | Microbivores | 3.77 | 86 |
| Wet | Detritivores | 2.63 | 52 | |
| Wet | Omnivorous | 1.6 | 5 | |
| Moist | Detritivores | 1.43 | 25 | |
| Flood | Detritivores | 1.18 | 14 | |
| Moderate | Dry Detritivores | 1.18 | 14 | |
| Flood | Fungivores | 1.16 | 11 | |
| Moderate | Dry Herbivores | 1.11 | 16 | |
| Moist | Fungivores | 0.86 | 11 |
Table 11: Multi-Response Permutation Procedure (MRPP) analysis, which categorizes combinations by their degree of dissimilarity (indicated by high delta values) or similarity (indicated by low delta values) in trophic level composition between groups. Sample sizes (n) associated with each group denote the number of observations or data points utilized to evaluate the trophic level composition within each category.
The MRPP analysis identified combinations with a high degree of dissimilarity (high delta values) in community structure within plant types, such as Shrub in Moderate Dry conditions at the Microbivores trophic level (delta=6.17), and in Wet conditions at the Omnivorous trophic level (delta=5.29). Fern in Moist conditions at the Microbivores trophic level (delta=4.36), and in Flood conditions at the Detritivores trophic level (delta=3.44). Conversely, more similar combinations include Grass in Flood conditions at the Fungivores trophic level and Shrub in Wet conditions at the Herbivores trophic level, both with a delta of 0.00, and Fern in Moist conditions at the Fungivores trophic level (delta=0.22).
Discussion
This research elucidates the significant impact of plant functional types on soil arthropods community structure and composition. By investigating the effects of litter cover variations— particularly its quantity and quality—on soil arthropod assemblages across different wetland hydroperiods, our research addresses the pivotal question of how traits of plant-derived litter influence these assemblages. The findings offer a detailed insight into the ecological interconnections among plant litter attributes, environmental hydroperiod variations, and their collective effects on the biodiversity of soil arthropods. The study makes a valuable contribution to the broader ecological questions, emphasizing the critical role of vegetation in determining the dynamics of soil ecosystems.
Substrate quality and quantity across plant functional type and hydroperiod
Significant variations in the contents of substrate fractions of carbon (C) and nitrogen (N), as well as in the carbon to nitrogen (C:N) ratios found under different plant functional types across hydroperiods, highlight the intricate relationships between vegetation and hydrological regimes. These relationships influence the quality and decomposition rate of plant litter, which varies among functional types, affecting the accumulation or depletion of soil nutrients. This underlines the complexity of soil ecosystems and the critical role of decomposition processes in shaping the habitat and nutrient availability for soil arthropods. C:N ratios within old litter-organic soil fractions were significantly lower compared to those in loose litter fractions, a pattern consistent across all plant types and hydroperiods. Such distinctions suggest varying decomposition stages or nutrient release patterns between the two soil fractions, contributing to a diverse mosaic of substrate qualities that influence soil arthropod dynamics [19,27]. Upon comparing these metrics across plant functional types under various hydroperiods, the shrub type, a nitrogen-fixing plant, consistently exhibited the highest carbon (C) and nitrogen (N) percentages under all conditions, indicating a richer nutrient profile in their litter compared to other types. This enhanced nutrient availability can accelerate microbial decomposition processes and subsequently influence soil nutrient dynamics. In contrast, trees exhibited the highest carbonto-nitrogen (C:N) ratios, marking them significantly different from other plant functional types. These high C:N ratios are indicative of a slower decomposition rate due to the more recalcitrant nature of their litter. High C:N ratios in tree litter result from a higher proportion of lignin and cellulose, which are more resistant to rapid breakdown and thus persist longer in the soil. The differentiation in litter quality among plant types (Table 2), highlights unique traits that significantly influence soil arthropod communities by modulating their interactions within the decomposition process. Litter quality is determined by their chemical and structural characteristics, such as nitrogen, lignin, and cellulose content, which affect the rate and manner of decomposition. These characteristics give rise to two primary energetic channels for decomposition: the bacterial channel and the fungal channel, which in turn shape the trophic assemblages of soil arthropods communities. In low C:N ratio litter, where lignin and cellulose are less abundant, decomposition is typically dominated by bacteria. This leads to a rapid transformation cycle of carbon, creating an environment where bacteria, along with protozoa, nematodes, and earthworms, thrive. The abundance of these primary decomposers also supports a diverse array of predators that depend on them for food, further modulating the community structure. Conversely, high C:N ratio litter, characterized by a higher content of complex compounds such as lignin, humic or phenolic acids, and cellulose, tends to decompose more slowly. This slower process is primarily facilitated by fungi, which can break down these resistant compounds. As a result, the fungal decomposition channel becomes more prominent, supporting a different structure of soil organisms, predominantly consisting of mesofauna such as mites and springtails. Thus, the quality of litter, dictated by the type of plant and its litter’s chemical and structural properties, plays a crucial role in determining the decomposition pathways and the associated trophic interactions among soil arthropods.
The results underscored the significant influence of hydroperiods on both the mass and composition of plant litter. A marked increase in plant litter mass was observed during moderate dry periods across all examined vegetation types, with the most substantial accumulations being recorded in microenvironments dominated by trees and shrubs. This pronounced accumulation can be attributed to the specific characteristics of leaf fall and senescence associated with these plant types. Trees and shrubs typically undergo a distinct leaf-fall season, which is notably observed in mangrove forests where seasonal patterns of leaf litter production are prominent during warmer months, often associated with air temperature increases [51], (Shang et al., 2015, as cited in Medina, 2024). This phenomenon contributes significantly to the litter mass, as observed in various ecosystems [39]. Unlike trees and shrubs, grasses and ferns do not have a distinct leaf-fall season [39,52]. They continuously grow new leaves from the base while older leaves die off gradually. This growth pattern results in a more constant but less noticeable contribution to the litter layer. Furthermore, the study revealed that during moist and flood periods, there was a notable redistribution of litter, particularly around ferns and grasses. Given the diverse decomposition rates of these residues, attributable to their distinct chemical compositions (Table 3), this redistribution leads to heterogeneous decomposition rates beneath both fern and grass vegetation.
The distinct leaf fall, litter redistribution, and decomposition patterns associated to these plant types at the study site, contribute to the spatial variability in litter quantity and quality.
Influence of loose litter mass, carbon and nitrogen content, and C:N ratio on soil arthropods communities
Loose litter mass, substrate carbon (%C) and nitrogen (%N) content demonstrate statistically significant correlations with the richness and abundance of soil arthropods, each influencing ecological metrics in distinct ways. Although %C exhibits relatively smaller coefficients (0.01 for richness and 0.02 for abundance), its significant impact, highlighted by high tvalues (13.70 for richness and 4.29 for abundance), suggests that even minor increases in carbon content can significantly affect species metrics. Conversely, litter mass exerts a more substantial quantitative effect with coefficients of 0.20 for richness and 0.93 for abundance, underlining its vital role in enhancing ecological diversity and abundance through nutrient provision and habitat creation. %N exhibits a dual effect on these ecological parameters. While it positively influences species abundance—likely due to its role in enhancing growth and reproductive rates among microflora, which soil arthropods help to regulate—it adversely affects arthropod richness. This negative impact could lead to reduced ecological diversity due to the competitive exclusion of less dominant species. The substantial difference in the tvalues for %N’s impact on abundance (1.97) versus richness (-7.8) underscores its complex role in ecological dynamics, suggesting that its effects are context-dependent and may vary across different ecological or environmental conditions.
The effect of the C:N ratio on soil arthropod communities, across trophic guilds such as detritivores, fungivores, herbivores, microbivores, omnivores, and predators, offers insightful observations into ecosystem dynamics. Densities of soil arthropod trophic guilds were notably higher within the equilibrium phase (C:N ratio between 20:1 and 30:1) and the immobilization phase (C:N ratio>30:1) of decomposition, with no significant differences observed either between these phases or among the trophic guilds within them. This pattern underscores the indirect influence of C:N ratios on soil arthropods by affecting the availability of microbial communities, thereby shaping the conditions that support a diverse spectrum of soil arthropods and sustain high densities across various trophic guilds [10,12,19,52]. The distinct separation from the mineralization phase (C:N ratio<20:1) highlights the sensitivity of soil arthropod communities to nitrogen availability, suggesting that nitrogen’s role in this context is mediated significantly by its effects on microbial decomposition processes and the subsequent availability of nutrients. In environments where nitrogen is more readily available for mineralization, significant shifts occur in the composition and interaction patterns among detritivores, fungivores, herbivores, microbivores, omnivores, and predators (Figure 19) [10,12,19]. The observed statistical differences within the mineralization phase, especially among herbivores, detritivores, and microbivores, as well as between microbivores and predators, highlight the nuanced changes in food web dynamics under nitrogen-rich conditions. These variances may point to competition for resources, as well as potential shifts in predator-prey relationships [52]. Furthermore, it emphasizes the critical role of primary and secondary decomposers, such as detritivores (Diplopoda, Blattodea) and microbivores (Oribatida and Collembola), in the initial stages of loose litter decomposition through mechanical and physical management, setting the stage for microbial action and further decomposition [19].

Figure 19: Soil arthropods belonging to different functional groups (groups of species with similar traits and effects on processes) involve in carbon and nutrient mobilization from litter (dead plant residues). Adapted from Bastow, 2013.
Hydroperiod influences on soil arthropod diversity across vegetation types
The two-way non-parametric analysis revealed significant dependencies of soil arthropods on their microhabitats (plant functional types), which vary with hydrological conditions. This variability underscores the complex interplay between hydrological conditions, plant functional types, and faunal diversity for both mesofauna and macrofauna.
Macrofauna densities significantly increased within fern, grass, and tree vegetation types during the flood and wet hydroperiods, including Detritivores (Diptera, Isopoda), Predators (Coleoptera, Araneae), Omnivores (Formicidae), and Herbivores (Hemiptera). Conversely, mesofauna densities were higher during flood, wet, and moist hydroperiods, particularly within grass and shrub vegetation types, with the highest densities observed in tree vegetation types during the flood period. This included Microbivores (Oribatida, CollembolaArthropleona) and Predators (Mesostigmata, Prostigmata). These distribution pattern can likely be attributed to the enhanced availability of habitats and resources inherent to each vegetation type, driven by fluctuations in substrate quality and quantity [12,14,27,52]. These fluctuations are intricately connected to the effects of the hydrological regime before the sampling intervals on litter redistribution [5,21]. The timing of the flood sampling aligned with the receding floodwaters following significant atmospheric events, leading to prolonged flooding. The moist period sampling occurred during a dry interval between flood events, while the wet hydroperiod was marked by a series of flooding and drying cycles preceding the sampling. Variations in these wetting cycles led to the redistribution of both loose and decomposed plant litter, creating patches of fresh and aged organic material from various plant sources, notably under fern and grass vegetation types [5,23]. This redistribution induced notable variations in substrate Carbon to Nitrogen (C:N) ratios across different vegetation types, linked to different decomposition stages, from immobilization and an equilibrium phase to mineralization. The mixing of litter and the presence of diverse decomposition stages prompted shifts in microflora communities, ranging from fungi, which break down more recalcitrant resources (such as cellulose and lignin), to bacteria that decompose more readily available components (Figure 19). This bacterial and fungal succession influences the composition of soil arthropod communities, which undergo significant changes as decomposition progresses. Initially, surface-dwelling macrofauna predominated, facilitating the physical breakdown of litter. As decomposition continues, the community composition shifts towards a dominance of soil-dwelling mesofauna, well-adapted to exploit the microhabitats created by litter breakdown [14,19,27]. Regarding soil arthropods’ richness and Menhinick’s Index, further analysis illustrates significant variations among macrofauna and mesofauna groups within the contexts of fern, shrub, and tree functional types exhibiting higher values during the wet, flood and moist periods, respectively. In contrast, grass types exhibited significantly higher values under moderate dry conditions. This variation underscores the adaptive strategies employed by different faunal groups in response to resource and habitat availability [10,12,19]. Certain groups seem to prefer drier conditions with abundance of resources (litter mass). which typify the moderate dry phase, succeeding months of reduced precipitation [53]. It should be emphasized that shrub vegetation exhibited higher diversity during moist and flood periods for both mesofauna and macrofauna. This suggests that shrubs play significant functional role in aiding the recovery of microhabitats for other vegetation types following disturbances.
The patterns of density, richness, and diversity among soil arthropods reveal a distinct interaction between faunal assemblages and their microhabitat conditions, emphasizing the importance of temporal changes in resource availability on biodiversity patterns. It is important to note that in the case of fern and grass vegetation types, these variations are influenced by litter mixing beneath them, where loose litter from shrubs and trees predominates following flooding events.
Relationships between soil arthropod taxa and the combined effects of plant functional types and hydroperiods
Biodiversity variations, classified into dominant, common, and rare taxa provided a distinctive understanding of species distribution across varying hydroperiods. The analysis revealed that common and rare taxa consistently outnumbered the dominant groups in all functional vegetation types across the different hydroperiods suggesting a high level of ecological diversity and niche specialization within these ecosystems. This diversity suggests efficient niche utilization and conditions of high-quality soil that are resilient to disturbances [18,52,54]. Sampling during the moist period, conducted immediately after the recession of floodwaters, revealed the greatest relative density of common taxa across all vegetation types (64% mean value), including diverse taxonomic groups such as Predators (Araneae, Coleoptera, Mesostigmata, Prostigmata), Detritivores (Diptera), Herbivores (Hemiptera), and Omnivores (Hymenoptera). The moist period can be seen as an early stage of ecological succession following the disturbance created by flooding. Early successional stages are often characterized by a higher presence of opportunistic species that can quickly take advantage of changing conditions [32]. The presence of exposed, nutrient-rich litter post-flooding provides an ideal environment for rapid colonization and growth [21,15,45]. Common taxa, with their generalist ecological requirements, are particularly well- equipped to prosper under such conditions [55]. Their capacity for rapid response and recovery, attributed to characteristics like large body size, dormancy in egg stage and high mobility, that enables species to quickly immigrate or recolonize sites post-flooding, and versatile dietary preferences, allows them to effectively utilize the newly available resources [5,56,57]. These traits play a pivotal role in ecosystem stability and recovery following disturbances. Furthermore, disturbances lead to the convergence of species traits due to uneven resource exploitation, thereby enhancing the ability of these adaptable taxa to fill available ecological niches amid low functional evenness [1,54]. Such dynamics during the critical window for ecosystem recovery and diversification highlight the significant impact of postdisturbance phases on community composition and biodiversity. In the wet sampling period, characterized by alternating flooding and drying cycles leading up to the sample collection, we observed the highest relative densities of rare taxa groups, averaging 39%. This period included a wide array of taxonomic groups, such as Predators (Araneae), Detritivores (Amphipoda, Spirobolida, Polyzoniida), Herbivores (Lepidoptera), Microbivores (Psocoptera), and Omnivores (Hymenoptera). The predominance of these rare taxa may be attributed to the specific environmental conditions prevalent during the wet period, which likely included a mix of detritus at various stages of decomposition due to the preceding cycles of prolonged drying and shorter flooding. This variability in detritus quality and quantity, further compounded by the unique litter traits of different plant functional types, may have created specialized niches. Rare taxa, though fewer in number, possess a unique combination of traits that enable them to exploit these niches, thriving in habitats or fulfilling dietary requirements not readily available to more generalized species [54]. Their success during this period underscores the complex interplay between hydrological dynamics, litter traits, and biodiversity, highlighting how specific environmental conditions can facilitate the flourishing of specialized organisms within soil ecosystems. During these intermediate stages, a gradual increase in diversity occurs as rare species begin to establish, supported by the stabilizing environment. This phase marks a critical period in ecological succession, where the conditions become increasingly conducive for a broader array of species to thrive, further enriching the ecosystem’s complexity and resilience.
It’s important to note that some taxa appear both as common and rare across different sampling periods, reflecting their adaptive strategies and ecological plasticity. These species possess a broad range of ecological tolerances that allow them to quickly capitalize on the post-flood abundance of resources, yet their presence as ‘rare’ in other periods indicates a sensitivity to changing environmental dynamics, such as alternating wet and dry conditions. This duality underscores the complexity of ecological niches and the flexibility of species in responding to the mosaic of habitat conditions presented by varying hydroperiods. The dominant taxa, characterized by Oribatida mites (Microbivores), account for an average of 48% across various vegetation types and hydroperiods. This significant dominance underscores their resilience and adaptability to a wide range of environmental conditions, terrestrial, aquatic, and semi-aquatic, and establishes their critical role as keystone species in regulating decomposition processes, nutrient cycling, and overall soil health [58]. They are instrumental within the soil food web, playing a pivotal role in shaping soil microbial communities, regulating their proliferation and diversity, and serving as a connecting node between microflora and a diverse array of invertebrate predators [18,43]. As the dominant group, Oribatida mites not only support ecosystem recovery and stability through their interactions within the soil food web but also play a vital role in preserving biodiversity and ecosystem services under varying environmental conditions. Overall, vegetation types exhibit approximately 32% similarity in soil arthropod taxa across hydroperiods, underscoring the presence of distinct ecological communities among vegetation types, with a significant 68% of taxa unique to specific assemblages. This diversity includes non-shared groups such as Amphipoda, Blattodea, Coleoptera, Dermaptera, Geophilomorpha, Hemiptera, Isopoda, Isoptera, Lepidoptera, Orthoptera, Polyzoniida, Psocoptera, Spirobolida, Symphypleona, and Thysanoptera, highlighting the rich biodiversity within these ecosystems. The Bray-Curtis Index and Non-metric Multidimensional Scaling (NMDS) analyses further uncover significant patterns in these community assemblages, revealing that combinations like Fern-Flood and Grass-Flood (64%), and Moderate Dry-Fern and Moderate Dry-Grass (74%), exhibit high similarity, in contrast to the low similarity observed between Wet- Tree and Moderate Dry-Shrub (16%). These findings emphasize the critical role that hydroperiods and vegetation types play in shaping the diversity and composition of soil arthropod communities, reflecting both the shared and unique environmental niches that these communities inhabit across different conditions.
Combined effects of vegetation types and hydroperiods on the community composition and structure of soil arthropods
The influence of hydroperiods and vegetation functional types on the assemblages and trophic structure of soil arthropods, explaining 43% and 22% of the observed variations, respectively, emphasizes the intricate bidirectional interactions between plant communities and soil arthropods, shaped by the wetland’s hydrological conditions across time and space (Figure 20) [9-13,21]. Complex ecological structures predominate during wet hydroperiods, whereas simpler structures are characteristic of flood periods (Figure 20). This pattern emerges from the cumulative effects of interspecific differences on vegetation type substrates, including their chemical and structural compositions, as well as hydrological regimes.

Figure 20: Soil arthropods community structure across vegetation types among hydroperiods. Note: Tropic level  Fungivore;
Fungivore;  Microflora.
Microflora.
Significant dissimilarities in community structure among different conditions were detected in combinations such as Shrub in Moderate Dry conditions at the Microbivores trophic level (delta=6.17), Fern in Moist conditions at the Microbivores level (delta=4.36), and Shrub in Wet conditions at the Omnivorous level (delta=5.29). These variations are attributable to the adaptation and specialization of arthropod communities within their unique habitats. In contrast, more homogeneous community structures were observed in specific conditions, such as Fern in Moist conditions at the Fungivore level (delta=0.22), suggesting that certain environmental conditions, particularly those following disturbance events, may promote more uniform community structures. Within the framework of soil ecosystems and the decomposition food web dynamics, the impact of disturbances on substrate availability and quality significantly influencing the activity of fungi on less labile (more difficult to decompose) materials. Such post-disturbance conditions create specific ecological niches that favor fungivores, as these organisms feed on and regulate fungal resources. Furthermore, the creation of new niches and resources, following disturbances, establishes fertile grounds for colonization, thereby encouraging the formation of more homogeneous community structures among soil-dwelling organisms, including fungivores [55,57].
Findings in relation to objectives, questions, and hypotheses
Soil biota’s reactions to variations in vegetation types and hydroperiods can be attributed to several mechanisms: 1) Variations in resource quantity and quality due to interspecific differences, 2) the interplay of bottom-up and top-down dynamics, and 3) the creation and alteration of microhabitats [28]. The observed concomitant changes in the soil arthropods community structure and decomposition substrates C:N ratios indicate the critical role resource quality in shaping microbial community availability and, consequently, supporting a diverse array of soil arthropods. Soil arthropod trophic guild densities peak in both the equilibrium (C:N ratio between 20:1 and 30:1) and immobilization (C:N ratio>30:1) phases of decomposition. The distinct separation from the mineralization phase (C:N ratio<20:1) underscores a) soil arthropod communities’ sensitivity to nitrogen availability, b) interactions and potential shifts in bottom-up and top-down regulatory mechanisms, c) the role of primary and secondary decomposers in ecosystem processes. A succession of taxa-dependent interactions related to decomposition stages, where common, rare, and dominant groups interact, illustrates the complex interplay among different arthropod groups that contribute to nitrogen cycling and organic matter breakdown in soil ecosystems. Common groups such as predators (Araneae, Coleoptera, Mesostigmata, Prostigmata), detritivores (Diptera), herbivores (Hemiptera), and omnivores (Hymenoptera), which colonize early in successional stages, are characterized by a higher presence of opportunistic taxa [32,54]. These taxa swiftly adapt to changing conditions, playing a vital role in contributing to ecosystem stability and recovery postdisturbance. Rare groups, including predators (Araneae), detritivores (Amphipoda, Spirobolida, Polyzoniida), herbivores (Lepidoptera), microbivores (Psocoptera), and omnivores (Hymenoptera), typically establish at intermediate stages. As these rare taxa begin to settle, a gradual increase in diversity is supported by the stabilizing environment, marking a critical period in ecological succession [19]. Dominant groups, particularly Oribatida mites (microbivores), emerge as key regulators of decomposition processes, nutrient cycling, and overall soil health, being instrumental within the soil food web by shaping soil microbial communities, regulating their proliferation and diversity, and acting as a bridge between microflora and various invertebrate predators [58-61]. The group interactions across vegetation and hydroperiods underline the integral role that each arthropod group—common, rare, and dominant—plays at different stages of decomposition, highlighting their collective contribution to the soil ecosystem’s dynamics [19,54]. The presence of taxa in both common and rare categories across different hydroperiods highlights their ecological versatility and the nuanced balance between competition, adaptation, and specialization. Shifts in taxa dominance and rarity accentuate the fluid nature of ecological communities, where taxa adjust their roles and abundances in response to the ever-changing environmental conditions and resource availability. The ongoing dialogue between taxa traits and ecosystem processes shapes the intricate web of life within these dynamic habitats [60-66]. An observed increase in litter mass during moderate dry periods, coupled with its redistribution in flood and moist periods, identified trees and shrubs as major contributors to litter across all hydroperiods [67-70]. Additionally, shrubs play a crucial functional role in aiding the recovery of microhabitats constituted by other vegetation types following disturbances within wetland ecosystems [71-75]. Litter mass, carbon and nitrogen concentration are pivotal determinants of soil arthropod richness and abundance, with variations attributed to hydroperiods and plant functional types [76-78]. The significant observed variations in soil arthropod diversity across different vegetation types and hydroperiods, collectively highlight the combined influence of plant-hydroperiod interactions on habitat and resource availability and its influence on soil arthropods assemblages and trophic structure. Complex ecological structures predominate during wet hydroperiods, whereas simpler structures are characteristic of flood periods (Figure 20). This pattern emerges from the cumulative effects of interspecific differences on vegetation type substrates, including their chemical and structural compositions, as well as hydrological regimes. These interactions highlight the dynamic adaptability of soil arthropods to fluctuating microhabitat conditions and the functional role of plant-soil dynamics in the resilience of the wetland ecosystem.
Conclusion
This research contributes to a deeper understanding of the intricate ecological interconnections between plant litter attributes, environmental hydroperiods, and soil arthropod biodiversity. It underscores the integral role of vegetation and water in shaping soil ecosystem dynamics. The findings bolster the hypothesis that synchrony and synlocation of soil arthropod communities are influenced by the specific plant functional type and the characteristics of its associated litter, with hydroperiod dynamics playing a crucial role. Directly addressing the research question regarding the modulation of arthropod assemblages by plant- derived litter characteristics, providing new insights into the ecological interconnections that shape soil ecosystem dynamics. By elucidating the complex interdependencies between plant functional types, litter quality and quantity, and soil arthropod assemblages, the study offers valuable insights for ecosystem management and conservation strategies aimed at preserving biodiversity and ecosystem functionality in wetland environments.
Declarations
Author contributions
GO-R and EC conceived and designed the study, analyzed the data, and wrote the manuscript. GO-R, EH, and SP-P field sampling and analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NSF CREST–Center for Innovation Research and Education in Environmental Nanotechnology (CIRE2N) HRD 1736093, NSF HRD 1806129, and the Center for Applied Tropical Ecology and Conservation (CATEC) of the University of Puerto Rico.
Acknowledgments
The authors would like to acknowledge the technical assistance provided by the Center for Applied Tropical Ecology and Conservation (CATEC). Appreciation is also extended to the coordinator of the Process and Functions Ecosystem Lab, Larry Díaz, and to the undergraduate students — Diego E. Otero-Santiago, Cecilia M. Fadhel-Alvarez, Isabel Alayon, Jorge L. Gonzalez- Santana, Thalia M. Rosa-Agosto, Alexis Roque Sotomayor, and Alexander Cántres — for their substantial contributions to both field and laboratory work.
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wardle DA, Bardgett RD, Klironomos JN, Setala H, Van Der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304(5677):1629–1633.
[Crossref] [Google Scholar] [PubMed]
- Whigham DF, Bayley SE. Wetland functions and values: The state of our understanding. Nutrient Dyn Freshwater wetland. 1978.
- Facelli JM, Pickett ST. Plant litter: Its dynamics and effects on plant community structure. The Botanical Review. 1991;57:1-32.
- Wardle DA, Jonsson M, Bansal S, Bardgett RD, Gundale MJ, Metcalfe DB. Linking vegetation change, carbon sequestration and biodiversity: insights from island ecosystems in a long-term natural experiment. J Ecol. 2012; 100(1): 16-30.
- Bardgett RD, Van Der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014; 515(7528):505-511.
- Mitsch WJ, Gosselink JG, Zhang L, Anderson CJ. Wetland ecosystems. John Wiley & Sons. 2009.
- Lavelle P, Blanchart E, Martin A, Martin S, Spain A. A hierarchical model for decomposition in terrestrial ecosystems: Application to soils of the humid tropics. Biotropica. 1993; 130-150.
- Lavelle P. Faunal activities and soil processes: Adaptive strategies that determine ecosystem function. Advanc Ecol Res. 1997;27:93-132.
- Lavelle P, Spain AV. Soil Ecology. Kluwer Academic Publishers. 2001.
[Crossref]
- Lavelle P, Decaens T, Aubert M, Barot S, Blouin M, Bureau F, et al. Soil invertebrates and ecosystem services. Europ J Soil Biology. 2006;42:3-15.
- Lavelle P, Mathieu J, Spain A, Brown G, Fragoso C, Lapied E, et al. (2022). Soil macroinvertebrate communities: A world-wide assessment. Global Ecol Biogeograph. 2022; 31(7): 1261-1276.
- Swift MJ, Heal OW, Anderson JM, Anderson JM. Decomposition in terrestrial ecosystems. Univ California Press. 1979; 5.
- Culliney TW. Role of arthropods in maintaining soil fertility. Agriculture. 2013;3(4):629-659.
- 14 Barberena-Arias MF, Cuevas E. Physicochemical foliar traits predict assemblages of litter/humus detritivore arthropods. Tropic Forest. 2018:1-9.
- Mulder C, Den Hollander HA, Vonk JA, Rossberg AG, Jagers op Akkerhuis GA, Yeates GW. Soil resource supply influences faunal size–specific distributions in natural food webs. Naturwissenschaften. 2009;96:813-826.
- Haynert K, Kiggen M, Klarner B, Maraun M, Scheu S. The structure of salt marsh soil mesofauna food webs–the prevalence of disturbance. PLoS One. 2017;12(12): 0189645.
- Tongkaemkaew U, Sukkul J, Sumkhan N, Panklang P, Brauman A, Roslan Ismail RI. Litterfall, litter decomposition, soil macrofauna, and nutrient content in rubber monoculture and rubber-based agroforestry plantations. Forest Society.2018.
- Menta C, Remelli S. Soil health and arthropods: From complex system to worthwhile investigation. Insects. 2020;11(1):54.
- Bastow J. Succession, resource processing, and diversity in detrital food webs. Soil Ecol Ecosystem Service. 2012;117-135.
- Chen Y, Wang B, Pollino CA, Cuddy SM, Merrin LE, Huang C. Estimate of flood inundation and retention on wetlands using remote sensing and GIS. Ecohydrology. 2014;7(5):1412-1420.
- Wu P, Zhang H, Wang Y. The response of soil macroinvertebrates to alpine meadow degradation in the Qinghai–Tibetan Plateau, China. Applied Soil Ecology. 2015;90:60-67.
- Schoenly K, Reid W. Dynamics of heterotrophic succession in carrion arthropod assemblages: Discrete seres or a continuum of change?. Oecologia. 1987;73:192-202.
- Batzer DP, Sharitz RR. Ecology of freshwater and estuarine wetlands. Univ California Press. 2014.
- Batzer D, Wu H, Wheeler T, Eggert S. Peatland invertebrates. Invertebrates in freshwater wetlands: an international perspective on their ecology. Springer.2016:219-250.
- Batzer DP, Wu H. Ecology of terrestrial arthropods in freshwater wetlands. Annual Rev Entomol. 2020;65:101-119.
- Lockaby BG, Murphy AL, Somers GL. Hydroperiod influences on nutrient dynamics in decomposing litter of a floodplain forest. Soil Sci Soc America J. 1996;60(4):1267-1272.
- Yang Y, Zhou H, Wang W, Zhu C, Cui D, Ye Z. Transient flooding and soil covering interfere with decomposition dynamics of populus euphratica leaf litter: Changes of mass loss and stoichiometry of C, N, P, and K. Forests. 2022;13(3):476.
- Zhang L, Liu J, Yin R, Xu Z, You C, Li H, et al. Soil fauna accelerated litter C and N release by improving litter quality across an elevational gradient. Ecologic Proces. 2023;12(1):47.
- Moore JC, de Ruiter PC. Temporal and spatial heterogeneity of trophic interactions within below-ground food webs. Agriculture, Ecosys Environ. 1991;34(1-4):371-397.
- Wardle DA. Communities and ecosystems: Linking the aboveground and belowground components (MPB-34). Princeton Univ Press. 2013.
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853-858.
- Gonzalez G, Barberena MF. Ecology of soil arthropod fauna in tropical forests: A review of studies from Puerto Rico. J Agri Univ Puerto Rico. 2017;101(2):185-201.
- Barberena-Arias MF, Cuevas E. Vertical arthropod dynamics across organic matter fractions in relation to microclimate and plant phenology. Arthropod Benef Mankind. 2019.
- Cuevas E. Personal communication. 2024.
- Barberena-Arias MF, Cuevas E. vertical arthropod dynamics across organic matter fractions in relation to microclimate and plant phenology. IntechOpen. 2021[ Crossref]
- Coleman DC, Wall DH. Fauna: The engine for microbial activity and transport. Soil Microbiol Ecol Biochemist. 2007; 163-191.
- Moco MK, Gama-Rodrigues EF, Gama-Rodrigues AC, Machado RC, Baligar VC. Relationships between invertebrate communities, litter quality and soil attributes under different cacao agroforestry systems in the south of Bahia, Brazil. Applied Soil Ecol. 2010;46(3):347-354.
- Ward SE, Orwin KH, Ostle NJ, Briones MJ, Thomson BC, Griffiths RI, et al. Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology. 2015;96(1):113-123.
- Ortiz-Ramírez G, Hernández E, Pinto-Pacheco S, Cuevas E. The dynamics of soil mesofauna communities in a tropical urban coastal wetland: Responses to spatiotemporal fluctuations in phreatic level and salinity. Arthropoda. 2024;2(1):1-27.
- Torres-Valcarcel A, Harbor J, González-Avilés C, Torres-Valcárcel A. Impacts of urban development on precipitation in the tropical maritime climate of Puerto Rico. Climate. 2014;2(2):47-77.
- Webb RM, Gómez-Gómez F. Synoptic survey of water quality and bottom sediments, San Juan Bay estuary system, Puerto Rico, December 1994-July 1995. US Geologic Survey.1998.
- Cuevas E. Personal communication. 2020.
- Medina E. Personal communication. 2024.
- Mehltreter K, Walker LR, Sharpe JM. Fern ecology. Cambridge University Press. 2010.
- National Weather Service. Climatological Data for La Puntilla Station (ID number 9755371), San Juan, Puerto Rico. 2020-2023.
- National Weather Service. Tropical Storm Laura August 21-23, 2020. Weather.gov. Tropical Storm Laura - August 21-23, 2020 (weather.gov). 2020.
- Pradisty NA, Amir AA, Zimmer M. Plant species-and stage-specific differences in microbial decay of mangrove leaf litter: The older the better?. Oecologia. 2021;195(4):843-858.
- Kennaway T, Helmer EH. The forest types and ages cleared for land development in Puerto Rico. GIScience & Remote Sens. 2007;44(4):356-382.
- Centro de IyD, Recinto de M. Pumarada-O’Neill: Los Puentes Históricos de Puerto Rico. Universidad de Puerto Rico. 1991.
- Hernandez Figueroa E. Ecophysiological responses of plant functional types to environmental conditions in a coastal urban wetland, Ciénaga Las Cucharillas in Northeastern Puerto Rico. 2021.
- Perez-Harguindeguy N, Diaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, et al. New handbook for standardised measurement of plant functional traits worldwide. Australia J Botany. 2013; 61: 167-234.
- Box EO. Macroclimate and plant forms. Springer. 1981;1.
- Native Plant Trust. Go Botany: Discover thousands of New England plants. 2024.
- Herrera FF, Cuevas E. Artropodos del suelo como bioindicadores de recuperacion de sistemas perturbados. Venesuelos. 2003;11(1-2):67-78.
- Nielsen UN. Soil fauna assemblages. Cambridge Univ Press.
- Potapov AM, Beaulieu F, Birkhofer K, Bluhm SL, Degtyarev MI, Devetter M, et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biologic Review. 2022;97(3):1057-1117.
- Potapov AM. Multifunctionality of belowground food webs: Resource, size and spatial energy channels. Biologic Review. 2022;97(4):1691-1711.
- Brust GE. Management strategies for organic vegetable fertility. Safety Pract Organic Food. 2019;193-212.
- Liu C, Sun X. A review of ecological stoichiometry: Basic knowledge and advances. Refer Modul Earth Sys Environment Sci. 2013.
- Tan Y, Yang W, Ni X, Tan B, Yue K, Cao R, et al. Soil fauna affects the optical properties in alkaline solutions extracted (humic acid-like) from forest litters during different phenological periods. Canada J Soil Sci. 2019;99(2):195-207.
- Zheng X, Wang H, Tao Y, Kou X, He C, Wang Z. Community diversity of soil meso-fauna indicates the impacts of oil exploitation on wetlands. Ecologic Indicat. 2022;144:109451.
- Li K, Bihan M, Yooseph S, Methe BA. Analyses of the microbial diversity across the human microbiome. PloS one. 2012;7(6): 32118.
- Medina E. Principios de ecofisiología vegetal. Caracas. 2024;414.
- Wardle DA. The influence of biotic interactions on soil biodiversity. Ecology Letter. 2006;9(7):870-886.
- Peng Y, Vesterdal L, Penuelas J, Peguero G, Wu Q, Hedenec P, et al. Soil fauna effects on litter decomposition are better predicted by fauna communities within litterbags than by ambient soil fauna communities. Plant Soil. 2023;487(1):49-59.
- Briones MJ. The serendipitous value of soil fauna in ecosystem functioning: The unexplained explained. Front Environment Sci. 2018;6:149.
- Dee LE, Cowles J, Isbell F, Pau S, Gaines SD, Reich PB. When do ecosystem services depend on rare species?. Trend Ecol Evolut. 2019;34(8):746-758.
- Coyle DR, Nagendra UJ, Taylor MK, Campbell JH, Cunard CE, Joslin AH, et al. Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biology Biochemist. 2017;110:116-133.
- Gerisch M, Agostinelli V, Henle K, Dziock F. More species, but all do the same: Contrasting effects of flood disturbance on ground beetle functional and species diversity. Oikos. 2012;121(4):508-515.
- Behan-Pelletier V, Lindo Z. Oribatid Mites: Biodiversity, Taxon Ecol. 2023.
- Garcia-Gomez A, Castano-Meneses G, Vazquez-Gonzalez MM, Palacios-Vargas JG. Mesofaunal arthropod diversity in shrub mangrove litter of Cozumel Island, Quintana Roo, Mexico. Applied Soil Ecol. 2014;83:44-50.
- Gergocs V, Hufnagel L. The effect of micro arthropods on litter decomposition depends on litter quality. Europo J Soil Biology. 2016;75:24-30.
- Guo J, Zhao C, Zhang L, Han Y, Cao R, Liu Y, et al. Water table decline alters arthropod community structure by shifting plant communities and leaf nutrients in a Tibetan peatland. Sci Tot Environ. 2022;814:151944.
- Moore JC, de Ruiter PC. Temporal and spatial heterogeneity of trophic interactions within below-ground food webs. Agri Ecosyst Environ. 1991;34(1-4):371-397.
- Weilhoefer CL, Williams D, Nguyen I, Jakstis K, Fischer C. The effects of reed canary grass (Phalaris arundinacea L) on wetland habitat and arthropod community composition in an urban freshwater wetland. Wetland Ecol Manag. 2017;25:159-175.
- Lonard RI, Judd FW, DeYoe HR, Stalter R. Biology and ecology of the halophyte Laguncularia racemosa (L.) Gaertn. f: A review. Handbook Halophyte. 2020:1-6.
- Krab EJ, Berg MP, Aerts R, van Logtestijn RS, Cornelissen JH. Vascular plant litter input in subarctic peat bogs changes Collembola diets and decomposition patterns. Soil Biol Biochemist. 2013;63:106-115.
- Alegre J, Alonso-Blázquez N, De Andrés EF, Tenorio JL, Ayerbe L. Revegetation and reclamation of soils using wild leguminous shrubs in cold semiarid Mediterranean conditions: Litterfall and carbon and nitrogen returns under two aridity regimes. Plant Soil. 2004;263:203-212.
Citation: Gloria OR, Elix HF, Solimar PP, Elvira C (2024) Effects of Plant Functional Types Substrates on Soil Arthropod Community in Coastal Urban Wetlands. J Coast Zone Manag. 27:620.
Copyright: © 2024 Gloria OR et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
