PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 10
Effects of Mesorhizobium ciceri and Biochar on the Growth, Nodulation and Antifungal Activity Against Root Pathogenic Fungi in Chickpea (Cicer arietinum L.)
Muhammad Shah Jahan1, Umbreen Shahzad2, Summar Abbas Naqvi3, Ibrahim Tahir2, Tahira Abbas2, Mudassar Iqbal4 and Phoebe Nemenzo Calica5*2College of Agriculture, Bahauddin Zakariya University, Bahadur Sub-campus Layyah, Pakistan
3Institute of Horticultural Sciences, University of Agriculture Faisalabad, Pakistan
4Institute of Vegetable, Ayyub Agriculture Research Institute, Faisalabad, Pakistan
5Department of Environmental Sciences, Ateneo de Davao University, Davao City, Philippines
Received: 01-Sep-2020 Published: 23-Oct-2020, DOI: 10.35248/2157-7471.20.11.520
Abstract
This study was conducted to investigate the effects of the inoculation of Mesorhizobium ciceri on the nodulation, growth and antagonistic expression against soil-borne fungal pathogens (Phytophthora medicaginis, Fusarium oxysporum and Fusarium solani) on Cicer arietinum L. or commonly known as chickpea grown in vermiculite medium amended with 5% green waste (GW) biochar. The combination of M. ciceri and biochar showed significant effect to chickpea compared with other treatments and control plants in terms of nodulation. The chickpea inoculated with M. ciceri and amended with biochar produced the highest nodule number with an average value of 110 nodules per plant and with an average nodule fresh weight of 57.90 mg per plant at 60 days harvest. The other treatments (M. ciceri only and biochar only) and the positive control (2 mM nitrate-treated plants) produced an average of 55, 65 and 15 nodules per plant with the corresponding average nodule weight of 39.5, 46.5 and 35.6 mg per plant, respectively, 60 days after seed sowing. The combined M. ciceri and biochar also enhanced the shoot length, and fresh and dry weights of chickpea. However, it was observed that the primary root length was shorter than the control but clusters of feeder roots were observed. The combination of M. ciceri and biochar also completely inhibited the colony development of all root pathogenic fungi of chickpea after three days of inoculation. Therefore, the inoculation of M. ciceri in vermiculite medium amended with green waste biochar enhanced the nodulation and growth conditions in chickpea as well as inhibited the growth of root pathogenic fungi P. medicaginis, F. oxysporum and F. solani.
Keywords
Mesorhizobium cicero; Biochar; Chickpea; Nodulation; Antifungal activity
Introduction
Chickpea (Cicer arietinum) (2n=2x=16) is a self-pollinated, diploid, annual grain legume crop that belongs to the family Fabaceae [1]. It is the world’s second most widely grown legume [2]. Chickpea is one of the major pulses crop grown in Pakistan and occupies nearly 76 percent of the total pulses area in Punjab [3]. Pakistan is the fourth largest producer of chickpeas in the world producing 323, 364 tonnes as of 2018 [4]. Globally, chickpea production ranks third after beans with a mean annual production of over 11.5 million tons and the land area devoted to chickpea has increased in recent years and now stands at an estimated 14.56 million hectares [5]. Chickpea provides multi-functionality in terms of high protein content source of nutrition, animal feed, soil fertility and cash income [6]. The chickpea production paved way against malnutrition, food security and global livelihood generation [7].
The nutritional value of chickpea in terms of nutrition and body health has been recently emphasized frequently by nutritionist in health and food area in many countries around the world. It is a major source of high quality protein in human diet and also provides high quality crop residues for animal feed [8]. Chickpea also has estimated 60–65% carbohydrates, 6% fat and is a good source of minerals and essential B vitamins [9]. Chickpea is a valued crop and provides nutritious food for an expanding world population and will become increasingly important with climate change.
Chickpeas are a cost-effective alternative for the animal protein in improving the diets of the poor in South-East Asia and Africa. They can fix their own nitrogen (140 kg N per hectare) from the atmosphere through symbiotic nitrogen fixation, which meets 80% of its nitrogen (N) requirement and partially benefit the following crops of the system by enriching soil [10]. The chickpea leaves contain a substantial amount of residual N for subsequent crops and adds plenty of organic matter to maintain and improve soil health [11].
Chickpeas and other grain legumes are frequently subjected to both abiotic and biotic stresses resulting in severe yield losses. Despite its high production potential, fungal root diseases are the major bottlenecks in chickpea production. The main root pathogenic fungi in chickpea include Fusarium oxysporum, Fusarium solani, and Phytophthora. Due to prolonged nature of survival of the pathogens, cultural control such as crop-rotation is not feasible and chemical control is not only costly but it also imposes serious implications to the environment. Misk and Franco [12] studied the antimicrobial activity of M. ciceri against Phytophthora medicaginis in a greenhouse experiment to control Phytophthora root rot on chickpea. Inoculation with M. ciceri enhanced vegetative growth of root and shoot dry weights and increased root and shoot dry weights of chickpea. Unlike our study, Misk and Franco [12] did not use biochar (M. ciceri only).
Global yields of legumes have been stagnant for the past five decades in spite of adopting various conventional and molecular breeding approaches [13]. Furthermore, the increasing costs and negative effects of pesticides and fertilizers for crop production necessitate the use of biological options of crop production and protection. The use of biofertilizers such as plant growthpromoting rhizobacteria (PGPR) for improving soil and plant health has become one of the attractive strategies for developing sustainable agricultural systems due to their eco-friendliness, low production cost and minimizing consumption of non-renewable resources. Beneficial microorganisms can be used effectively in enhancing the yield and controlling the pests and pathogens of chickpeas [13]. This study aimed to improve the growth of chickpea using beneficial microorganisms such as Mesorhizobium ciceri and soil amendment (biochar).
Beneficial microorganisms are already established to fix atmospheric N by symbiotic association with legumes [14,15]. Mesorhizobium ciceri has already been proven to increase the nodulation and enhance the yield in chickpea crops in non-control conditions and exposed to all kinds of biotic and abiotic stress factors [16]. Pandey et al. [17] analyzed M. ciceri for their multiple plant growth promoting traits, resistance to various environmental stresses such as temperature, pH and salt and tested them individually for growth and yield of chickpea. M. ciceri isolates exhibited siderophore production, solubilized the inorganic phosphate and zinc, produced ammonia, HCN and IAA and were found able to tolerate environmental stresses. M. ciceri may be an effective bioinoculant for the growth and yield enhancement of chickpea.
Biochar is a good biofertilizer, biopesticide and is a good carrier material for rhizobacteria. Biochar can be used in the disease suppression against root rot caused by pathogenic fungi such as F. oxysporum in asparagus [18], Rhizochtonia solani in cucumber, and Phytophthora sp. in red oak [19]. Bean crops were also protected from root pathogenic fungi through the use of biochar [20]. Biochar can also protect soil from root pathogenic fungi because it may persist for thousands of years in the soil unlike other peat and lignite which may only survive for several years [21]. Biochar helps microbes by changing soil ph, increasing cation exchange capacity (CEC), increasing water holding capacity, more aeration in soil, enhancing mycorrhizal competence colonization and supply nutrients onto the soil which increase soil fertility and crop productivity [22,23].
Considering the widespread advocacy to reduce the use of chemical pesticides and inorganic fertilizers in agriculture, this study is deemed significant especially in promoting the use of M. ciceri and biochar combination for successful chickpea production. This study may not be a pioneering work but it presented and discussed a new perspective about biofertilizers and other previous concepts. The old methods were modified to make them applicable in this study. Therefore, this research paper is different from related literature because this is a report of a study written by the authors who actually did the study, the hypothesis or research question was described, the purpose of the study was clearly stated, the detailed research methods were given, the results of the research were interpreted and reported, the possible implications were described, an old theory was challenged with evidence and lastly, a theory in a new context was formulated.
Materials and Methods
Biochar and plant growth medium
Biochar which was prepared from green wastes (GW) at a high treatment temperature of 450°C in pyrolysis system, were used throughout the research. Green waste was prepared from citrus wood. The pyrolysis was done in a traditional charcoal pit (lump charcoal), and the pyrolysed biochar was ground into a powder of less than 0.05 cm particles and stored in a sealed metal box until use. The physical and chemical characteristics of the biochar were similar to the study of Graber et al. [24]. Ash content of the biochar was 10.9%. The only definable mineral phases of the biochar were quartz and calcite, both at low, non-quantifiable levels. The surface area of the biochar, was 46.2 m2/g. Accounting for ash content, the elemental composition of the biochar was found to be 70.6% C, 0.6% N, 2.3% H, and 15.5% O, giving an O/C atomic ratio of 0.16, H/C ratio of 0.40, C/N ratio of 130.69, and an H/O ratio of 2.41. The extracts was nearly neutral, EC was lower than the EC of the fertigation solution (2.2 mS/m), and of the essential macronutrients (NPK), only K existed in substantial quantities (10%). By weight, only 50% of the biochar was digested in the hot concentrated HNO3; Ca and Fe were present in the greatest concentrations (1.8 and 1.1%, respectively). Potassium, Na, and S were the only elements released from the biochar to the aqueous solutions in relatively large amounts as compared with their amount in the HNO3 digest, and were likely present in the biochar as readily soluble salts. The extracts of the biochar also contained a number of identifiable organic compounds belonging to alkanoic acids, hydroxy and acetoxy acids, benzoic acids, diols, triols, and phenols.
The autoclaved pots (22.5 cm top diameter, 16.5 cm bottom diameter and 18 cm height) and grade 2 vermiculite (2-4 mm) were used in sterile growth conditions. Grade 2 vermiculite is an expanded vermiculite (granular) loose fill with a medium particle size and a finer grain. It is suitable for packing hazardous liquids and has great absorption, creating a tight fit around containers during storage and shipping. As a natural mica mineral, it works as a soil additive to provide aeration and water retention for the horticulture purposes. The 5% GW biochar was mixed with sterile vermiculite for biochar treatment experiment.
Plant growth and culture
In all experiments conducted, wild type chickpea (C. arietinum L.) was used. Seeds were surface-sterilized using 70% (v ⁄v) ethanol for 10 seconds followed by rinsing five times with sterile water, then were sown in sterile vermiculite in 5 L pots. Plants were grown in controlled glasshouse conditions (28°C and 24°C, day and night, respectively, with a 16-h day length). Plants were watered daily and supplemented with a B & D nutrient solution [25] twice per week. The B & D nutrient solution was composed of the following elements: Ca (in the form of 1000 μM of CaCl2·2H2O), P (500 μM of KH2PO4), Fe (10 μ M of Fe-Citrate), Mg (250 μ M of MgSO4·7H2O), K (1500 μM of K2SO4), S (500 μM), Mn (1 μM of MnSO4·H2O), B (2 μM of H3BO4), Zn (0.5 μM of ZnSO4·7H2O), Cu (0.2 μM of CuSO4·5H2O), Co (0.1 μM of CoSO4·7H2O) and Mo (0.1 μM of Na2MoO4·2H2O). The volume of B & D nutrient solution per pot was 150 ml.
Pots were filled with 4L of vermiculite. The weight of dry vermiculite was 0.075 kg per liter. The vermiculite was placed in autoclavable plastic bags and autoclaved at 121°C for 20 minutes under 15 psi of pressure. The vermiculite was purchased from a garden shop in Pakistan.
Growth conditions of M. ciceri and chickpea fungal pathogens
M. ciceri (isolated from the chickpea experimental area at the Department of Primary Industries, New South Wales, Australia) was grown for 48 h at 28°C in Yeast Mannitol Broth [26]. The isolated strain was confirmed by 16s rDNA sequence. Cultures were diluted with water to a final concentration of OD600=0.01 prior to inoculating plants. Approximately 150 ml of this final concentration was applied per pot. Chickpea pathogens P. medicaginis, F. oxysporum and F. solani (isolated from chickpea growing areas in New South Wales and Queensland, Australia) were cultured on Potato Dextrose Broth at 25°C with shaking for 150 rpm for 15 days.
Effect of M. ciceri and biochar on growth of chickpea
There were three (M. ciceri, biochar and M. cicero + biochar) treatments, plus two positive (2 mM nitrate) and a negative (water) control. There were studies that showed mineral nitrogen such as nitrate can increase the leaf water content, membrane stability, chlorophyll, leaf water potential, leaf area, nodule water content, nodule number and biomass in chickpea [27,28]. All the chickpea seedlings were watered with nitratefree B & D nutrient solution except the positive control which was supplied with 2 mM potassium nitrate twice a week. M. ciceri was inoculated at the third day after germination. M. ciceri was grown for 48 h at 28°C in Yeast Mannitol Broth [26]. Cultures were diluted with water to a final concentration of OD600=0.01 prior to inoculating plants. Approximately 150 ml of the final concentration (107 CFU/ml) was applied per pot. The experimental design used was completely randomized design (CRD). In each pot, four plants were grown and each treatment has three replicates. The experimental set-up has a total of 60 plants. There were 12 plants (4 plants per pot × 3 pots) per treatment or control. The pots used had the dimensions of 22.5 cm top diameter, 16.5 cm bottom diameter and 18 cm height and the volume was 5 liters. The weight of vermiculite per pot was 0.3 kg.
Plants were harvested after 20, 40 and 60 days after seed sowing and the following growth parameters were measured for each plant: fresh weight, dry weight, primary root length, shoot length, number of nodules and weight of nodule. The data were statistically analyzed using the analysis of variance (one way ANOVA) for independent samples using SPSS (SPSS Inc., Chicago, IL, USA). Standard errors (SEs) of the means were also calculated and diagrams were made using Excel (Microsoft 2010).
Antifungal effects of M. ciceri and biochar on pathogenic fungi
Chickpea pathogens P. medicaginis, F. oxysporum and F. solani were inoculated onto sterile vermiculite while the control was left uninoculated. Potato Dextrose Broth (PDB) (150 ml) in a 500 ml- Erlenmeyer flask was autoclaved for 20 min at 120°C. The PDB was inoculated with a fungal suspension to a final concentration of 106 conidia/ml. The suspension was incubated for 3 to 4 days on a rotary shaker at 150-170 rpm and 28 ± 1°C. The culture was filtered through three autoclaved milk filter discs into a 500 ml centrifuge bottle. The fungi were precipitated by centrifugation, at 10 xg for 20-30 min at 10 ± 4°C and washed twice by resuspending in sterile distilled water and centrifuging. The fungi were resuspended in 2-3 ml water. The suspensions were prepared in autoclaved distilled water for inoculation. The inoculum was kept chilled at 4°C until use on the same day.
The 15-day old chickpea seedlings were uprooted from vermiculite growing medium. The root tips were trimmed off. The roots were immersed into the fungal suspension 106 conidia/ml, water plus spores) for 2 min. The inoculated seedlings were then transplanted into an individual pot containing sterile vermiculite watered with sterile distilled water. A 10 ml fungal suspension (106 conidia/ ml) was then applied per pot. After four weeks, the plants were uprooted gently from the pots under a running water to remove the vermiculite and observed the roots.
Biochar, M. ciceri, and combination of M. ciceri + biochar treatments were tested for antagonistic effect against root pathogenic fungi of chickpea in vivo. Disease severity was observed. First symptoms (leaf yellowing and wilting) started appearing at about 14 days post inoculation. Plants of susceptible accessions were usually dead 4-5 weeks after inoculation. The proportion of wilted leaves was computed and compared among the treatments and control. Disease was evaluated on a severity scale based from Jimenez-Díaz et al. [29] depending on the percentage of affected leaves (0=0%, 1=1-33%, 2=34-66%, 3=67-100%, 4=dead plant) at 14 to 60 days after sowing [29].
The antagonistic effect of treatments against fungal pathogens in vitro was determined through poisoned food technique method [30]. In this method, the different treatments tested for antifungal property were mixed with PDA medium before pouring. Completely Randomized Design (CRD) was used for the experiment, with replications. Uniform inoculum size of pathogen were placed in the center of petri plates having “poison” PDA. One treatment were kept as control, to assess the efficacy of different treatments. The petri plates were kept at 25°C in incubator, for mycelial growth. Data were recorded by measuring colony diameter (cm) after five and ten days of incubation. All the recorded data were then statistically analysed.
For this study, the different treatments (biochar, M. ciceri and M. ciceri + Biochar) were mixed with PDA before dispensing into the petri plates. A 90 ml of double-strength PDA was poured into a sterile 250 ml Erlenmeyer flasks and maintained at 55°C in a water bath. The required volume of treatments was added to the sterile distilled water to obtain 90 ml aliquots, each double the concentration to be tested. Each of these was then added to one of the flasks of double-strength PDA maintained at 55°C, thus providing the required test concentration. After the agar and the treatments had been mixed together, approximately 15 ml was poured into each petri plate. The plates were allowed to cool before inoculating the center with a 4 mm diameter mycelial plug obtained from the periphery of growing fungal colonies (12-day old). The plates were incubated in room temperature. Each treatment was replicated four times. The control plates did not contain any kind of treatment to compare the colony growth of pathogen with those which contained the treatments. Data were taken after five days and the mycelial growth diameter was measured. The data were statically analyzed by using analysis of variance (ANOVA) and Least Significant Difference test to check the significant relationship between the different treatments and the control.
Results and Discussion
Effects of M. ciceri and biochar on plant growth
There were significant differences among the treatments, positive control (Nitrate-treated plants) and negative control (sterile water) in terms of biomass production. As shown in Figure 1, the M. cicero + biochar combination produced the highest fresh weight of chickpea. The statistical analysis proved that the treatments have significant differences in fresh weight from the control after 60 days (last harvest). The negative control and biochar alone have significant difference in fresh weight which was 12.71 g and 24.27g, respectively. The biochar only and positive control (nitrate) have no significant difference with each other but significantly different from the negative control. The M. ciceri inoculation alone did not significantly increase the fresh weight of chickpea and has no significant difference with the control plants. These results showed that plants treated with M. cicero + biochar gave the heaviest fresh weight (35 g, p<0.05) in chickpea, followed by the positive control (nitrate; 32 g, p<0.05) and biochar only (24.27 g, p<0.05), and then lastly, the M. ciceri only (21 g, p<0.05) and negative control (12.71 g, p<0.05). Hence, there is a synergistic effect M. ciceri + biochar to enhance the fresh weight in chickpea.
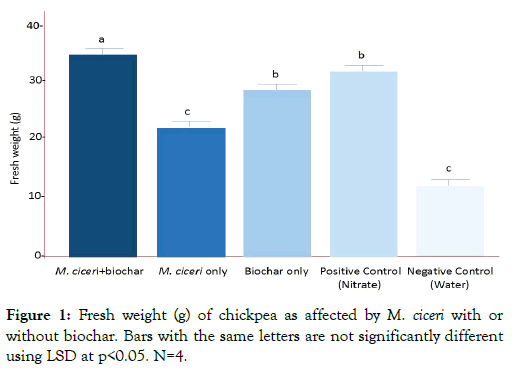
Figure 1: Fresh weight (g) of chickpea as affected by M. ciceri with or without biochar. Bars with the same letters are not significantly different using LSD at p<0.05. N=4.
As shown in Figure 2, the combination of M. ciceri + biochar produced the highest dry weight in chickpea compared with the other treatments and control plants. After 60 days, all treatments were significantly (p<0.05) different among each other. M. ciceri + biochar has the highest dry weight of 6.48 g while the positive control (nitrate) has a dry weight of 3.92g. The other treatments, M. ciceri only and biochar only, produced 3.59 g and 4.49 g, respectively. The treatments were all significantly different from the negative control plants with an average dry weight of 2.10 g. Hence, the best treatment to increase the dry weight of chickpea is the combination of M. ciceri + biochar while M. ciceri only, biochar only and nitrate have no effect on the dry weight of chickpea.

Figure 2: Dry weight (g) of chickpea as affected by M. ciceri with or without biochar. Bars with the same letters are not significantly different using LSD at p<0.05.
The effect of different treatments to the shoot length of chickpea was shown in Figure 3 while the effect on the root length was shown in Figure 4. For shoot length, M. ciceri + biochar produced the highest shoot length (81 cm, p<0.05) which was significantly different from the negative control (54 cm, p<0.05). However, this was not the case for root length in which the M. ciceri + biochar produced shorter roots (38 cm, p<0.05) compared with the negative control (42 cm, p<0.05). This was attributed to the formation of clusters of feeder roots. The presence of feeder roots was advantageous to chickpea even if it has shorter primary roots. The major function of feeder roots is the absorption of water and minerals. Hence, the results implied that the best treatment to increase shoot length in chickpea was M. ciceri + biochar (81 cm, p<0.05), followed by the positive control (nitrate; 70 cm, p<0.05), biochar only (67 cm, p<0.05), M. ciceri only (61 cm, p<0.05) and lastly the negative control (54 cm, p<0.05). On the other hand, M. ciceri + biochar was not shown to increase the root length but the roots developed clusters of feeder roots which are beneficial for chickpea.
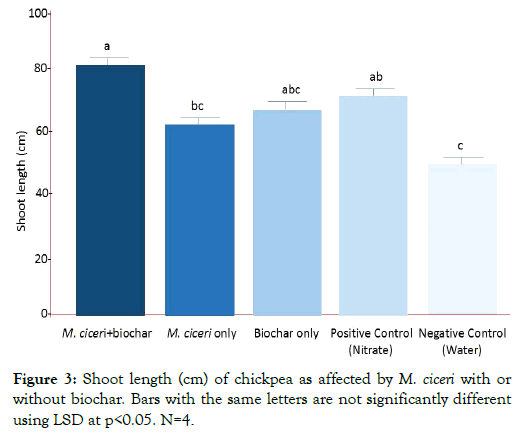
Figure 3: Shoot length (cm) of chickpea as affected by M. ciceri with or without biochar. Bars with the same letters are not significantly different using LSD at p<0.05. N=4.
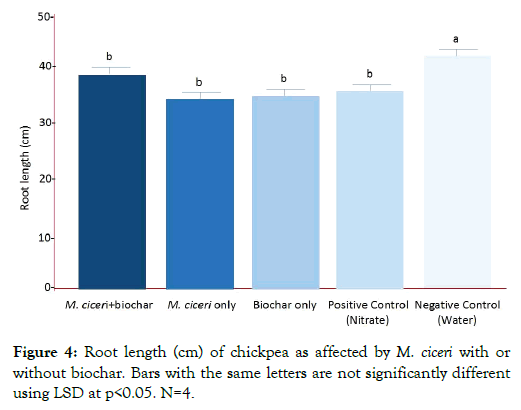
Figure 4: Root length (cm) of chickpea as affected by M. ciceri with or without biochar. Bars with the same letters are not significantly different using LSD at p<0.05. N=4.
Effects of M. ciceri and biochar on nodulation
Nodules are the site of symbiotic nitrogen fixation in legumes. Therefore, the higher the nodule number produced by chickpea, the more efficient is its nitrogen fixation activity. After 60 days from sowing (as shown in Figures 5 and 6), there were several mature nodules observed in chickpea plants treated with M. ciceri + biochar (110, p<0.05), followed by biochar only (65, p<0.05) and then M. ciceri only (55, p<0.05). Both positive and negative controls formed nodules that were immature and negligible. It was mainly due to cross-contamination (presumably air-borne) brought about by the prolong period of growing the chickpea in the experimental area. The effect of the different treatments on the nodule number of chickpea was the same for nodule weight. Chickpea plants treated with M. ciceri + biochar had the heaviest nodule fresh weight (57.90 mg, p<0.05), followed by biochar only (46.5 mg, p<0.05) and then M. ciceri only (39.5, p<0.05).
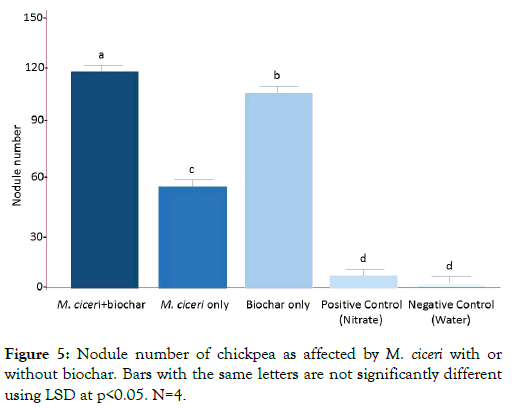
Figure 5: Nodule number of chickpea as affected by M. ciceri with or without biochar. Bars with the same letters are not significantly different using LSD at p<0.05. N=4.
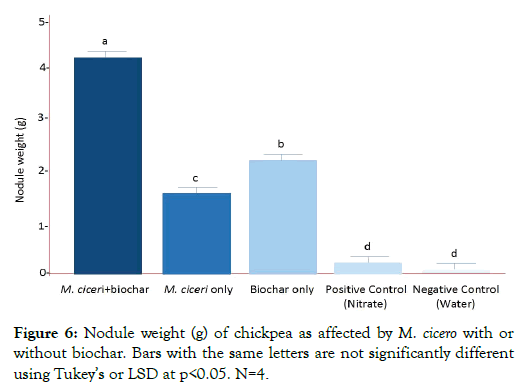
Figure 6: Nodule weight (g) of chickpea as affected by M. cicero with or without biochar. Bars with the same letters are not significantly different using Tukey’s or LSD at p<0.05. N=4.
As shown in Figure 7, the morphology of nodules was observed to be the same for all nodulated chickpea. The shape of the mature nodules was coralloid (Figures 7B-7E) while immature nodules were cylindrical (Figure 7 A). The size of the nodules was about 0.3-0.6 cm and the color was light brown. The cross-sections of the functional nodules (Figure 7F) were pinkish red which implied efficient nitrogen fixation activity inside the legumes. The pinkish red color of the internal tissues of the legumes was due to the presence of leghemoglobin. The formation of mature and functional nodules in chickpea was therefore improved by the combination of M. ciceri and biochar.
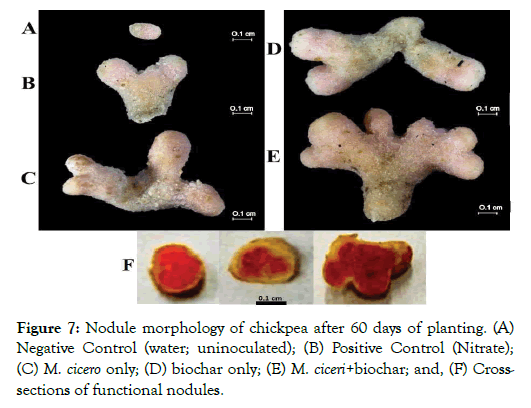
Figure 7: Nodule morphology of chickpea after 60 days of planting. (A) Negative Control (water; uninoculated); (B) Positive Control (Nitrate); (C) M. cicero only; (D) biochar only; (E) M. ciceri+ biochar; and, (F) Crosssections of functional nodules.
Antifungal effects of M. ciceri and biochar on pathogenic fungi
Biochar, M. ciceri, and the combination of M. ciceri + biochar treatments were tested for antagonistic effect against root pathogenic fungi of chickpea in vivo. Leaf yellowing and wilting started to appear at about 14 days post inoculation. Plants with biochar only and M. ciceri only were dead 4-5 weeks after inoculation while plants with M. ciceri + biochar survived. The proportion of wilted leaves for M. ciceri + biochar plants was lower than the other treatments.
The antagonistic effect of M. ciceri with and without biochar against F. oxysporum, F. solani and P. medicaginis was confirmed in vitro. The root pathogens of chickpea were completely inhibited by M. Ciceri + biochar after two to seven days. Control plates had overgrown pathogens within a week. Colony inhibition data were statistically analyzed as shown in Table 1.
| Treatment | Colony diameter (cm) | ||
|---|---|---|---|
| F. oxysporum | F. solani | Phytophthora spp. | |
| Biochar only | 1.43 b | 1.20 b | |
| M. ciceri only | 1.21 c | 1.18 b | 1.14 b |
| M. ciceri + biochar | 0.99 d | 0.86 c | 0.64 c |
| Control | 4.45 a | 3.98 a | 2.80 a |
Table 1: Colony diameter (cm) of F. oxysporum, F. solani and P. medicaginis after seven days of incubation.
The combination of M. ciceri and biochar was proven to significantly enhance the growth of chickpea by increasing its fresh weight, dry weight and shoot length. M. ciceri is a natural biofertilizer that improves the growth of chickpea and its beneficial effects can be further enhanced with biochar amendment (synergistic effect). Biochar is a good alternative for synthetic fertilizers which supports the growth of symbiotic microbial community in soil and in the rhizosphere [31]. It increases the relative abundance of rhizobacteria with antagonistic activity to be used as biocontrol [32]. In this study, biochar alone was not sufficient to enhance the growth of chickpea but when combined with M. ciceri, chickpea plants exhibited the best growth conditions.
Since worldwide agricultural food production has to double to feed the global increasing population while reducing dependency on conventional chemical fertilizers plus pesticides, it is therefore significant to use biofertilizers and soil amendments in food crop production. The combination of their potentials when fully harnessed under agricultural scenario will help to sustain agriculture and boost food security globally.
M. ciceri and biochar also improved chickpea nodulation by increasing the nodule number and nodule weight significantly. Nodules are the sites of symbiotic nitrogen fixation. Hence, an improved nodulation in chickpea may be an indication of efficient nitrogen fixation ability. Moreover, cross-sections of the chickpea nodules in this study showed reddish internal tissues which established the presence of leghemoglobin - an essential component for nitrogen fixation by legumes [33]. Leghemoglobin has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in colour. It was originally thought that the heme prosthetic group for plant leghemoglobin was provided by the bacterial symbiont within symbiotic root nodules. According to Singh & Varma [33], leghemoglobin is a hemeprotein found in micromolar concentrations in infected cells of legume roots. This leg-hemoglobin is produced as a result of symbiotic association between bacteroid and plant. The major role of leghemoglobin involves protection of nitrogenase enzyme from denaturation, if exposed to atmospheric concentration of oxygen, but at the same time supply of ample amount of oxygen to bacteria for respiration. The synthesis of leghemoglobin starts shortly after nodule initiation and before nitrogenase synthesis. Hence, leghemoglobin is very important for the symbiotic nitrogen fixation in chickpeas. The enhanced nodulation and the presence of leghemoglobin in chickpea may imply that chickpea production does not require the application of expensive synthetic chemicalbased nitrogen fertilizers to improve growth because chickpea can fix its own nitrogen through symbiotic nitrogen fixation.
This study used vermiculite medium as inert material for growing the chickpea plants. Vermiculite is commonly used in nodulation studies for legumes [14]. The benefits of using vermiculite as growing media includes better aeration, better water holding capacity and better absorption of nutrients as described by Indrasumunar and Gresshoff [34]. Vermiculite is a suitable medium to study the effect of different treatments on nodulation development in legumes. Nascimento et al. [16] also cited the use of vermiculite medium to grow the chickpea plants inoculated with M. ciceri. The study showed a 127% increase in the nodule number and 125% increase in the biomass. In this study, the nodule number was increased by 100% while the biomass was increased by 68%.
The combination of M. ciceri and biochar was proven to have antagonistic effect against F. oxysporium, F. solani and P. medicaginis. Beneficial microorganisms can promote the growth of plants not only by improving their growth conditions but also suppressing the pathogens [35]. Elicitation of defense related enzymes like L-phenylalanine ammonia lyase, peroxidase and polyphenol oxidase was observed to be higher in M. ciceri treated plants as compared to uninoculated plants under pathogen challenged soil [36]. Meanwhile, Yao et al. [37] emphasized the long-term effects of biochar as a soil amendment including its effects on fungal community composition. The relative abundances of several potential crop pathogens such as Fusarium decreased with biochar addition, suggesting that biochar amendment may be beneficial in terms of suppressing the occurrence of crop disease over the long term.
Conclusion and Recommendations
This study has established the synergistic effect of M. ciceri and biochar in the improved production of chickpea. Chickpea production can be improved by the application of M. ciceri with biochar amendment which was established to enhance the growth conditions by increasing the biomass and improve the nodulation by increasing the nodule number and nodule weight as well as exhibit antagonistic activity against fungal root pathogens such as P. medicaginis, F. oxysporum and F. solani. The synergistic effect of biofertilizers and soil amendments should be used widely in agriculture because they promote the growth of plants without harming our environment.
For future research, it is recommended to pursue studies that will determine the success rate of M. ciceri application and biochar amendment in field conditions. It is also recommended to conduct further in-vivo experiments to establish the mechanism of the antifungal property of M. ciceri and biochar. Lastly, comparative assessment of symbiotic nitrogen fixation in chickpea inoculated with M. ciceri and grown in bochar-amended soils must be done.
Acknowledgments
The researchers would like to thank the following collaborators: Dr. Jonathan M. Plett (Assistant Professor at Western Sydney University) in Australia for the provision of M. ciceri pure culture; Dr. Kevin Moor of the Department of Primary Industry, Tamworth Agricultural Institute, New South Wales, Australia for providing the P. medicaginis pure culture; and the Queensland Plant Pathology Herbarium, Brisbane, Australia for providing the Fusarium isolates.
REFERENCES
- Chandora R, Shekhawat N, Malhotra N. Chickpea genetic resources: collection, conservation, characterization, and maintenance. In: Chickpea: Crop Wild Relatives for Enhancing Genetic Gains. Academic Press. 2020;37-61.
- Sinha R, Gupta A, Senthil-Kumar M. Concurrent drought stress and vascular pathogen infection induce common and distinct transcriptomic responses in chickpea. Front Plant Sci. 2017;8:333.
- Mushtaq K, Ali A, Ghafoor A, Hussain M, Hameed S. Evaluating The efficiency of chickpea markets in punjab, pakistan. Pak J Agri Sci. 2020;57(2):585-590.
- Food and Agriculture Organization (FAO). FAOSTAT. 2020.
- Merga B, Haji J. Economic importance of chickpea: Production, value, and world trade. Cogent Food & Agriculture. 2019;5(1):1615-1718.
- Verkaart S, Munyua BG, Mausch K, Michler JD. Welfare impacts of improved chickpea adoption: A pathway for rural development in Ethiopia? Food Policy. 2017;66:50-61.
- Wani SP, Garg KK, Chander G, Anantha KH. Improving water use in tropical rain-fed systems: the situation in India Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India.
- Bar-El Dadon S, Abbo S, Reifen R. Leveraging traditional crops for better nutrition and health-The case of chickpea. Trend Food Sci Technol. 2017;64:39-47.
- Muehlbauer FJ, Sarker A. Economic importance of chickpea: production, value, and world trade. In The chickpea genome. Springer, Cham, UK. 2017;5-12.
- Gogoi N, Baruah KK, Meena RS. Grain legumes: impact on soil health and agroecosystem. In Legumes for Soil Health and Sustainable Management. Springer, Singapore. 2018;511-539.
- Panchal P, Patel PH, Patel AG, Desai A. Effect of Panchagavya on growth, yield and economics of chickpea (Cicer arietinum). Int J Chem Stud. 2017;5(2):265-267.
- Misk A, Franco C. Biocontrol of chickpea root rot using endophytic actinobacteria. Bio Control. 2011;56(5):811-22.
- Sathya A, Vijayabharathi R, Gopalakrishnan S. Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech. 2017;7(2):1-10.
- Nemenzo-Calica P, Indrasumunar A, Scott P, Dart P, Gresshoff PM. Nodulation and symbiotic nitrogen fixation in the biofuel legume tree Pongamia pinnata. Atlas J Biol. 2016:274-291.
- Calica PN. Nodulation and nitrogen fixation of Pongamia pinnata. J Trop Crop Sci. 2017;4(1):1-10.
- Nascimento F, Brígido C, Alho L, Glick BR, Oliveira S. Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant soil. 2012;353:221-230.
- Pandey RP, Srivastava AK, Gupta VK, O’Donovan A, Ramteke PW. Enhanced yield of diverse varieties of chickpea (Cicer arietinum L.) by different isolates of Mesorhizobium ciceri. Environ Sustain. 2018;1(4):425-435.
- Elmer WH, Pignatello JJ. Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. Plant Dis. 2011;5(8):960-966.
- Zwart DC, Kim SH. Biochar amendment increases resistance to stem lesions caused by Phytophthora spp. in tree seedlings. Hort Sci. 2012;47(12):1736-1740.
- Jaiswal AK, Elad Y, Graber ER, Frenkel O. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol Biochem. 2014;69:110-118.
- Zimmerman AR. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol. 2010;44(4):1295-1301.
- Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant soil. 2010;337:1-8.
- Hossain MK, Strezov V, Chan KY, Ziolkowski A, Nelson PF. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J Environ Manag. 2011;92(1):223-228.
- Graber ER, Elad Y. Biochar impact on plant resistance to disease. CRC Press, Boca Raton, FL, USA, 2013.
- Broughton WJ, Dilworth MJ. Control of leghaemoglobin synthesis in snake beans. Biochem J. 1971;125(4):1075-1080.
- Somerville JE, Kahn ML. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J Bacteriol. 1983;156(1):168-176.
- Bahavar N, Ebadi A, Tobeh A, Jamaati-E-Somarin S. Effects of mineral nitrogen on water use efficiency of chickpea (Cicer arietinum L.) under water deficit condition. Res J Environ Sci. 2009;3(3):332-338.
- Namvar A, Sharifi R, Khandan T, Moghadam M. Organic and inorganic nitrogen fertilization effects on some physiological and agronomical traits of chickpea (Cicer arietinum L.) in irrigated condition. J Cent Eur Agri. 2013.
- Jiménez-Díaz RM, Castillo P, Del Mar Jiménez-Gasco M, Landa BB, Navas-Cortés JA. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Protect. 2015;73:16-27.
- Grove RK, Moore JD. Toximetric studies of fungicides against brown rot organism Sclerotina fruticola. Phytopathol. 1962;52:876-880..
- Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. Biochar effects on soil biota–A review. Soil Biol Biochem. 2011;43(9):1812-1836..
- Kolton M, Harel YM, Pasternak Z, Graber ER, Elad Y, Cytryn E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl Environ Microbiol. 2011;77(14):4924-30..
- Singh S, Varma A. Structure, function, and estimation of leghemoglobin. In Rhizobium Biology and Biotechnology. Springer, Cham. 2017;309-330.
- Indrasumunar A, Gresshoff PM. Vermiculite’s strong buffer capacity renders it unsuitable for studies of acidity on soybean (Glycine max L.) nodulation and growth. BMC research notes. 2013;6(1):1-8..
- Nemenzo PS, Rivero GC, Rivera WL. Characterization of potential plant growth-promoting rhizobacterial isolates from sago (Metroxylon sagu Rottb.) palms. The Philippine Agricultural Scientist. 2012;95(1).
- Das K, Rajawat MV, Saxena AK, Prasanna R. Development of Mesorhizobium ciceri based biofilms and analyses of their antifungal and plant growth promoting activity in chickpea challenged by Fusarium wilt. Indian J Microbiol. 2017;57(1):48-59.
- Yao Q, Liu J, Yu Z, Li Y, Jin J, Liu X, et al. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol Biochem. 2017;110:56-67.
Citation: Jahan MS, Shazad U, Naqvi SA, Tahir I, Abbas T, Iqbal M, et al. (2020) Effects of Mesorhizobium ciceri and Biochar on the Growth, Nodulation and Antifungal Activity Against Root Pathogenic Fungi in Chickpea (Cicer arietinum L.). J Plant Pathol Microbiol 11:520. doi: 10.35248/2157-7471.20.11.520.
Copyright: © 2020 Jahan MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

