Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 10, Issue 11
Effects of Dietary Zinc Amino Acid Complex and Zinc Sulfate on Growth Performance, Digestive Enzyme Activity and Immune Response in Asian Seabass (Lates calcarifer)
Kanokwan Sansuwan1, El-Orapint Jintasataporn2* and Srinoy Chumkam22Faculty of Agricultural Technology, Valaya Alongkorn Rajabhat University, Thailand
Received: 04-Jul-2019 Published: 30-Aug-2019, DOI: 10.35248/2155-9546.19.10.572
Abstract
Zinc is an essential trace mineral to fish and vital to various biological processes and function. The artificial diets offered to intensively cultivated fish must possess the zinc content required by the animal metabolism for health maintenance and high weight gain. However, essential elements must also be in an available form to be utilized by the organism. Thus, this study was designed to evaluate the effects of different zinc forms, including organic zinc (zinc amino acid complex) and inorganic zinc (zinc sulfate) as feed additives in diets on in vitro protein digestibility, growth performance, feed utilization, digestive enzyme activity, immune response and muscle quality of Asian seabass (Lates calcarifer). The study was assigned in CRD with 3 treatments and 3 replicates. Three groups of fish with mean weight 22.54 ± 0.80 g, were given a basal diet either unsupplemented (control) or supplemented with 50 mg Zn kg-1, as zinc sulfate (ZnSO4) or zinc amino acid complex (ZnAA). The fish were fed experimental diets 3.0% of their body weight per day, twice daily at 08.00 and 16.00 h, for 10 weeks. At the end of the experiment, no significant differences were observed on protein digestibility, survival, growth performance and feed utilization across the three dietary treatments (P>0.05). Fish fed ZnSO4 diets exhibited a significant increase in the specific activities of total protease, pepsin and trypsin compared with ZnAA and the control (P<0.05). Hematocrit, lysozyme and superoxide dismutase activities of fish fed ZnAA diets were significantly higher compared with all other groups (P<0.05). However, no significant differences were observed for muscle quality and whole body composition (P>0.05). The results of the present work allowed us to conclude that there was no difference in the growth between the two zinc sources but ZnAA supplementation exhibited a higher immunity response in Asian seabass.
Keywords
Asian seabass; Zinc amino acid complex; Zinc sulfate; Growth performance; Immunity
Introduction
Aquaculture is one of the largest food producing sectors next to agriculture. Asian seabass (Lates calcarifer) is a commercially important fish species for aquaculture in Thailand and Southeast Asia. It is a fast growing fish and can grow well in marine, brackish or freshwater [1]. It fetches a high market price due to its delicatelyflavoured white meat [2].
Minerals play an essential role in biological, physiological, and immunological responses of an organism [3]. Among the minerals, the trace element zinc (Zn) is an essential mineral required by fish. It promotes growth and plays a vital role in numerous cellular functions comprising cell proliferation, co-factor reproduction, immune function and guard against free radicals [4-8]. Signs of impaired growth, increased mortality, cataracts, short body dwarfism, and low tissue Zn may occur in fish fed a Zn-deficient diet [9]. However, the absorption of trace elements often limits their utilization. One of the factors that affect mineral absorption and utilization is their chemical form. Hence, mineral sources with higher bioavailability should be considered in feed formulation [10].
Organic minerals are important trace mineral sources because they protect trace elements from forming insoluble complexes in the digestive tract and facilitates transport across the intestinal mucosa [11]. It was confirmed that organic zinc had higher bioavailability than inorganic zinc in aquatic animals, such as abalone [12], shrimp [10], channel catfish [13], rainbow trout [14] and hybrid striped bass [15]. On the other hand, some other studies suggested that absorption from inorganic and organic zinc sources might vary depending on the species. Research with tilapia [16,17] and turbot [18] indicated no significant differences in the availability of Zn from either source. However, this has not been studied in Asian seabass. Thus, the current study was designed to evaluate the application of different zinc sources, including inorganic zinc (zinc sulfate) and organic zinc (zinc amino acid complex, ZnAA), as feed additives in diets for Asian seabass.
Materials and Methods
Preparation of the experimental diets
The basal diets compositions are shown in Table 1. The diets were formulated to be isonitrogenous (42% protein) and isolipidic (18% lipid). For zinc supplementation, 50 mg kg-1 of zinc was added to the basal diet either in the form of zinc sulfate (ZnSO4, Merck, Germany) or Availa® zinc (zinc amino acid complex, ZnAA Zinpro, USA). The ingredients were ground and sieved to less than 150 μm size before they were mixed thoroughly, pelleted using a farm extruder and dried at 60°C for 10 h. After drying, the diets were sieved into convenient pellet sizes and stored at 4°C until being used. The dietary Zn concentration in the basal diet was determined by inductively coupled plasma optical emission spectrophotometer (ICP-OES) to be 58.7 mg kg-1.
| Ingredients | % (Dry weight) |
|---|---|
| Fish meal | 15 |
| Poultry meal | 15 |
| Poultry liver hydrolysate | 5 |
| Wheat gluten | 10 |
| Corn gluten75 | 10 |
| Fermented soybean meal (ESP 500) | 8 |
| Krill meal | 10 |
| Wheat flour | 10 |
| Casava | 3.5 |
| Lecitin | 3 |
| Fish oil | 3.5 |
| Soya oil | 3 |
| Palm oil | 3 |
| Vitamin premix | 0.5 |
| Mineral premix | 0.5 |
Table 1: Composition of the basal diet.
Proximate chemical composition of diets
The chemical compositions of the experimental diets were analyzed for moisture, crude protein, crude lipid, crude ash and crude fiber, according to standard methods of AOAC [19]. Nitrogen free extract (NFE) was calculated from 100 – (crude protein + crude lipid + crude ash + crude fiber). The chemical compositions of the experimental diets are shown in Table 2.
| Chemical composition | Experimental diet | ||
|---|---|---|---|
| Control | ZnSO4 | ZnAA | |
| Moisture (% FW) | 8.87 | 8.96 | 9.17 |
| Crude protein (% DM) | 42.08 | 43.19 | 42.43 |
| Crude lipid (% DM) | 18 | 18.33 | 18.24 |
| Crude ash (% DM) | 8.14 | 8.32 | 7.94 |
| Crude fiber (% DM) | 1.87 | 1.58 | 1.63 |
| Nitrogen free extract (% DM) | 29.91 | 28.58 | 29.76 |
| FW: Fresh Weight; DM: Dry Matter | |||
Table 2: The proximate chemical composition of experimental diet for rearing Asian seabass. The values represent triplicate determination.
In vitro protein digestibility of diets
In vitro reaction of the experimental diets was performed according to the method described by Supalug et al. [20]. The protein digestibility was determined by measuring the reactive amino group by using the ninhydrin assay [21]. A solution 1.0 ml of undigested control (0 h) or the digested mixtures (24 h) were mixed thoroughly with 1.0 ml of cd-ninhydrin reagent. The mixture was incubated at 84°C for 5 minutes and rapidly cooled on ice. The supernatant was measured at 507 nm and the concentration of the reaction amino group was calculated by using tyrosine as the standard.
Fish preparation and feeding trial
The experiment was conducted at Aquatic Animal Nutrition Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University in Bangkok, Thailand. The juvenile Asian seabass, reared in 30 ppt seawater, were purchased from a local farm in Chachoengsao province of Thailand. They were acclimatized to freshwater by gradually lowering salinity by 3ppt per day with the addition of freshwater to the rearing tanks. During the acclimatization period, the fish were fed to satiation using commercial pellets for marine carnivorous fish (Profeed 906; Thai Union Feedmill Co., Ltd., Samut Sakhon, Thailand). Subsequently, the fish of 22.54 ± 0.80 g initial body weight was randomly distributed into 9 aquaria (50 cm width × 120 cm length × 36 cm height) each containing 200 L freshwater, at a stocking density of 10 fish per aquarium. The experiment was conducted for 10 weeks, under 12-h light/12-h dark cycle. The fish were fed experimental diets 3.0% of their body weight per day, twice daily at 08.00 and 16.00h. The amount of feed consumed in each tank was recorded daily.
During the experimental period, feces and uneaten feed were removed to maintain water quality every day. The rearing water was exchanged by 70-80% daily. Water temperature, pH and dissolved oxygen were recorded daily. Ammonia and nitrite were recorded once a week by standard methods of APHA, AWWA and WPCF [22]. The water temperature varied within 27-29°C, pH within 7.5-7.7, and dissolved oxygen was more than 6.0 mgL-1. The mean values for total ammonia and nitrite were 0.1-0.2 mgL-1 and 0.01– 0.03 mgL-1, respectively
Sampling and analytical methods
All the fish were individually weighed at the beginning of the experiment and every 2 weeks thereafter. At the end of the feeding experiment, the fish were starved for 24 h before weighting to reduce handling stress and allow for digestion track evacuation, and their body weight and total length were measured. Two fish per tank were randomly selected for the determination of whole body composition. Three fish from each replicate were anesthetized with clove oil and blood was collected from the caudal vein using a syringe with EDTA as an anticoagulant. For serum, another three fish from each replicate were anesthetized and blood was collected without anticoagulant and left to clot. Then the clotted blood was centrifuged at 4000 × g for 10min to obtain serum. The fish were then dissected to obtain samples of digestive tract and muscle.
Measurement of growth performance and feed utilization
At the end of the feeding trial, the following indices were calculated as the follows:
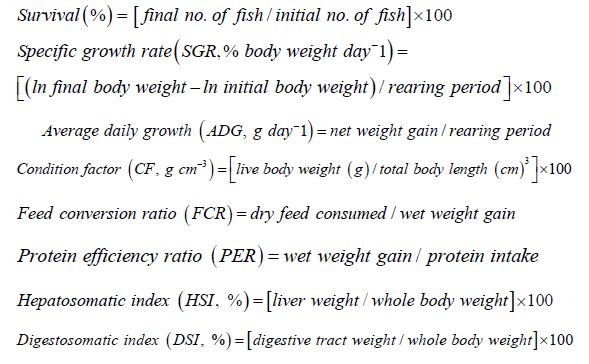
Digestive enzymes
Digestive enzyme extraction and protein quantification: Fish stomach and whole digestive tract were homogenized on ice in 0.2 M phosphate buffer pH 8 (1:3 w/v) using a micro-homogenizer (THP-220, OMNI International, USA). The homogenate was centrifuged at 15000 × g for 15 min at 4°C and then the supernatant was collected and kept at -20°C until use for determination of digestive enzymes. The protein concentration of a crude enzyme extract was compared to a standard curve of bovine serum albumin (BSA), according to the standard method of Lowry et al. [23].
Digestive enzyme assays: Stomach extract was only used for assaying pepsin activity (EC 3.4.23.1) while whole digestive tract was used for activities of total protease (EC 3.4.-.-), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1). The activity of pepsin was determined based on the method of Worthington [24], using hemoglobin as the substrate. The total protease was determined using azocasein as substrate based on Areekijseree et al. [25]. Activities of trypsin and chymotrypsin (EC 3.4.21.1) were determined according to the method of Rungruangsak-Torrissen et al. [26], using 1.25 mM N-α-benzoyl-L-arginine-p-nitroanilide (BAPNA) and 0.1 mM N-succinyl-ala-ala-pro-phe-p-nitroanilide (SAPNA) as the substrates, respectively.
Immunological assays
Hematological studies: The blood samples were used to analyze red blood cells (RBC), white blood cells (WBC), hemoglobin and hematocrit. RBC and WBC were counted with a hemacytometer under the light microscope. The estimation of hemoglobin was done according to the method of Drabkin and Austin [27]. Hematocrit was measured using hematocrit capillary tubes spun in Gemmy model KHT-410E Hematocrit Centrifuge. Total serum protein was measured following the method of Lowry et al. [23], using BSA as the standard protein. Albumin was quantified using bromocresol green binding method by Doumas et al. [28].
Lysozyme activity assay: Serum lysozyme activity was modified by turbidity method as described by Shugar [29]. 250 μL of Micrococcus lysodeikticuc suspension (0.2 mg mL-1 of the bacterium in 50 mM potassium phosphate buffer, pH 6.2) was added to 10 μL of fish serum in 96-well flat-bottom microplates. Control was set by adding bacterial suspension into 10 μL of potassium phosphate buffer. Turbidity reduction was measured after adding bacteria suspension at 1 and 4min by OD 540 measurement.
Lysozyme activity (Unit mL-1)=[Δ Absorbance (sample) – Δ Absorbance (control)]/( 0.00 × 0.01)
Superoxide dismutase assay: Serum superoxide dismutase (SOD) activity was measured by its ability to inhibit superoxide anion generated by xanthine and xanthine oxidase reaction system according to Wang and Chen [30] using a SOD detection kit (Nanjing Jiancheng Bioengineering Institute, China). The optical density was measured at 450 nm. One unit of SOD was defined as the amount required for inhibiting the rate of xanthine reduction by 50% in 1 ml reaction system.
Muscle RNA and protein concentration
RNA and protein concentrations in the white muscle were determined using TRIzol® reagent (Invitrogen, Carlsbad CA, USA), as described in Rungruangsak-Torrissen [31]. The extinction coefficients for calculating RNA and protein were E260=40 μg RNAmL-1 and E280=2.1 mg protein mL-1, respectively. The concentration ratios were calculated from the amounts of RNA and protein in the same sample.
Proximate chemical composition of whole body and muscle
The whole body and muscle were homogenized for proximate analysis on moisture, crude protein, crude lipid, and crude ash, similarly as in the analysis of diet composition.
Statistical analysis
The experiment followed a completely randomized design (3 treatments × 3 replications). The nine experimental units had 10 fish each. Data analyses were performed using statistical software. All data were expressed as mean ± SD. Means were compared using One-Way ANOVA and statistically significant differences between means were ranked using Duncan's Multiple Range Test at 95% confidence level (P<0.05).
Results
In vitro protein digestibility of diets
The in vitro protein digestibility of experimental diets differing in the zinc source is shown in Figure 1. There was no significant effect of dietary zinc sources on protein digestibility of diets at the end of the experiment (P>0.05).
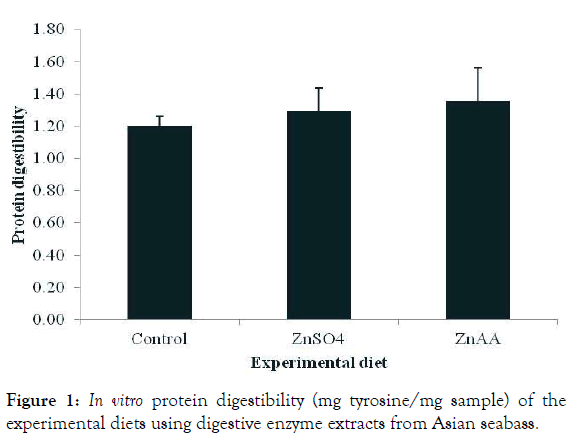
Figure 1: In vitro protein digestibility (mg tyrosine/mg sample) of the experimental diets using digestive enzyme extracts from Asian seabass.
Survival, growth performance and feed utilization
The survival, growth performance and feed utilization of Asian seabass fed difference the experimental diets for 10 weeks are shown in Table 3. There were no differences in survival (83.33%) across the three experimental diets (P>0.05). No differences in growth performance and feed utilization were observed among the three experimental diets (P>0.05). However, higher final weight, weight gain, ADG, SGR, PER, HSI and DSI were found in fish fed ZnAA diets, followed by fish fed ZnSO4 diets, whereas lower values were found in control treatment.
| Parameters | Experimental diet | P-value | ||
|---|---|---|---|---|
| Control | ZnSO4 | ZnAA | ||
| Survival rate (%) | 83.33 ± 5.77 | 83.33 ± 5.77 | 83.33 ± 5.77 | 1 |
| Average initial weight (g) | 22.63 ± 0.87 | 22.97 ± 0.47 | 22.77 ± 0.55 | 0.461 |
| Average final weight (g) | 87.99 ± 8.36 | 95.61 ± 9.29 | 98.23 ± 6.51 | 0.344 |
| Weight gain (g fish-1) | 65.36 ± 8.13 | 72.64 ± 9.43 | 75.46 ± 5.99 | 0.344 |
| Total length (cm) | 19.24 ± 1.61 | 19.79 ± 0.42 | 19.28 ± 0.50 | 0.767 |
| Condition factor (g cm-3) | 1.26 ± 0.03 | 1.35 ± 0.04 | 1.30 ± 0.04 | 0.083 |
| Average daily gain (g day-1) | 0.87 ± 0.11 | 0.97 ± 0.13 | 1.01 ± 0.08 | 0.334 |
| Specific growth rate (% day-1) | 1.81 ± 0.12 | 1.90 ± 0.14 | 1.95 ± 0.06 | 0.377 |
| Feed conversion ratio | 1.30 ± 0.11 | 1.22 ± 0.14 | 1.20 ± 0.04 | 0.525 |
| Protein efficiency ratio | 1.84 ± 0.16 | 1.91 ± 0.22 | 1.96 ± 0.06 | 0.661 |
| Hepatosomatic index (%) | 3.19 ± 0.55 | 3.27 ± 0.54 | 3.69 ± 1.08 | 0.493 |
| Digestosomatic index (%) | 10.21 ± 1.75 | 10.74 ± 0.60 | 11.58 ± 1.87 | 0.318 |
Different superscripts in the same row indicate significant difference (P<0.05).
Table 3: The survival, growth performance and feed utilization of Asian seabass fed the different zinc source diets for 10 weeks.
Digestive enzyme specific activities
The digestive enzyme specific activities of Asian seabass fed difference experimental diets for 10 weeks are illustrated in Table 4. Significant increases in specific activities of total protease, pepsin and trypsin were observed for the fish fed ZnSO4 diets (P<0.05). However, there were no differences in specific activities of chymotrypsin and the ratio of trypsin to chymotrypsin (T/C ratio) across the three dietary treatments (P>0.05).
| Digestive enzymes | Experimental diet | P-value | ||
|---|---|---|---|---|
| Control | ZnSO4 | ZnAA | ||
| Total Proteases (U mg protein-1) | 1.48 ± 0.05b | 1.61 ± 0.02a | 1.27 ± 0.08c | 0 |
| Pepsin (U mg protein-1) | 1.84 ± 0.16ab | 2.00 ± 0.09a | 1.72 ± 0.13b | 0.045 |
| Trypsin (U mg protein-1) | 7.00 ± 0.09b | 7.33 ± 0.08a | 6.96 ± 0.14b | 0.001 |
| Chymotrypsin (U mg protein-1) | 7.81 ± 0.05 | 7.92 ± 0.13 | 7.90 ± 0.25 | 0.6 |
| T/C ratio | 0.90 ± 0.01 | 0.93 ± 0.02 | 0.88 ± 0.03 | 0.064 |
Data are expressed as mean ± SD.
Different superscripts in the same row indicate significant difference (P<0.05).
Table 4: The specific activities of digestive enzymes of Asian seabass fed the different zinc source diets for 10 weeks.
Immune response
The immuno-hematological parameters are presented in Table 5. Significant increase in hematocrit was observed in fish from both ZnAA and ZnSO4 treatments, relative to the control treatment (P<0.05). However, no significant differences were observed across the three treatments in RBC, WBC, hemoglobin, total serum protein and albumin.
| Parameters | Experimental diet | P-value | ||
|---|---|---|---|---|
| Control | ZnSO4 | ZnAA | ||
| Red blood cells (Mcell mL-1) | 3.70 ± 0.44 | 3.70 ± 0.30 | 4.03 ± 0.40 | 0.511 |
| White blood cells (cell mL-1) | 3930.33 ± 567.33 | 4123.33 ± 460.04 | 4913.33 ± 534.54 | 0.126 |
| Hematocrit (%) | 41.00 ± 2.65b | 42.33 ± 1.53ab | 46.00 ± 1.00a | 0.039 |
| Hemoglobin (g dL-1) | 5.10 ± 0.40 | 5.40 ± 0.10 | 5.47 ± 0.23 | 0.289 |
| Serum protein (g dL-1) | 3.40 ± 0.10 | 3.57 ± 0.15 | 3.43 ± 0.32 | 0.623 |
| Albumin (g dL-1) | 1.10 ± 0.10 | 1.13 ± 0.06 | 1.20 ± 0.10 | 0.422 |
Different superscripts in the same row indicate significant difference (P<0.05).
Table 5: Hematological parameters of Asian seabass fed the different zinc source diets for 10 weeks.
Data on the lysozyme and SOD activities in serum are shown in Table 6. Fish fed ZnAA diets had the highest lysozyme activity, followed by fish fed ZnSO4 diets, and lowest in fish fed the diets with the control (P<0.05). Significant increase in SOD activity was observed in fish from both ZnAA and ZnSO4 treatments, relative to the control treatment (P<0.05).
| Parameters | Experimental diet | P-value | ||
|---|---|---|---|---|
| Control | ZnSO4 | ZnAA | ||
| Lysozyme (units mL-1) | 433.33 ± 57.73b | 666.67 ± 28.87b | 1033.33 ± 230.94a | 0.005 |
| SOD (% inhibition) | 66.77 ± 2.63b | 74.46 ± 11.62ab | 77.48 ± 2.00a | 0.047 |
Data are expressed as mean ± SD.
Different superscripts in the same row indicate significant difference (P<0.05).
Table 6: The lysozyme and SOD activities of Asian seabass fed the different zinc source diets for 10 weeks.
Muscle quality
No significant differences in RNA, protein and RNA/protein ratio in muscle were found across the three dietary treatments (Table 7).
| Muscle quality | Experimental diet | P-value | ||
|---|---|---|---|---|
| Control | ZnSO4 | ZnAA | ||
| RNA (µg g-1) | 2348.71 ± 167.71 | 2514.48 ± 167.07 | 2640.97 ± 18.36 | 0.102 |
| Protein (mg g-1) | 77.00 ± 5.81 | 84.26 ± 7.42 | 85.66 ± 6.71 | 0.306 |
| RNA/protein ratio (µg g-1) | 30.51 ± 0.13 | 30.03 ± 3.67 | 29.47 ± 0.27 | 0.839 |
Different superscripts in the same row indicate significant difference (P<0.05).
Table 7: The muscle quality of Asian seabass fed the different zinc source diets for 10 weeks.
Whole body and muscle composition
Dietary treatment did not significantly influence moisture, crude protein, crude lipid and crude ash in the whole body. Significant increase in muscle crude lipid contents was observed in fish from both ZnSO4 and ZnAA treatments (P<0.05). However, no significant differences in moisture, crude protein and crude ash in muscle were detected among the experimental diets (Table 8).
| Composition | Experimental diet | P-value | ||
|---|---|---|---|---|
| Control | ZnSO4 | ZnAA | ||
| Whole body | ||||
| Moisture (%) | 7.71 ± 1.46 | 7.00 ± 0.74 | 7.66 ± 1.75 | 0.789 |
| Crude protein (%) | 52.38 ± 0.69 | 53.24 ± 1.33 | 53.68 ± 0.27 | 0.26 |
| Crude lipid (%) | 28.92 ± 2.23 | 29.65 ± 3.52 | 25.79 ± 0.98 | 0.207 |
| Crude ash (%) | 14.77 ± 0.23 | 16.52 ± 1.32 | 14.57 ± 1.28 | 0.123 |
| Muscle | ||||
| Moisture (%) | 7.58 ± 0.50 | 8.32 ± 1.46 | 6.53 ± 0.21 | 0.125 |
| Crude protein (%) | 72.63 ± 0.32 | 71.32 ± 1.11 | 71.68 ± 0.89 | 0.218 |
| Crude lipid (%) | 7.02 ± 0.16b | 10.12 ± 0.22a | 9.94 ± 0.02a | 0 |
| Crude ash (%) | 5.51 ± 0.02 | 5.63 ± 0.14 | 5.50 ± 0.16 | 0.414 |
Different superscripts in the same row indicate significant difference (P<0.05).
Table 8: The whole body and muscle composition of Asian seabass fed the different zinc source diets for 10 weeks (% Dry matter).
Discussion
Zinc plays an essential role in growth, development and maintenance of physiological activities, and functions as a cofactor of many enzymes and an integral part of more than 20 metalloenzymes [4]. Among the factors that influence zinc bioavailability is the chemical form of the supplemental zinc source [10]. Previous studies have shown that organic zinc had higher bioavailability than inorganic zinc in aquatic animals [10,12-15].
In vitro digestion is being used to predict the quality of diets. Many in vitro methods have been developed and tested for measuring digestibility of different dietary proteins [32]. In the current study, there was no significant difference in the in vitro protein digestibility of diets between the supplemental ZnSO4 and ZnAA. The result was similar to the study on growth performance. This study indicated that in vitro digestibility based on fish digestive enzyme extracts is related to the results from growth trial [33,34].
The in vitro digestibility in this research was mainly focused on protein. This is due to the Asian seabass diet contains highly variable amounts of protein. Therefore, protein plays a significant impact on the overall nutritional quality of diets and also acts as the primary factor for digestibility in fish [35].
In the present study, growth performance, digestive enzyme activity, immune response and muscle quality were improved by dietary ZnSO4 or ZnAA supplementation. There was no significant difference between the two zinc sources, for all growth parameters and feed utilization variables. The weight gain results in this study indicated that there was enough available zinc in the non-zincsupplemented basal diet to allow good weight increase. This could be due to the high level of zinc from animal protein sources in the basal diet provided zinc as 58.7 mg kg-1 that was enough for the zinc requirement in Asian seabass [36]. Furthermore, as the diets were isoproteic, no significant difference for PER results was expected because this index is just a relation between weight gain and protein intake. In brief, results of the present study indicated that the chemical form of supplementary zinc source utilized, inorganic or organic included in the basal diet at 50 mg Znkg-1, do not affect the growth performance and feed utilization of Asian seabass. The growth trial indicated that the dietary zinc from the two zinc sources could be utilized by fish. Similar results have been reported in turbot [18] and Nile tilapia [17]. However, some studies reported that the growth performance of zinc from organic zinc was better than inorganic zinc for channel catfish [13], rainbow trout [14] and hybrid striped bass [15]. The difference could be related to different species, diet types or organic zinc sources [16].
Better utilization of the feed is depending on both digestive and absorptive capacity [37]. Digestive enzymes play a key role in digesting nutrients for fish, of which the activity can directly reflect the digestive ability [38]. In this study, total protease, pepsin and trypsin activities in the whole digestive tract enhanced with the supplemental dietary ZnSO4. It is well established fact that zinc acts as cofactors for several metabolic pathways in many enzymatic systems [39]. ZnSO4 is completely water-soluble and broken down in the digestive tract [40]. This may explain why the supplementation of ZnSO4 can enhance some digestive enzyme activities. However, information on the relationship between dietary zinc sources and digestive enzyme activity in fish has not yet been reported. The mechanism needs further investigation.
The immune system of fish plays a vital role in the resistance against environmental and disease stressors [41,42]. Hematological parameters can be used to assess health status, indicating physiological and pathological changes of the fish [43]. Moreover, qualitative and quantitative variations in hematological parameters are the most significant findings in regard to diagnosis [44]. In the current study, hematocrit was significantly higher in the fish from both ZnAA and ZnSO4 treatments. It was reported that zinc could act as a signal to stimulate red blood formation in the common carp [45,46]. However, no significant effect on red blood cells of the Asian seabass was observed in this study. This could be due to the differences among hematological values are affected by a multitude of intrinsic and extrinsic factors [47].
Serum lysozyme activity is a chief innate immune defense index of fish [48]. Lysozymes inhibit bacterial proliferation and colonization by attacking cell wall polysaccharides, leading to cell wall breakdown and eventual death [49,50]. In the current study, fish fed ZnAA diets had the highest lysozyme activity, followed by fish fed ZnSO4 diets, and lowest in fish fed the diets with the control. A similar result was found in Nile tilapia [51]. They showed an increased lysozyme activity after feeding with the zinc diets. The increased serum lysozyme activity in the current study might indicate improved innate immune response of the fish. Zinc supplementation has intracellular signaling molecules which play an important role in cell mediated immune functions [52,53]. This indicated that supplementation of zinc can enhance some innate immune response. However, ZnAA showed better lysozyme activity compared to ZnSO4. This implied that ZnAA was effective in enhancing innate immune responses of Asian seabass. Likewise, this corresponds to the previous study [54] wherein the immune activity of shrimp from the organic zinc was markedly higher compared to the zinc sulfate.
Zinc plays an important role in enhancing the antioxidant status and decreasing lipid peroxidation [55]. Organism defense systems against the reactive oxygen species (ROS) consist of antioxidant enzymes [56]. Superoxide dismutase (SOD) is the first enzyme to respond against oxygen radicals [57]. It is widely used as nonspecific immune indices in fish as well as their activities are key indicators of the antioxidant capability of cells [58]. In the present study, serum SOD activities were significantly increased by dietary zinc, regardless of the zinc sources. This could be due to the fact that zinc is a cofactor in several enzyme systems. It is a component of a large number of metalloenzymes including Cu–Zn–SOD [59]. Similar findings were also reported in Nile tilapia [7], turbot [18], Jian carp [60] and shrimp [10]. In addition, in the present study, crude lipid contents in the muscle significantly increased by dietary ZnSO4 and ZnAA. This could be due to the increase in anti-oxidative activity in Asian seabass which decreased lipid peroxidation [61].
Protein synthesis capacity and protein turnover rate were observed via RNA concentration and RNA/protein ratio. Low flesh RNA and RNA/protein ratio indicate a faster growth rate of the fish on comparing to a slower growth rate group [62]. However, in the current study, no significant effect on RNA, protein and RNA/ protein ratio in muscle were observed. It was positively correlated with the result of growth performance. Moreover, no significant differences in muscle and whole body compositions were found across the three dietary treatments, suggesting sufficient amount of diet for maintaining growth as well as for deposition in muscle and the whole body of Asian seabass.
Conclusion
The results suggest that supplemental dietary ZnAA enhances immune responses for Asian seabass. Dietary ZnSO4 supplementation improved digestive enzyme activities. However, there were no significant differences in the growth performance, feed utilization and muscle quality of dietary zinc sources from either ZnSO4 or ZnAA when the diets contained a high level of zinc from 45% animal protein and high energy from 18% lipid.
Acknowledgments
This work was supported by the Aquatic Animal Nutrition Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University, Thailand. The authors sincerely appreciate Zinpro Corporation, Thailand, for providing zinc amino acid complex.
REFERENCES
- Harpaz S, Hakim Y, Slosman T, Eroldogan TO. Effects of adding salt to the diet of Asian sea bass Lates calcarifer, reared in fresh or salt water recirculating tanks, on growth and brush border enzyme activity. Aquaculture. 2005;248:315-324.
- Venkatachalama S, Kandasamy K, Krishnamoorthy L, Narayanasamy R. Survival and growth of fish. Lates calcarifer;under integrated mangrove aquaculture and open-aquaculture systems. Aquacult Res. 2018;9:18-24.
- Muralisankar T, Bhavan SP, Srinivasan V, Radhakrishnan S, Seenivasan CS. Effects of dietary copper on the growth physiology and biochemistry of the freshwater prawn Macrobrachium rosenbergii post larvae. Int J Pure App Biosci. 2015;3:509-518.
- Watanabe T, Viswanath K, Satoh S. Trace minerals in fish nutrition. Aquaculture. 1997;151:185-207.
- Gammanpila M, Age AY, Bart AN. Evaluation of the effects of dietary vitamin C, E and Zinc supplementation on reproductive performance of Nile tilapia, Oreochromis niloticus. Sri Lanka J Aquat Sci. 2007;12:39-60.
- Feng L, Tan LN, Liu Y, Jiang J, Jiang WD. Influence of dietary zinc on lipid peroxidation protein oxidation and antioxidant defence of juvenile Jain carp, Cyprinus carpio var Jain. Aquacult Nutr. 2011;17:875-882.
- Huang F, Jiang M, Wena H, Wu F, Liua W. Dietary zinc requirement of adult Nile tilapia. Oreochromis niloticus;fed semi-purified diets, and effects on tissue mineral composition and antioxidant responses. Aquaculture. 2015;439:53-59.
- Moazenzadeh K, Rajabi IH, Zamini A, Soltani M. Dietary zinc requirement of Siberian sturgeon. Acipenser baerii, Brandt 1869; juveniles, based on the growth performance and blood parameters. Aquac Int. 2017;9:25-35.
- National Research Council. Nutrient requirements of fish. National Academy Press, Washington DC, USA. 2011.
- Lin S, Lin Z, Yang Y, Li F, Luo L. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp, Litopenaeus vannamei. Aquaculture. 2013;407:79-84.
- Ashmead H. The roles of amino acid chelates in animal nutrition. Noyes Publications, New Jersey, USA. 1993;419.
- Tan B, Mai K. Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture. 2001;192:67-84.
- Paripatananont T, Lovell RT. Chelated zinc reduces the dietary requirement of channel catfish, Ictalurus punctatus. Aquaculture. 1995;133:73-82.
- Apines MJ, Satoh S, Kiron V, Watanabe T, Nasu N. Bioavailability of amino acids chelated and glass embedded zinc to rainbow trout, Oncorhynchus mykiss, fingerlings. Aquac Nutr. 2001;7:221-228.
- Buentello AJ, Goff BJ, Gatlin MD. Dietary zinc requirement of hybrid striped bass, Morone chrysops × Morone saxatilis, and bioavailability of two chemically different zinc compounds. J World Aquacult Soc. 2009;40:687-694.
- Do Carmo e Sa MV, Pezzato LE, Barros MM, Padilla PM. Optimum zinc supplementation level in Nile tilapia Oreochromis niloticus juveniles diets. Aquaculture. 2004;238:385-401.
- Zhao HX, Cao JM, Liu XH, Zhu X, Chen SC, Lan. Effect of supplemental dietary zinc sources on the growth and carbohydrate utilization of tilapia Smith 1840, Oreochromis niloticus × Oreochromis aureus. Aquac Nutr. 2011;17:64-72.
- Ma R, Hou H, Mai K, Bharadwaj SA, Ji F, Zhang W. Comparative study on the bioavailability of chelated or inorganic zinc in diets containing tricalcium phosphate and phytate to turbot, Scophthalmus maximus. Aquaculture. 2014;421:187–192.
- AOAC. Official Methods of Analysis. 18th edn. Association of Official Analytical Chemists, Washington, DC, USA. 2005.
- Supalug K, Orapint J, Wanchai W, Srinoy C. pH Characterization of Digestive Enzyme and In vitro Digestibility of Red Bee Shrimp, Caridina cantonensis, Decapoda: Atyidae. J Aquac Res Dev. 2018;9:1-6.
- Nankervis L, Southgate PC. Enzyme and acid treatment of fish meal for incorporation into formulated microbound diets for barramundi. Lates calcarifer larvae. Aquacult Nutr. 2009;15:135-143.
- APHA, AWWA, WPCF. Standard methods for the examination of water and wastewater. 20th ed. Water Pollution Control Federation, Washington DC, USA. 1998.
- Lowry HO, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265-275.
- Worthington V. Worthington Enzyme Manual. Enzymes and Related Biochemicals. Worthington Chemical, New Jersey, USA. 1993.
- Areekijseree M, Engkagul A, Kovitvadhi U, Thongpan A, Mingmuang M. Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis bialatus. Simpson 1900. Aquaculture. 2004;234:575-587.
- Rungruangsak-Torrissen K, Moss R, Andresen LH, Berg A, Waagbo R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon, Salmo salar L.. Fish Physiol Biochem. 2006;32:7-23.
- Drabkin DL, Austin JH. Spectrophotometric studies: II. Preparations from washed blood cells, nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem. 1935;112:51-65.
- Doumas BT, Watson W, Biggs HG. Albumin standards and measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87-96.
- Shugar D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952;8:302-309.
- Wang SH, Chen JC. The protective effect of chitin and chitosan against Vibrio alginolyticus in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2005;19:191-204.
- Rungruangsak-Torrissen K. Digestive efficiency, growth and qualities of muscle and oocyte in Atlantic salmon. Salmosalar L.; fed on diets with krill meal as an alternative protein source. J Food Biochem. 2007;31:509-540.
- Rungruangsak-Torrissen K, Rustad A, Sunde J, Eiane SA, Jensen HB. In vitro digestibility based on fish crude enzyme extract for prediction of feed quality in growth trials. J Sci Food Agric. 2002;82:644-654.
- Supannapong P, Pimsalee T, Akomol T, Engkakul A, Kovitvadhi U. Digestive enzymes and in vitro digestibility of different species of phytoplankton for culture of the freshwater pearl mussel, Hyriopsis. Hyriopsis;bialatus. Aquacult Int. 2008;16:437- 453.
- Sultana Z, Ahmed MS, Iqball MS, Chisty MAH. Determination of in vitro protein digestibility of different feed ingredients for Oreochromis nilotica. Bangladesh Res Pub J. 2010;4:87-94.
- Areekijseree M, Engkagul A, Kovitvadhi S, Kovitvadhi U, Thongpan A. Development of digestive enzymes and in vitro digestibility of different species of phytoplankton for culture of early juveniles of the freshwater pearl mussel, Hyriopsis bialatus. Simpson, 1900. Invert Reprod Develop. 2006;49:255-262.
- Sapkale PH, Singh RK. Dietary Zinc and Cobalt Requirements of Fry of Seabass, Lates calcarifer and Catfish. Clarias batrachus. ISR J Aquacult-Bamid. 2011;63:1-6.
- Tengjaroenkul B, Smith BJ, Cacec T, Smith SA. Distribution of intestinal enzymes activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture. 2000;182:317-327.
- Wen ZP, Zhou XQ, Feng L, Jiang J, Liu Y. Effect of dietary pantothenic acid supplement on growth, body composition and intestinal enzyme activities of juvenile Jian carp, Cyprinus carpio var. Jian. Aquacult Nutr. 2009;15:470-476.
- Salgueiro MJ, Zubillaga M, Lysionek A, Sarabia MI, Caro R, et al. Zinc as an essential micronutrient: a review, Nutr Res. 2000;20:737-755.
- Mohamed MAW, El-Houseiny W, Ibrahim ER, Yasmina M, Abd-Elhakim. Palliative effects of zinc sulfate against the immunosuppressive, hepato- and nephrotoxic impacts of nonylphenol in Nile tilapia, Oreochromis niloticus. Aquaculture. 2019;504:227-238.
- Adel M, Yeganeh S, Dadar M, Sakai M, Dawood MA. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon. Huso huso Linnaeus, 1754. Fish Shellfish Immunol. 2016;56:436-444.
- Anderson DP. Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annu Rev Fish Dis. 1992;2:281-307.
- Firouzbakhsh F, Noori F, Khalesi MK, Jani-Khalili K. Effects of a probiotic, protexin, on the growth performance and hematological parameters in the Oscar, Astronotus ocellatus fingerlings. Fish Physiol Biochem. 2011;37:833-842.
- Martins ML, Tavares-Dias M, Fujimoto RY, Onaka EM, Nomura DT. Haematological alterations of Leporinus macrocephalus, Osteichthyes: Anostomidae; naturally infected by Goezia leporine. Nematoda: Anisakidae in fish pond. Arq Bras Med Vet Zootecnia. 2004;56:640-646.
- Chen YH, Fang SW, Jeng SS. Zinc transferrin stimulates red blood cell formation in the head kidney of common carp, Cyprinus carpio. Comp Biochem Physiol A. 2013;166:1-7.
- Chen YH, Shiu JR, Ho CL, Jeng SS. Zinc as a signal to stimulate red blood cell formation in fish. Int J Mol Sci. 2017;18:1-12.
- Clauss TM, Dove ADM, Arnold JE. Hematologic disorders of fish. Vet Clin North Am Exot Anim Pract. 2008;11:445–462.
- Mahmoud HK, Al-Sagheer AA, Reda FM, Mahgoub SA, Ayyat MS. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture. 2017;475:16-23.
- Sotirov L, Dimitrov I, Djorbinava M. Serum lysozyme concentration in different sheep breed. Bulgarian J Vet Med. 2005;8:83–89.
- Talpur AD, Ikhwanuddin M. Azadirachta indica. neem; leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol. 2013;34:254-264.
- Hung SW, Tu CY, Wang WS. In vivo effects of adding singular or combined anti-oxidative vitamins and/or minerals to diets on the immune system of tilapia, Oreochromis hybrids; peripheral blood monocyte-derived, anterior kidney-derived, and spleen-derived macrophages. Vet Immunol Immunopathol. 2007;115:87-99.
- Rink L, Kirchner H. Zinc-altered immune function and cytokine production. J Nutr. 2000;130:1407-1411.
- Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1-44.
- Lin SM, Mao SH, Guan Y, Lin X. Effects of different Zn Mn Cu sources on growth performance and non-specific immunity of Litopenaeus vannamei. Freshwater Fisheries. 2011;41:64–69.
- Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:212-218.
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620-650.
- Winston GW, Di-Giulio RT. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol. 1991;19:137-161.
- Shiau SY, Gabaudan J, Lin YH. Dietary nucleotide supplementation enhances immune responses and survival to Streptococcus iniae in hybrid tilapia fed diet containing low fish meal. Aquacult Repo. 2015;2:77-81.
- Shiau SY, Jiang LC. Dietary zinc requirements of grass shrimp, Penaeus monodon, and effects on immune responses. Aquaculture. 2006;254:476-482.
- Feng L, Tan LN, Liu Y, Jiang J, Jiang WD. Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defence of juvenile Jian carp, Cyprinus carpio var. Jian. Aquac Nutr. 2011;17:875-882.
- Pathak A, Mahmood A, Pathak R, Dhawan D. Role of zinc on lipid peroxidation and antioxidative enzymes in intestines of ethanol-fed rats. Biol Trace Elem Res. 2004;100:247-257.
- Saekhow S, Thongprajukaew K, Phromkunthong W, Sae-khoo H. Minimal water volume for intensively producing male Siamese fighting fish, Betta splendens Regan, 1910. Fish Physiol Biochem. 2018;44:1075-1085.
Citation: Sansuwan K, Jintasataporn O, Chumkam S (2019) Effects of Dietary Zinc Amino Acid Complex and Zinc Sulfate on Growth Performance, Digestive Enzyme Activity and Immune Response in Asian Seabass (Lates calcarifer). 10:572. doi: 10.35248/2155-9546.19.10.572
Copyright: © 2019 Sansuwan K, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sources of funding : This work was supported by the Aquatic Animal Nutrition Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University, Thailand. The authors sincerely appreciate Zinpro Corporation, Thailand, for providing zinc amino acid complex.

