Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2024) Volume 14, Issue 4
Effectiveness of Ferrous Gluconate Plus Multivitamins and Minerals by Iron Deficiency Anemia Status, Menstrual Blood Flow and Pregnancy Status: A Subgroup Analysis of the SANOIN Study
NS Comia1, Christopher Joseph L Soriano2, Roger Gibb3, Poonam Sule4 and James Wun Chiang Sia5*2Department of Obstetrics and Gynecology, Ateneo de Manila University School of Medicine and Public Health, Metro Manila, Philippines
3Personal Health Care, The Procter and Gamble Company, Mason, Ohio, United States of America
4Personal Health Care, Procter and Gamble Health Limited, Mumbai, India
5Singapore Innovation Center, 70 Biopolis Street, Queenstown, Singapore
Received: 26-Jul-2024, Manuscript No. CPECR-24-26594; Editor assigned: 29-Jul-2024, Pre QC No. CPECR-24-26594 (PQ); Reviewed: 12-Aug-2024, QC No. CPECR-24-26594; Revised: 19-Aug-2024, Manuscript No. CPECR-24-26594 (R); Published: 26-Aug-2024, DOI: 10.35248/2161-1459.24.14.430
Abstract
Background: The Phase IV, open-label, single arm, prospective, non-interventional SANOIN study demonstrated that an oral fixed dose combination of ferrous gluconate, multivitamins and minerals (Sangobion® IRON+) was effective and well-tolerated in treating mild to moderate Iron Deficiency Anemia (IDA) in women aged 15-55 years.
Methods: We present a subgroup analysis of the SANOIN Hemoglobin (Hb) and serum ferritin data over the 90-day treatment period based on menstrual flow (normal vs. heavy vs. very heavy) and pregnancy status (pregnant vs. non- pregnant). The frequency and severity of IDA symptoms at baseline and improvement at Days 30, 60 and 90 were assessed in these subgroups, and by IDA status (mild vs. moderate).
Results: Within the intent-to-treat population (n=87), 78 subjects were evaluated according to menstrual flow (normal, 44.9%; heavy, 47.4%; very heavy, 7.7%). Eight out of 87 subjects were pregnant. All subgroups demonstrated similar Hb normalization and increase in ferritin levels after 90 days. Overall, 72.4% of the subjects were anemia-free by Day 90. The most frequently occurring IDA symptoms at baseline were headache, dizziness and fatigue in most subgroups, while tachycardia and shortness of breath were frequent in the small pregnant subgroup. IDA symptoms significantly improved by Day 90 across all subgroups.
Conclusion: This subgroup analysis demonstrated the broad efficacy of an oral fixed dose combination of ferrous gluconate, multivitamins and minerals in providing rapid and sustainable improvement in IDA symptoms, Hb and ferritin levels in the diverse range of baseline IDA status, menstrual flow, and pregnancy status.
Keywords
Iron+multivitamin and mineral; Sangobion; Iron deficiency anemia; Ferrous gluconate; Menstruation; Pregnancy
Clinical trial registration
PFDA Registry No: PHRR200413-002602
Introduction
Iron Deficiency (ID), one of the most common micronutrient deficiencies in the world, clinically manifests as a spectrum [1]. While the early stages of ID involve the depletion of iron stores with no anemia, if left unaddressed, further progression can lead to impaired erythropoiesis and result in anemia [1]. Notably, ID has a disproportionately greater impact on women [2]. In fact, the World Health Organization reports that 30% of women in the reproductive age range (15-49 years) have anemia [3], with the prevalence in this population being as high as 50% in low or middle-income countries [4], most cases attributable to ID [1].
The etiology of ID or Iron Deficiency Anemia (IDA) in women is multifactorial. Some of the factors putting women at a considerably higher risk of ID/IDA include blood loss due to menstruation, higher iron requirements during pregnancy and bleeding during childbirth [2]. Therefore, diagnosis, prevention and treatment of ID/IDA must consider the specific etiological factors.
The primary publication for the “Sangobion® IRON+ Non-Interventional Study to Evaluate Changes in Hemoglobin Status in Subjects with Mild to Moderate Iron Deficiency Anemia (SANOIN)” has previously demonstrated that an oral fixed dose combination of ferrous gluconate, multivitamins and minerals significantly improves hematological parameters, IDA symptoms and quality of life, accompanied by good tolerability [5]. The SANOIN study which recruited female subjects, included those with mild to moderate IDA [5]. The primary efficacy endpoint for the study was the change from baseline in mean Hemoglobin (Hb) concentration after 90 days of treatment. Secondary efficacy endpoints included change from baseline in Hb concentration at Days 14, 30, 60 and 90, as well as change from baseline in serum ferritin concentration at Day 90 [5]. These assessments were performed in the total trial population as well as in the mild IDA and moderate IDA subgroups [5]. Here, we report an analysis of the SANOIN study in key subgroups of interest: menstrual bleeding flow (normal or heavy or very heavy) and pregnancy status (pregnant or non-pregnant).
At present, there is an opportunity for better understanding of the symptoms and severity of patients experiencing mild versus moderate IDA. A common misconception is that IDA symptoms only occur in patients with moderate to severe IDA. In this subgroup analysis, we examined whether IDA symptoms occurred early in the IDA trajectory even among subjects with mild IDA. We also examined whether the IDA symptoms improved to the same extent in the SANOIN study treatment, regardless of IDA status. The choice of the initial dose of either 30 mg or 60 mg elemental iron and the dose progression over time was also examined in the respective subgroups. The proportion of subjects who became ID-free and symptom-free with the study drug was also evaluated.
Heavy Menstrual Bleeding (HMB) is a known risk factor for ID/IDA in women as the average blood loss due to HMB is 5-6 times more than in normal menstruation, resulting in depleted iron stores [6]. Considering that HMB affects approximately 18%- 38% of women of reproductive age, there is a need for women of child-bearing age to be screened for HMB and ID/IDA [6]. Even normal menstrual flow and the associated 300 mg of iron lost annually can lead to the development of ID when there is low dietary iron intake or iron absorption is insufficient [7]. Consequently, we investigated if ferrous gluconate plus multivitamins and minerals is equally effective in subjects with heavy or very heavy menstrual flow compared to those with normal flow.
Another significant risk factor for women for ID/IDA is pregnancy where increased iron requirements for fetal growth and development, as well as the expansion of maternal blood volume, raises the iron requirements [8]. While several international guidelines have recommended daily doses of 100-200 mg of elemental iron for the treatment of IDA in pregnancy [9], recent studies have indicated that lower doses of 30-60 mg may be sufficient due to proven effectiveness, coupled with improved tolerability and hence adherence to therapy [10]. This subgroup analysis presented an opportunity to assess the effectiveness of the prescribed ferrous gluconate plus multivitamins and minerals dose in treating IDA in pregnancy.
Iron is involved in several cellular mechanisms including gene expression, cell growth, signalling and regulation, energy metabolism and immune response [1]. Therefore, ID can cause diverse symptoms like hair loss, fatigue, headache, dizziness, pica, shortness of breath and tachycardia [1]. Other less common symptoms include restless legs syndrome, edema and brittle nails. It is imperative to recognize these symptoms early to enable prompt initiation of iron supplementation, thereby providing timely improvement of hematological parameters and symptoms relief.
Materials and Methods
Study design
The SANOIN study was a Phase IV/post-marketing, open-label, single arm, prospective, non-interventional study conducted at 6 sites in Philippines to evaluate the efficacy and safety of ferrous gluconate, multivitamins and minerals capsule in the real world [5]. The study was approved by the Philippine Food and Drug Administration (PFDA) prior to initiation and each site was approved by an Institutional Ethics Committee or Institutional Review Board. All subjects provided informed consent prior to participation. The study was conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki and was registered on the local registry for clinical trials, PFDA-Registry.
Study participants
Detailed inclusion and exclusion criteria of the study participants have been previously reported [5]. The study enrolled females (including women of reproductive age, and pregnant women) aged ≥ 15 years to ≤ 55 years with known mild to moderate IDA and a serum ferritin value of <30 ng/mL at baseline. The criteria for mild to moderate IDA was based on the criteria defined by the World Health Organization. Briefly, mild and moderate IDA in non-pregnant women was defined by an Hb level of 11.0 g/dL–11.9 g/dL and 8.0 g/dL–10.9 g/dL, respectively. Mild IDA in pregnant subjects was defined by Hb levels ranging from 9.5 g/dL to 10.5 g/ dL or 11.0 g/dL, depending on the stage of pregnancy. Moderate IDA in pregnant subjects was defined as Hb levels of 8.0 g/dL–9.4 g/dL. The menstrual flow categorization of normal, heavy and very heavy flow was based on the discretion of the investigators, which included clinical assessment on the frequency of change of sanitary napkins, amount of staining and use of adult diaper.
Treatment
All subjects were started with either 1 or 2 capsules of the study drug daily at baseline (30 mg or 60 mg elemental iron, respectively), depending on their baseline IDA status or as per the clinical judgment of the prescribing physician. The dosing regimen was maintained or modified by the investigator, based on tolerability and relevant healthcare data collected at subsequent visits. Further details on dose adjustment or modification have been previously reported [5].
Study endpoints and assessments
The following assessments were performed for the key subgroups based on IDA status (mild or moderate), menstrual blood flow (normal or heavy or very heavy) and pregnancy status (pregnant or non-pregnant): mean Hb at baseline and on Days 14, 30, 60 and 90; median serum ferritin levels at baseline and on Day 90; the proportion of subjects assigned to 30 mg vs. 60 mg daily dosing at baseline, Day 30 and Day 60; the proportion of subjects with varying number of IDA symptoms at baseline and subsequent visits; frequency and severity of IDA symptoms (tiredness, shortness of breath, dizziness, headache, swelling, fast heartbeat, hair fall, restless legs, brittle nails, cold hands or feet and pica) according to the Visual Analogue Scale (VAS) at baseline and at Days 30, 60 and 90. Assessments performed for the total population were as follows: the frequency of IDA symptoms at baseline; the proportion of IDA-free subjects on Days 14, 30, 60 and 90 and ID-free subjects on Day 90.
Statistical analysis
All analyses were performed on the Intent-to-Treat (ITT) population and were exploratory in nature. Per the statistical analysis plan written prior to database lock, missing primary endpoint data for subjects with at least one post-baseline value were imputed using the last observation carried forward approach. This resulted in the imputation of 13 (3.8%) post-baseline Hb observations in this subgroup analysis. No other data were imputed.
For continuous variables, descriptive statistics including the mean, standard error, median and median absolute deviation were calculated. For binary outcomes, descriptive statistics included frequencies and percentages. The statistical significance of change from baseline results was assessed with the Wilcoxon signed-rank test at a significance level of 5% (2-sided). Statistical analyses were performed with SAS version 9.4. Additional details are provided in the primary analysis of the SANOIN study [5].
Results
Study participants
Overall, 97 subjects were enrolled in the SANOIN study. A total of 87 subjects had Hb data available for analysis at one or more post- baseline visits. All 87 subjects were evaluated by IDA status with 30 (34.5%) having mild IDA and 57 (65.5%) having moderate IDA at baseline [5]. In this subgroup analysis, 78 subjects (excluding pregnant subjects) were evaluated by menstrual flow, where 35 (44.9%) had normal flow, 37 (47.4%) had heavy flow and 6 (7.7%) had very heavy flow. For the evaluation by pregnancy status, 8 (9.2%) subjects of the total 87 subjects with post-baseline visit Hb data were pregnant. Other baseline demographics of the SANOIN study population have been previously described [5].
Dose progression
At baseline, all subjects with none (n=1) or mild IDA (n=32) were started on a daily elemental iron dose of 30 mg. Among the 64 subjects with moderate IDA, 52% and 48% were started on 30 mg and 60 mg doses, respectively, with 68% of subjects receiving a final daily dose of 30 mg. The majority (82%) of subjects with normal menstrual flow received 30 mg daily doses. Among subjects with heavy menstrual flow, 44% were first started on 60 mg but this reduced to 5.6% by Day 60. Subjects with very heavy flow were prescribed 60 mg daily doses at the beginning of the study and all of them were receiving lower doses by Day 60. All pregnant subjects were prescribed 30 mg throughout the study duration. The majority of the non-pregnant subjects (64%) were prescribed 30 mg at the beginning of the study, with this proportion increasing to 73% by Day 60.
Efficacy
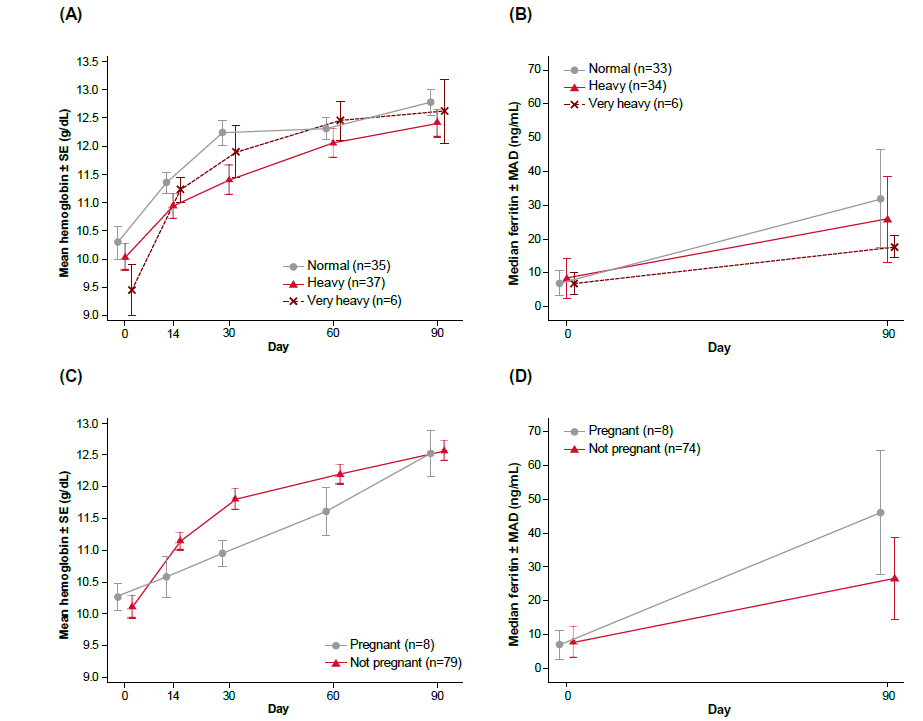
Hemoglobin and ferritin levels: Hb and ferritin levels across visits for the respective mild and moderate IDA subgroups were reported in the primary publication and will not be discussed here [5]. For the analysis based on menstrual flow, the study drug was demonstrated to be effective in improving Hb level irrespective of menstrual flow at baseline (Figure 1A, Supplementary Table 1), with statistically significant improvements in all subgroups across all post-baseline visits (p<0.05). In subjects with heavy menstrual flow, Hb increased by 0.9 g/dL by Day 14 (p<0.0001). In subjects with very heavy flow, Hb increased by 1.8 g/dL by Day 14 with 60 mg of daily study drug (p=0.0019). Median ferritin levels improved by Day 90 and the change from baseline was statistically significant across all 3 menstrual flow subgroups (p<0.05) (Figure 1B, Supplementary Table 2). The study treatment normalized mean Hb levels to a similar extent by Day 90 regardless of pregnancy status (Figure 1C, Supplementary Table 3). The mean Hb level in the pregnancy subgroup was normalized by Day 30 to 11.0 g/dL with a statistically significant increase of 0.7 g/dL (p=0.0255). Both pregnant and non-pregnant subgroups showed improved ferritin levels at Day 90, with higher levels observed in the pregnant subgroup (Figure 1D, Supplementary Table 4). The median ferritin level increased significantly from a baseline level of 7.0 ng/dL to 46.0 ng/dL by Day 90 (p=0.0078) in pregnant women, indicating repletion of iron stores above the normal cut-off of >30 ng/dL.
Figure 1: Improvement in hemoglobin and serum ferritin levels, according to menstrual flow and pregnancy status; (A) mean hemoglobin concentration by menstrual flow, (B) mean ferritin concentration by menstrual flow, (C) mean hemoglobin concentration by pregnancy status, (D) mean ferritin concentration by pregnancy status. Note: MAD: Mean Absolute Deviation; SE: Standard Error.
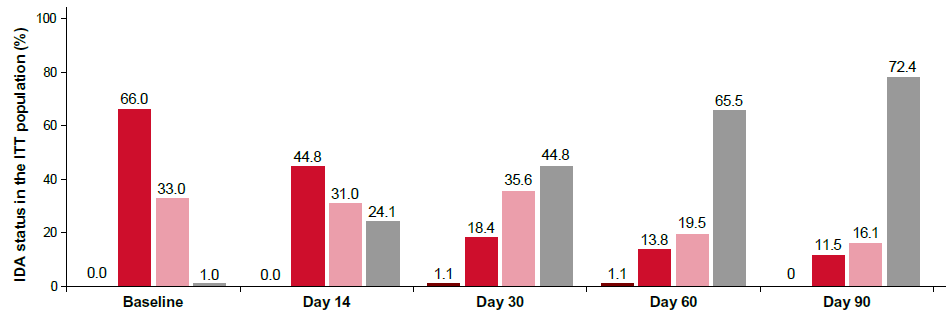
IDA status: Evaluation of IDA status in the total population demonstrated that 44.8% and 72.4% of subjects no longer had anemia by Day 30 and Day 90 respectively (Figure 2). The proportion of subjects with moderate IDA reduced from 66.0% at baseline to 18.4% on Day 30. Additionally, the proportion of subjects who became ID-free (based on Hb and ferritin levels cut- offs) was assessed, which revealed that 39% of subjects were ID-free by Day 90 (based on both Hb and ferritin levels at Day 90; n=82).
Figure 2: IDA status by visit in the ITT population. Note: IDA: Iron Deficiency Anemia; ITT: Intent-to-Treat;  None.
None.
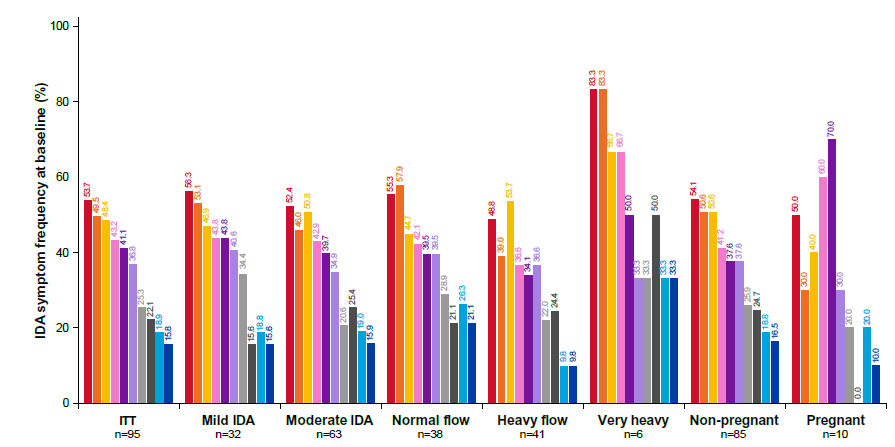
Number of IDA symptoms per subject and frequency and resolution of each IDA symptom: The occurrence of each IDA symptom according to the VAS score was analysed in each subgroup to identify the commonly occurring symptoms. Headache, dizziness and tiredness were the most common symptoms reported at baseline (Figure 3). The distribution of the number of IDA symptoms per subject at baseline was similar between the mild and moderate IDA subgroups (Figure 4A), with ~80% of both mild and moderate IDA subjects having ≥ 1 IDA symptoms at baseline. The prevalence of IDA symptoms observed at baseline among subjects with mild or moderate IDA subgroups are as shown in Figure 3, with 56.3% and 52.4% of subjects experiencing headache, 53.1% and 46.0% of subjects reporting dizziness and 46.9% and 50.8% of subjects experiencing tiredness, respectively. The VAS scores for the symptoms experienced at baseline were similar between the mild and moderate IDA subgroups (Supplementary Figure 1A). All symptoms improved considerably (according to VAS scores) throughout the 90-day study period, with similar resolution observed for both mild and moderate IDA subgroups (Supplementary Figure 1A). Among the subjects with mild and moderate IDA, 53.8% and 76.8% of subjects, respectively had no symptoms by Day 90 (Figure 4A).
Figure 3: Frequency of each IDA symptom at baseline in the following populations: ITT; mild IDA; moderate IDA; normal menstrual flow; heavy menstrual flow; very heavy flow; non-pregnant; and pregnant. Note: IDA: Iron Deficiency Anemia; ITT: Intent-to-Treat;  Dizziness;
Dizziness;  Fast heartbeat;
Fast heartbeat;  Restless legs;
Restless legs;  Cold hands or feet.
Cold hands or feet.
The distribution of symptoms experienced by subjects with normal and heavy menstrual flow at baseline were similar. Most subjects in these two subgroups experienced headache, dizziness and tiredness (Figure 3). The prevalence of IDA symptoms at baseline for subjects with very heavy menstrual flow is higher for all symptoms (except hair loss) compared to heavy and normal flow women, where the most frequently reported symptoms were headache (83.3%), dizziness (83.3%), shortness of breath (66.7%) and tiredness (66.7%) (Figure 3). Baseline VAS scores for the symptoms were similar regardless of menstrual flow (Supplementary Figure 1B). A similar extent of symptom resolution (by VAS) was achieved in all subgroups (Supplementary Figure 1B). The proportion of subjects who became symptom-free following 90 days of treatment (Figure 4B) was 67.6% in both the subgroups with normal and heavy menstrual flow. Among subjects with very heavy menstrual flow, 83.3% were symptom-free at the end of the study (Figure 4B). Hence, IDA symptoms as assessed by number, frequency and severity improved to a similar extent regardless of menstrual flow.
Pregnancy status was not associated with any significant difference in the number of symptoms experienced per subject at baseline (Figure 4C). Eighty percent or more of both pregnant and non- pregnant subjects experienced ≥ 1 IDA symptoms at baseline. The most commonly reported IDA symptoms experienced at baseline by subjects who were pregnant were fast heartbeat (70%), shortness of breath (60%) and headache (50%) (Figure 3). Baseline VAS score for most IDA symptoms were similar in the pregnant and non-pregnant subgroups (Supplementary Figure 1C) and improved to a similar extent by Day 90 (Supplementary Figure 1C). A total of 69.3% of non-pregnant subjects and 62.5% of pregnant subjects were symptom-free by Day 90 (Figure 4).
Figure 4: Frequency of IDA symptoms at baseline and after 90 days of treatment in (A) mild or moderate IDA; (B) normal, heavy or very heavy menstrual flow; (C) pregnant or non-pregnant subjects. Note: IDA: Iron Deficiency Anemia.
Safety
The safety analysis was performed only on the ITT population in the primary analysis and not for the individual subgroups as only a total of 3 adverse events (abdominal pain, hyperchlorhydria, and dizziness) were suspected to be related to the study drug in the ITT population.
Discussion
This subgroup analysis demonstrated the broad efficacy of an oral fixed dose combination of ferrous gluconate, multivitamins and minerals in providing rapid and sustainable improvement in IDA symptoms, Hb and ferritin levels, in the diverse range of baseline IDA status, menstrual flow, and pregnancy status. These findings enrich our understanding and approach in meeting the unique challenges and treatment requirements of a diverse patient population, encompassing varying degrees of IDA, menstrual flow, and pregnancy status.
Ferrous gluconate with multivitamin and minerals improved hematological parameters irrespective of IDA status, menstrual flow and pregnancy status
Patients with moderate IDA often receive a prescription for a higher dosage of elemental iron. This study demonstrated the effectiveness of improved IDA status with decreased prescribed dose over the course of treatment with 48% of subjects prescribed a 60 mg dose at baseline, reducing to 5.4% by Day 60. Similarly, the efficacy of an oral fixed dose combination of ferrous gluconate, multivitamins and minerals on the improvement of hematological parameters across all menstrual flow subgroups was demonstrated. A statistically significant rise in Hb levels was observed, even among subjects with heavy or very heavy menstrual flow, as early as Day 14 of treatment. This increase was maintained consistently throughout the 90-day duration of the study. In addition, significant improvement in serum ferritin levels was noted in both heavy and very heavy menstrual flow subgroups. However, it is important to note that despite the improvement in Hb and IDA status, the ferritin levels in individuals with moderate IDA and heavy or very heavy menstrual flow remained below the ideal cut-off of 30 ng/mL [5]. Therefore, further studies may be needed to investigate the hypothesis that longer duration of higher dose 60 mg elemental iron can raise the ferritin level and replete body iron stores more effectively in these subgroups.
By Day 30, pregnant subjects achieved a mean Hb level of 11.0 g/dL, which, although a smaller increase of 0.7 g/dL compared to non-pregnant subjects, met the normal Hb level cut-offs for the 2nd and 3rd trimesters of pregnancy. By Day 90, pregnant subjects had higher median ferritin levels compared to non-pregnant subjects, potentially due to pro-inflammatory changes in pregnant women and a faster replenishment of iron stores following Hb normalization [2]. In this study, all pregnant subjects were prescribed and maintained on a lower dose of 30 mg, likely as a precautionary measure by the investigators to minimize gastrointestinal side effects associated with iron supplementation during pregnancy. Although the 30 mg daily dose proved effective in this limited sample of pregnant women, consideration can be given to a higher dose of 60 mg during pregnancy, if necessary, based on updated guideline recommendations, to achieve faster Hb correction [11,12].
Early identification and understanding of IDA symptoms can guide early diagnosis and intervention
In contrast to the belief that individuals with mild IDA may be symptom-free, this subgroup analysis demonstrated that the mild IDA group showed similar number, frequency and severity of IDA symptoms compared to the moderate IDA group. Headache, dizziness and tiredness were the most prevalent symptoms in both subgroups. Similarly, the difference in menstrual flow volume did not seem to be associated with a significant difference in the pattern of symptom occurrence. The pregnant subjects in the study reported a higher frequency of shortness of breath and fast heartbeat at baseline which is consistent with the previously reported finding that these are common overlapping symptoms among healthy pregnant women in general [13]. This occurrence can be attributed to increased oxygen demand for fetal needs and expanded maternal blood volume, resulting in reduced oxygen supply to tissues, which leads to breathing difficulties and tachycardia [14]. IDA can worsen the reduced oxygen delivery, increasing the likelihood of these symptoms. The improvement in the VAS scores for these symptoms after treatment indicates that in the SANOIN study, these symptoms are, at least partially if not entirely, caused by IDA (Supplementary Figure 2).
By understanding the possible links between the mentioned symptoms and ID or IDA in individuals at risk, one can improve the early detection of these conditions. This in turn allows for prompt intervention through oral iron supplementation, helping to prevent the progression of ID or IDA to a more severe stage.
The case for optimal elemental iron dose for oral supplementation
The overall findings across the subgroups from this analysis add to the growing evidence supporting the efficacy of lower daily elemental iron dose of 30-60 mg along with multiple micronutrients. This study shows a preference for lower doses among physicians particularly for subjects with mild IDA, normal menstrual flow or pregnant, which reflects the real-world clinical practice, aiming to minimize side effects while maintaining an effective dosage for the patients. A higher initial dose of 60 mg, followed by maintenance with the same dosage can be considered for individuals who might benefit from faster Hb correction, such as those with moderate IDA, heavy to very heavy menstrual flow or pregnant women.
Study limitations
One of the limitations of this subgroup analysis of the SANOIN study is the single-arm, observational nature of the original study. A study design including an active comparator or placebo would offer more comprehensive insights on the benefits of the study drug in the study populations. Another limitation was the small sample sizes in the very heavy menstrual flow and pregnant subgroups. While analyses performed on these subpopulations revealed interesting insights on the effectiveness of the study drug in improving hematological parameters and providing IDA symptoms relief, these results need to be interpreted carefully owing to the small sample size. Further investigation is warranted to evaluate the benefits of a higher dose of the study treatment in pregnant women.
Conclusion
In this post-hoc analysis of the pivotal SANOIN study, it was found that recognition and evaluation of IDA symptoms play a significant role in the early identification of IDA, including patients with mild IDA. This observation debunks the common perception that IDA symptoms only occur in more advanced stages of IDA such as those with moderate or severe condition. Ferrous gluconate at 30-60 mg elemental iron with multivitamins and minerals (Sangobion® IRON+) was demonstrated to be fast and effective in improving IDA symptoms, Hb and ferritin levels across key subgroups irrespective of the subject’s IDA status, menstrual flow, and pregnancy status. The effectiveness of ferrous gluconate plus multivitamins and minerals in resolving IDA symptoms and hematological parameters of IDA even in subgroups at relatively higher risk (moderate IDA, heavy menstrual flow, pregnant women) was demonstrated. The findings from this subgroup analysis demonstrate the benefits of early intervention for anemia correction, and further support the consideration for longer treatment duration in certain subgroups to fully replete iron stores.
Acknowledgments
We would like to express our sincere appreciation to all the investigators who participated in this study. Their invaluable contributions and dedication have been instrumental in the successful execution and completion of the research. We would also like to extend our heartfelt appreciation to the P&G Personal Health Care team members, particularly Jass Liew and Sue Aspley, who were involved in this study. Their expertise, dedication, and hard work have been indispensable in the successful execution and analysis of the research. Medical writing assistance was provided by The Other Health Group Pte Ltd., the work was funded by P&G. The authors are fully responsible for all content and editorial decisions for this manuscript.
Conflicts of Interest
NS Comia and Christopher Joseph L. Soriano have no conflicts to report. Roger Gibb, Poonam Sule and James WC Sia are employed by Procter and Gamble. Roger Gibb and Poonam Sule own stocks in Procter & Gamble.
Funding
This study was funded by the Research & Development department of Personal Healthcare Division of Procter and Gamble.
References
- Fernandez-Jimenez MC, Moreno G, Wright I, Shih PC, Vaquero MP, Remacha AF. Iron deficiency in menstruating adult women: Much more than anemia. Womens Health Rep (New Rochelle). 2020;1(1):26-35.
[Crossref] [Google Scholar] [PubMed]
- Pai RD, Chong YS, Clemente-Chua LR, Irwinda R, Huynh TN, Wibowo N, et al. Prevention and management of iron deficiency/iron-deficiency anemia in women: An Asian expert consensus. Nutrients. 2023;15(14):3125.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Accelerating anaemia reduction: A comprehensive framework for action. 2023.
- Kinyoki D, Osgood-Zimmerman AE, Bhattacharjee NV, Kassebaum NJ, Hay SI. Anemia prevalence in women of reproductive age in low-and middle-income countries between 2000 and 2018. Nat Med. 2021;27(10):1761-1782.
[Crossref] [Google Scholar] [PubMed]
- Soriano CJL, Abalos V, Romero CM, Manalastas R, Villanueva G, Sule P, et al. Management of iron deficiency anemia with fixed dose combination of ferrous gluconate, multivitamins and multi-mineral capsule: A 12 week, open label single arm prospective, non-interventional (observational) study in Philippines. J Clin Exp Pharmacol. 2023;13:385.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
[Crossref] [Google Scholar] [PubMed]
- MacLean B, Sholzberg M, Weyand AC, Lim J, Tang G, Richards T. Identification of women and girls with iron deficiency in the reproductive years. Int J Gynaecol Obstet. 2023;162:58-67.
[Crossref] [Google Scholar] [PubMed]
- Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020;223(4):516-524.
[Crossref] [Google Scholar] [PubMed]
- O'Toole F, Sheane R, Reynaud N, McAuliffe FM, Walsh JM. Screening and treatment of iron deficiency anemia in pregnancy: A review and appraisal of current international guidelines. Int J Gynaecol Obstet. 2024;166(1):214-227.
[Crossref] [Google Scholar] [PubMed]
- Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: How much and how often?. Mol Aspects Med. 2020;75:100865.
[Crossref] [Google Scholar] [PubMed]
- Republic of the Philippines. Updated Guidelines on Micronutrient Supplementation (Vitamin A, Iron and Iodine). Department of Health. 2021.
- Fakultas Kedokteran Universitas Indonesia. Anemia Defisiensi Besi Pada Kehamilan. Departemen Obstetri dan Ginekologi. 2021.
- Goland S, Perelman S, Asalih N, Shimoni S, Walfish O, Hallak M, et al. Shortness of breath during pregnancy: Could a cardiac factor be involved?. Clin Cardiol. 2015;38(10):598-603.
[Crossref] [Google Scholar] [PubMed]
- Abu-Ouf NM, Jan MM. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med J. 2015;36(2):146-149.
[Crossref] [Google Scholar] [PubMed]
Citation: Comia NS, Soriano CJL, Gibb R, Sule P, Sia JWC (2024) Effectiveness of Ferrous Gluconate Plus Multivitamins and Minerals by Iron Deficiency Anemia Status, Menstrual Blood Flow and Pregnancy Status: A Subgroup Analysis of the SANOIN Study. J Clin Exp Pharmacol. 14:430.
Copyright: © 2024 Comia NS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.