Indexed In
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 13, Issue 3
Effectiveness of Convalescent Plasma to Treat COVID-19
Ephrem Awulachew*, Kuma Diriba, Asrat Anja and Firehiwot BelaynehReceived: 20-Apr-2020, Manuscript No. CMO-24-3932; Editor assigned: 23-Apr-2020, Pre QC No. CMO-24-3932 (PQ); Reviewed: 07-May-2020, QC No. CMO-24-3932; Revised: 15-Jul-2024, Manuscript No. CMO-24-3932 (R); Published: 12-Aug-2024, DOI: 10.35248/2327-5073.24.13.401
Abstract
Background: Currently, coronavirus disease (COVID-19) was reported in more than 204 countries. As of April 10, 2020, a total of 1,605,729 confirmed cases and 95,766 deaths had been reported worldwide. There are no approved specific antiviral agents targeting the novel virus. Convalescent plasma transfusion might be effective against the infection. Food and Drug Administration (FDA) has also approved the emergency use of investigational COVID-19 convalescent plasma to treat severely ill COVID-19 patients.
Objective: The aim of this study was to systematically review disease outcome and effectiveness of convalescent plasma to treat COVID-19.
Method: We searched literature published in English from December 20/2019 to April 10/2020 on electronic databases. Using R software we have conducted a systematic analysis, frequency, mean, standard deviation and chisquare test.
Result: The average age of the participants in the included was 55.7 with a standard deviation of 13.9. The average days of recovery or test negative for the COVID-19 PCR test after convalescent plasma therapy were 9.6 days (95% CI 2-30 days). About 43% (9/21) had a history of comorbidity. The average date of recovery of patients with co-existing chronic diseases infected by COVID-19 after convalescent plasma therapy was about 12 days relatively prolonged than patients without the co-existing disease (7.6 days). No series adverse effects have been demonstrated in patients who have received convalescent plasma transfusion.
Keywords
COVID-19; Convalescent plasma; Plasma; SARS CoV-2; Severe acute respiratory syndrome
Introduction
Background
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV- 2) is a novel strain of coronavirus that was first detected in the city of Wuhan, China. It was named as coronavirus disease 2019 (COVID-19) by World Health Organization (WHO) [1]. The outbreak SARS-CoV-2 was named as a pandemic disease by WHO on March 11, 2020. Currently, the disease was reported in more than 204 countries. As of April 10, 2020, a total of 1,605,729 confirmed cases and 95,766 deaths had been reported worldwide. Currently, due to an alarmingly spreading of the virus worldwide, the incidence and deaths are increasing.
However several efforts made to treat COVID-19, there are no approved specific antiviral agents targeting the novel virus and no vaccines have been fully tested for safety and efficacy while some drugs are still under investigation [2].
Convalescent Plasma (CP) therapy is an immunotherapy that has been applied to the prevention and treatment of many infectious diseases for more than one century. So, the use of convalescent plasma is not a new method rather it was used for poliomyelitis, measles, mumps and influenza, Severe Acute Respiratory Syndrome (SARS), several hemorrhagic fevers such as Ebola and other viral infections treatments. Even more recently during H1N1 influenza virus pandemics in 2009-2010, convalescent serum antibody preparations obtained by apheresis were used to treat individuals with severe H1N1 infection requiring intensive care. This was the baseline lesson for convalescent plasma to treat COVID-19 [3].
Plasma from people who have recovered from COVID-19 may contain antibodies to the virus that causes the disease and might be effective against the infection. Food and Drug Administration (FDA) has also approved the emergency use of investigational COVID-19 convalescent plasma to treat severely ill COVID-19 patients. The general principle of passive antibody therapy is that it is more effective when used for prophylaxis than for treatment of disease.
When used for therapy, the antibody is most effective when administered shortly after the onset of symptoms. So it is required to evaluate the effectiveness of convalescent plasma therapy. So this study planned to conduct systematic analysis of literatures on convalescent plasma treatment in severely ill COVID-19 patients [4].
Objective
The main aim of this study is to systematically review the effectiveness of convalescent plasma to treat COVID-19.
Materials and Methods
Types of participants
The study population of interest was human subjects of any age or sexes who were hospitalized or were in ICU with COVID-19 infection. The intervention of interest was convalescent plasma transfusion. Outcomes of disease progress after convalescent therapy that was extracted from included studies was used predict the clinical effectiveness of therapy [5].
Search strategy
We searched literatures published in English from December 20/2019 to April 10/2020 on electronic data bases PubMed, Cochrane library, EMBASE, Escopus, Hinari, CINAHL, Open Access Journals (OAJ) and Google Scholar, for studies conducted trial of convalescent plasma treatment of COVID-19. We simultaneously searched reference lists of all recovered articles for potentially eligible studies.
All identified keywords and mesh terms were combined using the “OR” operator and “AND” operator for searching literatures. Keywords used in the search included were those that explain infection of severe acute respiratory syndrome 2 (SARS CoV-2) (i.e., COVID-19, severe acute respiratory syndrome 2, SARS CoV-2, novel corona virus). Full-text articles were retrieved after review of the title and abstract.
Inclusion and exclusion criteria
Inclusion criteria: All studies published in English from December 20/2019 to April 10/2020 were included in the review. There was no restriction to study design made.
Data extraction
Data were extracted from included study by two investigator (EA and KD) using a standardized data extraction form. Then theextracted data were merged together for systematic analysis. Primary outcomes extracted from each study were, the citation details, sample size, number of cases, year of publication, location of study, method of identification of COVID-19. Secondary outcomes for this study included clinical data including the stage of disease, treatment received, co-morbidities, co-infection and dates of recovery after convalescent plasma transfusion, progress opacities in the lung [6].
Statistical analysis
The R software was used to conduct systematic analysis, frequency, mean, standard deviation and chi-square test.
Results
Identified studies
Following the initial search of data bases four eligible studies have been accessed and were reviewed by the authors. Following methodological quality assessment, all 4 articles were eligible and included in this systematic review and meta-analysis. In the included studies the total of 21 patients infected with COVID-19 were obtained convalescent plasma therapy. All 4 papers were published in English [7].
Characteristics of included study
Of the 4 studies included in the systematic review and metaanalysis, all studies reported the detail case report of COVID-19 patients. All patients were severely ill and were put to Intensive Care Unit (ICU). All 4 studies included in the systematic review and meta-analysis, infections of COVID-19 were examined using PCR which is of high quality diagnostic method. Most of the studies conducted are of higher quality because of method of diagnosis using highly sensitive and specific PCR techniques.
Systematic analysis outcome
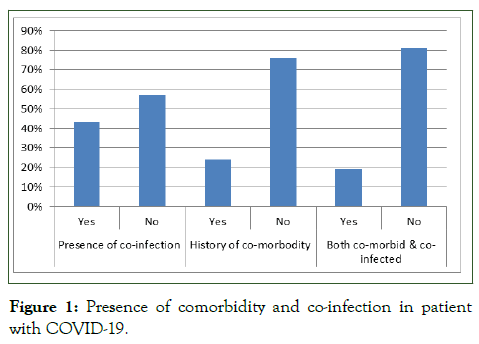
The average age of the participants in the included was 55.7 with standard deviation of 13.9. From a total of 21 patients 12 of them were male. The average days between admissions to start of convalescent plasma therapy was 10 ± 4.5 days and the average days of recovery or test negative for COVID-19 PCR test after convalescent plasma therapy was 9.6 days (95% CI 2-30 days). About 43% (9/21) had history of comorbidity and about 24% (5/21) have co-infections, while 19% (4/21) patients are have both history of co-existing disease and co-infections (Figure 1) [8].
Figure 1: Presence of comorbidity and co-infection in patient with COVID-19.
Common sign and symptoms and complications of in patients of COVID-19
In this systematic review the most common symptoms at disease onset were fever, cough with clear sputum and shortness of breath, while less commonly observed sign and symptoms were; chest pain, sore throat, myalgia and arthralgia (Figure 2). Majority of complications observed in the study participants were severe acute respiratory distress (85.7%), pneumorrhagia, cystorrhagia and gastrointestinal bleeding, myocardial damage and cardiac dysfunction [9].
Figure 2: Most common clinical features for admission of COVID-19.
Co-morbidity, co-infection and rate recovery of patients with COVID-19 after treatment by CP
According to data of systematic review and meta-analysis there was significant rate of recovery between patients with comorbidity and patients without co-morbidity. Patients without co-existing chronic diseases infected by COVID-19 relatively recovered earlier than those who have co-existing disease after convalescent therapy.
They test negative for COVID-19 PCR test after an average of 7.6 days. The average date of recovery of patients with coexisting chronic diseases infected by COVID-19 after convalescent plasma therapy was about 12 days. On the other hand patients with COVID-19 infection and who have both co-existing chronic diseases and co-infection relatively have prolonged time of recovery or require relatively long time to test negative for COVID-19 PCR test.
Reduction of pulmonary lesions on chest X-ray examinations
According to chest CTs, all patients showed different degrees of absorption of pulmonary lesions after convalescent plasma transfusion. With average range of 1-10 days after convalescent plasma therapy, a chest X-ray revealed further resolution of lung infiltrates which is from partial to persistent absorption of consolidation or absorption of interstitial pneumonia was observed.
Major anti-viral agents used for treatment of COVID-19
Most frequently used antiviral agents were Arbodol 62% (13/21), Lopinavir-ritonavir 43% (9/21) and Interferon alpha 43% (9/21) and the other antiviral drugs used were: Oseltamivir, ribavirin, favipiravir, hydroxychloroquine, remdesivir and peramivir. In more than 76% (16/21) patients antiviral agents were discontinued due to gradual recovery of patients after convalescent plasma transfusion. The other treatment given for the patients was anti-inflammatory drug methylprednisolone 71% (15/21) [10].
Convalescent plasma adverse effects
None of the studies analyzed in this systematic review reported serious adverse events. During convalescent plasma transfusion to severely ill COVID-19 patients, no serious adverse effects were reported. Only one patient (1/21) showed minor facial red spot.
Discussion
The therapeutic benefits of convalescent plasma transfusion for infectious diseases began in the 20th century. Lesson learnt from proven effectiveness of convalescent plasma as a potential treatment of MERS-CoV, SARS-CoV and H5N1 influenza in the last decades can hold true that convalescent plasma transfusion can be an important optional treatment in patients with critical clinical disease stage in the absence of specific treatment of COVID-19 infection.
In this review we have demonstrated that there was significant difference of rate of recovery between patients with co-morbidity and patients without co-morbidity. Patients without co-existing chronic diseases infected by COVID-19 relatively recovered earlier than those who have co-existing disease after convalescent therapy [11].
Majority of complications observed in the study participants were severe acute respiratory distress, pneumorrhagia, cystorrhagia and gastrointestinal bleeding, myocardial damage and cardiac dysfunction. This could be reason to death in patients infected by COVID-19.
According to data this systemic review to evaluate the clinical effects of convalescent plasma therapy after evidence recovery of patients, partial to persistent absorption of consolidation within the lung, ex-tubation of mechanical ventilation within 1-3 days of CPT showed that convalescent plasma can be a promising treatment option for severe COVID-19 patients.
As a limitation there is no well-designed study conducted to see effectiveness of convalescent plasma transfusion therapy we included systematic analysis of detail case reports of patients who have received convalescent plasma transfusion therapy for COVID-19 and the clinical outcome after transfusion. The other limitation is that this study is a systematic review of case series which does not have control [12].
Conclusion
This study indicated that convalescent plasma transfusion might be a potential treatment for critically ill patients infected with COVID-19 and it can be helpful to reduce the risk of mortality of critically ill patients. We demonstrated no serious adverse reactions associated with the transfusion of convalescent plasma. This review suggests that convalescent plasma from patients who have recovered from COVID-19 infection might be an option to treat patients without causing severe adverse effects. Adequately assessing safety and efficacy of convalescent plasma therapy is essential. So, this study recommends that safety and efficacy of convalescent plasma transfusion in SARS-CoV-2 infected patients should be studied using well-designed clinical trial.
Conflicts of Interest
There are no conflicts of interests to declare.
Availability of Data and Materials
All the datasets generated and analyzed during the review are included in this article.
Author's Contribution
Ephrem Awulachew, Kuma Diriba, Asrat Anja, Firehiwot Belayneh designed the study, extracted, critically reviewed and analyzed data and wrote the first draft of the manuscript and approved the manuscript.
Funding Source
This manuscript was prepared independently without any funding support.
Consent for Publication
Not applicable.
Ethics Approval and Consent to Participate
Not applicable.
Acknowledgments
None.
References
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: A pilot study. MedRxiv. 2020:2020-2023.
- Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14):e149.
[Crossref] [Google Scholar] [PubMed]
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582-1589.
[Crossref] [Google Scholar] [PubMed]
- Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158(1):e9-e13.
[Crossref] [Google Scholar] [PubMed]
- Robbins JB, Schneerson R, Szu SC. Perspective: Hypothesis: Serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171(6):1387-1398.
[Crossref] [Google Scholar] [PubMed]
- Tiberghien P, de Lamballerie X, Morel P, Gallian P, Lacombe K, Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID‐19 treatment: Why and how? Vox Sang. 2020;115(6):488-494.
[Crossref] [Google Scholar] [PubMed]
- Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-456.
[Crossref] [Google Scholar] [PubMed]
- Roback JD, Guarner J. Convalescent plasma to treat COVID-19: Possibilities and challenges. Jama. 2020;323(16):1561-1562.
[Crossref] [Google Scholar] [PubMed]
- Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: Passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38:e66-73.
[Crossref] [Google Scholar] [PubMed]
- Rambar AC. Mumps: Use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child. 1946;71(1):1-3.
[Google Scholar] [PubMed]
- Park WH, Freeman RG. The prophylactic use of measles convalescent serum. J Am Med Asso. 1926;87(8):556-558.
- Park WH. Therapeutic use of antipoliomyelitis serum in preparalytic cases of poliomyelitis. J Am Med Asso. 1932;99(13):1050-1053.
Citation: Awulachew E, Diriba K, Anja A, Belayneh F (2024) Effectiveness of Convalescent Plasma to Treat COVID-19. Clin Microbiol. 13:401.
Copyright: © 2024 Awulachew E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.