Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
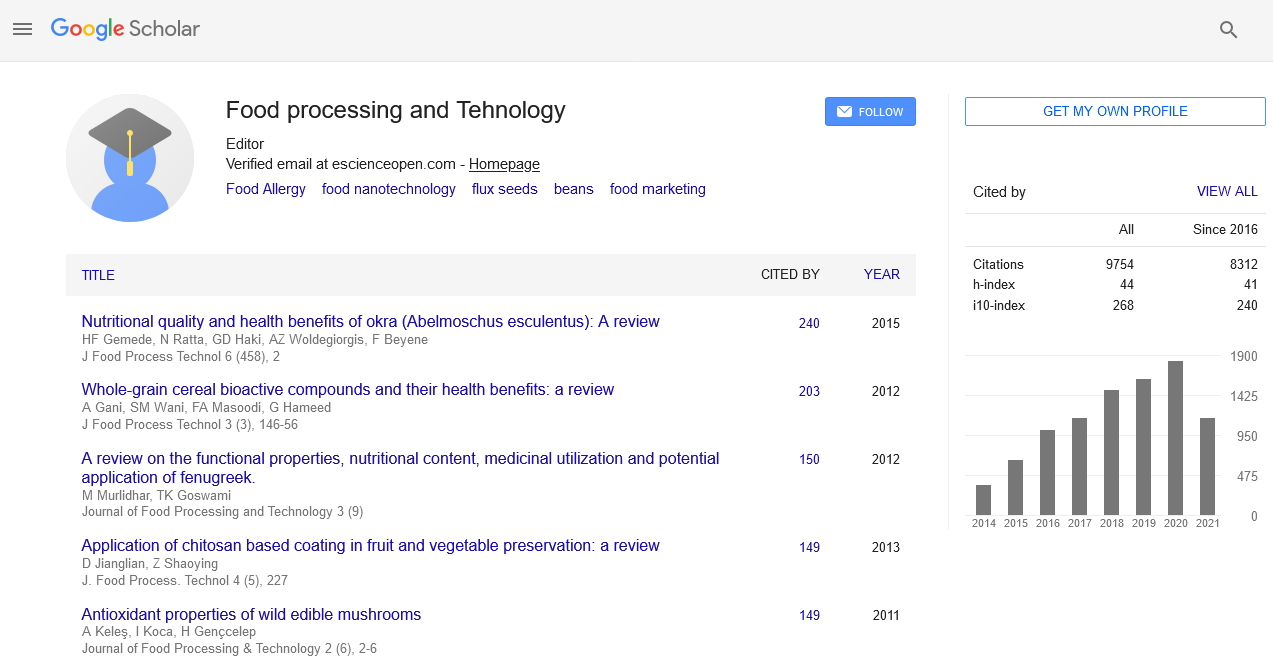
Research Article - (2020) Volume 11, Issue 5
Effect of Long-Term Storage, Heat and High Pressure Processing on Patulin Reduction in Tomato Products
Beatrice Scaccabarozzi1, Andrea Brutti1 and Elettra Berni2*2Stazione Sperimentale per l’Industriadelle Conserve Alimentari, SSICA, Viale F. Tanara, 31/A, 43121 Parma, Italy
Received: 21-Apr-2020 Published: 15-May-2020, DOI: 10.35248/2157-7110.20.11.829
Abstract
In this paper, the effect of long-term storage on patulin reduction in different tomato products (juice, puree, pulp, paste, and ketchup) was assessed, together with the evaluation of patulin heat-resistance and baro-tolerance in tomato juice. For these purposes, different extraction methods for patulin detection in tomato products using an RP-HPLC apparatus were preliminarily assessed. Concerning the effect of long-term storage (up to six months) on patulin, at 25°C a non-linear, progressive reduction of the toxin was observed for all tomato products tested during storage. Tomato paste was the matrix where the most marked decrease was observed, patulin being reduced to undetectable levels just after one month at 25°C. It was followed by tomato puree, where the toxin was reduced to unquantifiable levels after six months at 25°C. Differently, the toxin was always present at detectable levels in tomato pulp, puree, and ketchup where it was respectively reduced by 64%, 81%, and 88% after six months at 25°C. Concerning the effect of thermal treatment and High-Pressure Processing on patulin, the DT value (D95=270 min) calculated for tomato juice was much higher than times usually applied in the industrial practice on nonconcentrated tomato products, and the highest time/pressure combination applicable at an industrial level on tomato juice (600 MPa for 10 min) did not give any decimal reduction to patulin concentration. Since all the strategies applied did not prove sufficient to inactivate patulin in all products considered, tomato products other than pastes could represent a risk for this toxin, in case substantial spoilage by patulin-producing fungi occurred on tomato fruits and an insufficient amount of detoxing substances such as L-ascorbic acid was present in the above-mentioned products.
Keywords
Patulin; Tomato products; Solid phase extraction; Heat-resistance; HPP
Introduction
Fresh tomatoes are highly susceptible to spoilage by a wide range of fungal genera, due to their thin skin and to the influence that factors as bad weather or detrimental harvesting conditions can have during their production process. Their associated mycobiota is constituted by both phytopathogenic and saprophytic species, Alternaria being the most occurring genus on moldy tomatoes and prevailing over genera such as Penicillium, Stemphylium, Cladosporium, Rhizopus, Mucor, Geotrichum, Drosophila, Rhizoctonia, and Fusarium [1-4]. Next to them, heat resistant species such as Byssochlamys nivea and Neosartorya fischeri can be sometimes detected on these fruits [5], their contamination is a matter of concern for food industries because of their ability to survive to pasteurization treatment and spoil tomato-based processed products [6]. Among the associated mycobiota, close attention must be paid to toxigenic fungi, as they can spoil lesioned fruits and then produce a wide range of secondary metabolites or mycotoxins [1], highly toxic substances that are considered carcinogenic, mutagenic or teratogenic toward animals or humans [7]. About this, Penicillium expansum and Byssochlamys nivea are considered the main responsible for Patulin (PAT) production [2,3], whereas Alternaria alternata, Alternaria tenuissima sp.-group, and Alternaria arborescens sp.- group proved able to prevalently and simultaneously produce alternariol, alternariol monomethyl ether and tenuazonic acid in fresh tomatoes [4,8-11]. Among these mycotoxins, only PAT is regulated only at European level in tomato juice and tomatobased products for children under three-years-old, where the threshold value is respectively equal to 50 μg/kg and 10 μg/kg [12] since from 2012 tomato is considered as a fruit by the European legislation [13]. On the contrary, for Alternaria toxins, no limits have been so far set in tomato products by the European Commission or in the rest of the world.
PAT is highly reactive and therefore a very unstable molecule. Its stability proved to be affected by a wide range of chemical molecules, L-ascorbic acid, and thiol compounds being the more effective in toxin degradation. In fruit juices, a little reduction during short-term storages was observed in apple juices [14-16], while a higher degradation was registered in orange juice [14] and apricot or tropical juice [17]. Similarly, in tomato juice, it has been shown that PAT was partially reduced just within the first 24 h of storage, no further significant reduction being observed after five days [18]. In any case, no literature data exist about the effect that long-term storage can have on PAT reduction on fruit or tomato products.
Despite a low risk to find tomato products contaminated with PAT [18-21], toxin detection in such products must be monitored, also due to the conflicting results that continue to be generated by different authors about its heat-stability [17,22-24] and baro-tolerance [25-28] in fruit-based foods other than tomato products.
In 2000, an AOAC official method has been validated for PAT detection in clear or cloudy apple juices and apple purees [29,30], but it proved applicable just at matrices with PAT concentrations over 25 ng/g. In addition to this, for more complex matrices such as tomato products it could not be considered the most suitable analytical method, due to the presence of various molecules that might interfere with the toxin detection and impurities that could furtherly be retained on the column.
For these reasons, the aim of this work was: (i) to assess different extraction methods for PAT detection in tomato products, to find out the most efficient for our purposes using an HPLC apparatus; (ii) to assess the effect of long-term storage on PAT degradation in different tomato products artificially contaminated with the toxin; and (iii) to evaluate PAT heatresistance and baro-tolerance in tomato juice.
Materials and Methods
Samples
Five tomato products available on the market were collected in January 2017. A tomato juice (5.2°Brix, pH=4.34), a tomato pulp (6.7°Brix, pH=4.27), a tomato puree (8.7°Brix, pH=4.27), tomato ketchup (18.4°Brix, pH=3.95), and a tomato paste (30.3°Brix, pH=4.23) were separately transferred into 50 ml PYREX® round-bottom sterile tubes with screw cap. Each aliquot was then spiked with PAT in sterile conditions, to avoid fungal spoilage from indoor fungi, and used for tests.
Chemicals and standards
PAT stock solution (24.50 μg/ml) was purchased from RBiopharm (Glasgow, Scotland) as dissolved in acetonitrile and stored at 4°C no longer than one year. Working PAT solutions were prepared by properly diluting the solvent mixture of the stock solution in the RP-HPLC mobile phase to give the final desired concentration. The Pectinase Enzyme (4261 units/ml) used for sample clarification was purchased by R-Biopharm (Glasgow, Scotland) and maintained at (2-8)°C no longer than one year.
MycoSep®228 AflaPat and MultiSep®228 AflaPat clean-up columns were obtained from ROMER Labs® Inc. (Union, MO, USA). Paper filters were purchased from Filter-Lab by Filtros Anoia (Barcelona, Spain). Ethyl acetate, acetonitrile, acetic acid glacial, and methanol were HPLC grade and were obtained from Carlo Erba (Milan, Italy), whereas sodium sulfate anhydrous was obtained from Sigma-Aldrich (St. Louis, MO, USA). Doubledistilled water was daily produced in our laboratory by a Millipore water purification device (Billerica, MA, USA).
Extraction and clean-up procedure for PAT determination
Different extraction procedures were used as a comparison for PAT determination in different tomato products.
SSICA method: This procedure was based on a method developed by Spotti and Berni [31] for apple-based products. Briefly, 25 g of sample was supplemented with 0.15 ml of Pectinase Enzyme and left overnight at room temperature (this preliminary step was carried out just for tomato puree, tomato pulp, and ketchup). Each sample was then centrifuged at 1957 × g for 10 min and 7 ml of the clarified phase were transferred into a 20 × 200 mm glass test tube with 14 ml of ethyl acetate. The mixture was vortexed at high speed for two minutes and then transferred into a separating funnel where both the aqueous and the organic phase were let to separate. The lower aqueous layer was collected in the same glass test tube previously used, added with other 14 ml of ethyl acetate and the extraction process was repeated for a second and a third time. Once that glass tube was rinsed with 5 ml of ethyl acetate and the solvent collected in the separating funnel, the aqueous phase was discharged, whereas the organic phase containing the toxin was filtered through a filter paper containing 1.5 g of anhydrous sodium sulfate and collected in a 250 ml glass flask, to be dried by a rotary evaporator (Büchi Italia, Cornaredo, Italy) at 38°C. Residues were then dissolved with 7 ml of a solution of acetonitrile: water (84:16) and passed through a clean-up Mycosep®228 AflaPat columns at a rate of 5 ml/min. A total of 2 ml of filtered solution were then evaporated to dryness under an N2 stream and rapidly re-dissolved in 0.4 ml of water acidified with acetic acid glacial (pH=4). Each sample was finally collected in a 2.0 ml glass vial (Microcolumn, Desio, Italy) for HPLC injection.
Mycosep®228 AflaPat method: This procedure was based on a general procedure obtained by the clean-up column’s manufacturer with little modifications.
For tomato puree, tomato pulp, and ketchup, 10 g of ground sample previously diluted with 8 ml of distilled water were supplemented with 0.15 ml of pectinase and left overnight at room temperature. Then, 32 ml of acetonitrile were added to the above mixture and vortexed at high speed for two minutes. After filtration on a paper filter, 8 ml of the extract were passed through a clean-up Mycosep®228 AflaPat columns at a rate of 5 ml/min. A total of 2 ml of filtered solution were then evaporated to dryness under an N2 stream and rapidly redissolved in 0.4 ml of water acidified with acetic acid glacial (pH=4). Each sample was finally collected in a 2.0 ml glass vial (Microcolumn, Desio, Italy) for HPLC injection.
For tomato juice, 5 g of sample was supplemented with 20 ml of acetonitrile and vortexed at high speed for one minute. Then, 8 ml of the top layer of the extract were passed through a clean-up Mycosep®228 AflaPat columns at a rate of 5 ml/min. A total of 2 ml of filtered solution were then evaporated to dryness under an N2 stream and rapidly re-dissolved in 0.4 ml of water acidified with acetic acid glacial (pH=4). Each sample was finally collected in a 2.0 ml glass vial (Microcolumn, Desio, Italy) for HPLC injection.
For tomato paste, 5 g of sample was supplemented with 20 ml of acetonitrile: water (84:16) and vortexed at high speed for two minutes. Then, 8 ml of the top layer of the extract were passed through a clean-up Mycosep®228 AflaPat columns at a rate of 5 ml/min. A total of 2 ml of filtered solution were then evaporated to dryness under an N2 stream and rapidly redissolved in 0.4 ml of water acidified with acetic acid glacial (pH=4). Each sample was finally collected in a 2.0 ml glass vial (Microcolumn, Desio, Italy) for HPLC injection.
Multisep® 228 AflaPat method: This procedure was based on a general procedure obtained by the clean-up column’s manufacturer with little modifications and was identical to Mycosep® method, except for the use of Multisep® 228 AflaPat columns instead of Mycosep®228 AflaPat columns.
HPLC analysis
Chromatographic analyses were performed using a Jasco Model PU-1580 pump equipped with a Tracer Extrasil ODS-2 standard bore column (150 × 4.6 mm, 5 μm particle size, Teknokroma, Barcellona, Spain), a Jasco autosampler (Model AS-1555; 0.1 ml loop) and a Jasco UV detector (Model UV-1575, λ=276 nm). The system was computer-controlled by a Jasco LC-NetII/ADC for data handling. Double-distilled water was used as a mobile phase, at a flow rate of 1.0 ml/min. The calibration curve was based on the analysis of working standard solutions in the range (3.1-24.8) ng (3.1, 6.2, 9.3, 12.4, 18.6, 24.8) ng/0.1 ml loop, as triplicate. Experimental data were corrected by the proper Dilution Factor (DF), to find the corresponding concentration (ng/g). The Limit of Detection (LOD) and the Limit of Quantification (LOQ) were calculated according to the following equations: LOD=3 × (sa/b) and LOQ=10 × (sa/b), where sa is the standard deviation of the intercept of the regression line obtained from the calibration curve, and b is the slope of the line [32]. LOD and LOQ were equal to 0.8 ng/g and 2.6 ng/g for SSICA Method, whereas they amounted to 4.0 ng/g and 13 ng/g for Mycosep® and Multisep® methods. Recovery experiments were performed in triplicate on PAT-free samples. After 1 h, samples were analyzed using the protocols previously described.
Effect of long-term storage on PAT content
Tomato products listed in the “Samples” paragraph were used. They were previously spiked with PAT as previously described, to obtain a final concentration of 98 ng/g for tomato juice, tomato puree, tomato pulp or ketchup, and 245 ng/g for tomato paste. All spiked products were then incubated at 25°C for up to a maximum of six months. Each inoculated aliquot was vortexed just after the inoculum and every two weeks, to assure homogeneity of spiked tomato products.
Heat treatment
A tomato juice (5.2°Brix, pH=4.34) previously spiked with 735 ng/g of PAT was used. Polythene bags (130 × 78 mm) containing 10.0 mL of sample were sealed with no air present and plunged into a stirring water bath (FA90, Falc, Treviglio, Italy) equipped with a Platinum-sensor probe (Delta HOM, Padua, Italy) for a continuous temperature check. Thermal treatment was carried out at 95°C up to a maximum of 240 min. After heat treatment, bags were removed from the water bath, rapidly cooled in the water at 4°C, and opened under sterile conditions.
High Pressure Processing (HPP)
Hyperbaric treatment was carried out using an Avure QFP 35® system (Avure Technologies, JBT Group, Middletown, OH, USA). The system has a seating capacity of 35 l and can reach a pressure of 600 MPa. The compression times to reach the maximum pressure are equal to two min (starting with an empty pressurization chamber), whereas decompression is instantaneous. The system allows treatments in adiabatic conditions under controlled temperatures (from 20 to 90)°C.
A tomato juice (5.2°Brix, pH=4.34) previously spiked with 349.4 ng/g of PAT was used. Polythene bags (130 × 78 mm) containing 10.0 mL of sample were sealed with no air present and treated at 600 MPa up to 10 min. The temperature started at 10°C and then increased 3°C every 100 MPa, up to a final value of 28°C at 600 MPa.
Statistical analysis
StatGraphicsPlus Professional 16.0.03 (Statpoint Technologies, Inc., Warrenton, VI, USA) was used for the statistical elaboration of PAT levels in different tomato products during storage. Data were presented as mean values (ng/g) ± Standard Deviation (SD). The method used to discriminate among the means was Fisher’s Least Significant Difference (LSD) procedure. Significant differences were calculated at the 0.05 level.
SPSS® Version 11.5 (SPSS Inc., Chicago, IL, USA) was used for the statistical elaboration of heat-resistance data that were presented as mean values (Log ng/g) ± Standard Deviation (SD). Conversion of detected values into logarithmic data allowed us to use the linear regression function for the determination of the D value, defined as the time required bringing a 1-log reduction in a concentration at a given temperature.
Microsoft Excel 2013 (Microsoft, Redmond, WA, USA) was used for the graphical and statistical elaboration of HPP data that were presented as mean values ± standard deviation (SD). Significant differences were calculated using the ANOVA model at a 95% confidence level (p>0.05).
Results
Extraction and clean-up procedure for PAT determination
SSICA method was checked on tomato products since in 2003 it proved to give very good recoveries (82%-96%, respectively on apple or pear concentrated juices and purees) and a very good definition of the chromatographic peaks [31]. Simultaneously, a comparison with other extraction techniques was carried out to find, if any, a more efficient method in terms of recoveries, time consumption, and solvent reduction using an HPLC apparatus.
The results of recovery tests are reported in Figure 1. Among the methods used, for those products where clarification was needed (tomato puree, tomato pulp, and ketchup) SSICA method proved the most effective in terms of sample purification, as the synergic effect of ethyl acetate as the extraction solvent and Solid-Phase Extraction (SPE) resulted in a clear chromatogram and in the absence of interfering substances that could mask PAT. For these products, mean recoveries ranged between 51% and 53 %. On the contrary, for tomato products that can be analyzed without being clarified (tomato juice and tomato paste), Mycosep® method proved the most efficient in terms of recoveries, separation of the chromatographic peaks and reduction in the intensity of HMF peak, thus allowing us to analyze juice samples that were thermally over-treated when assessing PAT heat-resistance. For tomato paste and tomato juice, mean recoveries respectively amounted to 54% and 73 %. Mycosep®228 AflaPat columns proved essential for PAT detection for all tomato products tested.
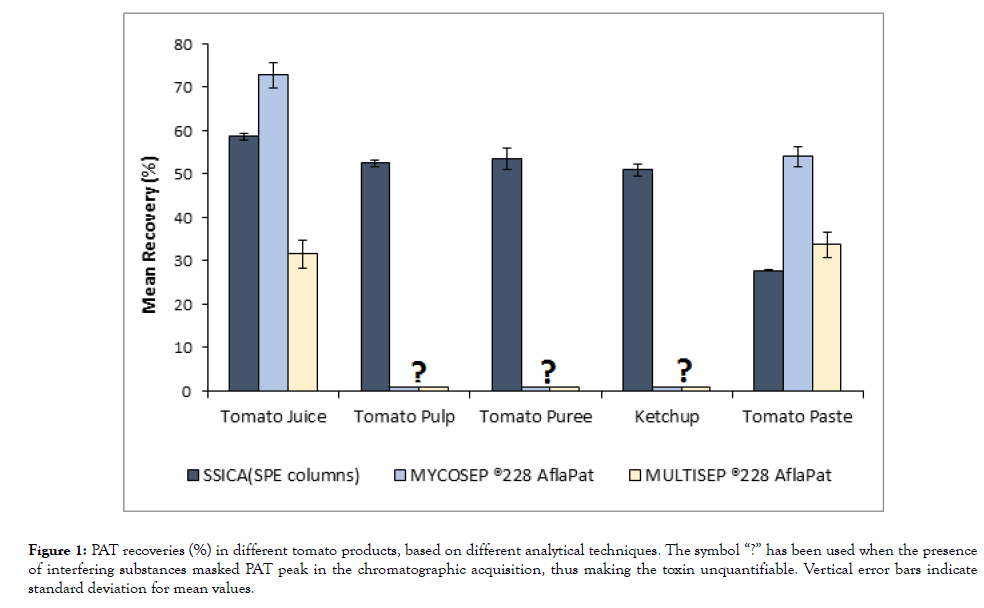
Figure 1: PAT recoveries (%) in different tomato products, based on different analytical techniques. The symbol “?” has been used when the presence of interfering substances masked PAT peak in the chromatographic acquisition, thus making the toxin unquantifiable. Vertical error bars indicate standard deviation for mean values.
Recoveries were lower than those obtained by Spotti and Berni [31] in pomaceous fruit products or indicated in SPE manufacturer’s specifications for apple products. This could be due to the complexity of the matrices tested. Compared to apples and pears, tomato fruits contain a higher amount of liposoluble molecules, such as phenolic compounds, vitamin E, lycopene, and related carotenoids. These molecules are usually concentrated during the production process, so their presence in tomato products assessed could have interfered with PAT during the extraction process, thus reducing the amount of toxin extracted.
Effect of long-term storage on PAT
The results are reported in Figure 2. As Figure shows, a nonlinear, progressive reduction of PAT was observed for all tomato products tested during storage. Tomato paste was the matrix where the most marked effect was observed, PAT being reduced to undetectable levels just after one month at 25°C. It was followed by tomato puree, where the toxin content was unquantifiable (more than 98% reduction) after six months at 25°C. On the contrary, PAT was always present at detectable levels in tomato juice, pulp, and ketchup, where it was respectively reduced by 64%, 81%, and 88% after six months at 25°C. The statistical analysis performed on toxin concentrations using Fisher’s Least Significant Difference (LSD) test showed that PAT values registered for each tomato products were statistically different at each sampling time considered, except for data: (i) at time 0 and after one month for tomato juice; (ii) at one, two, and four months for ketchup; (iii) at two, four, and six months on both tomato pulp or tomato juice.
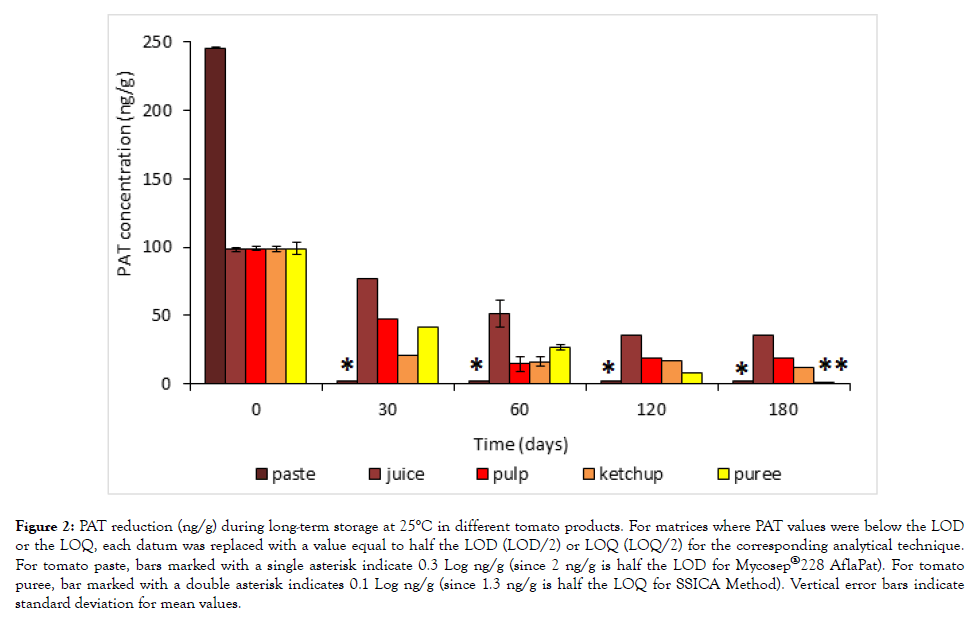
Figure 2: PAT reduction (ng/g) during long-term storage at 25°C in different tomato products. For matrices where PAT values were below the LOD or the LOQ, each datum was replaced with a value equal to half the LOD (LOD/2) or LOQ (LOQ/2) for the corresponding analytical technique. For tomato paste, bars marked with a single asterisk indicate 0.3 Log ng/g (since 2 ng/g is half the LOD for Mycosep®228 AflaPat). For tomato puree, bar marked with a double asterisk indicates 0.1 Log ng/g (since 1.3 ng/g is half the LOQ for SSICA Method). Vertical error bars indicate standard deviation for mean values.
The progressive PAT reduction observed in all tomato products tested could be due to the action of free radicals generated by degradation of L-ascorbic acid to dehydroascorbic acid, as pointed out by Brackett and Marth [15] and more recently by Baert et al. [33] and Drush et al. [16]. The former study observed that PAT disappearance increased with an increasing concentration of L-ascorbic acid in apple juice, whereas the latter study registered a decrease by 70% in PAT content of a model-system added with ascorbic acid, but only by 30% in a model-system without added ascorbic acid after a 34 d period.
A comparison with literature data seems difficult because papers concerning this specific topic are scarce. Concerning fruit juices, the only work concerning PAT degradation in apple juice and apricot or tropical nectars was carried out by Mutti and Quintavalla [17] which observed that a time-lapse between one and three months was sufficient to reduce PAT at undetectable levels in all matrices considered. Drusch et al. [16], which registered a 30% reduction of PAT after a 34 d period in an acid model system, obtained the same results. More recently, also Perre et al. [18] observed a partial reduction in PAT content of tomato juice in the first 24 h, with no further decrease in the next five days.
Effect of heat treatment on PAT reduction
The results of heat treatments are reported in Figure 3. As the figure shows, a progressive reduction in PAT concentration was detected as treatment time increased. The regression analysis of the best fit allowed us to define an equation describing the relation between the logarithmic value of the residual PAT and treatment time. A DT value at 95°C was calculated in tomato juice, being equal to 270 min.
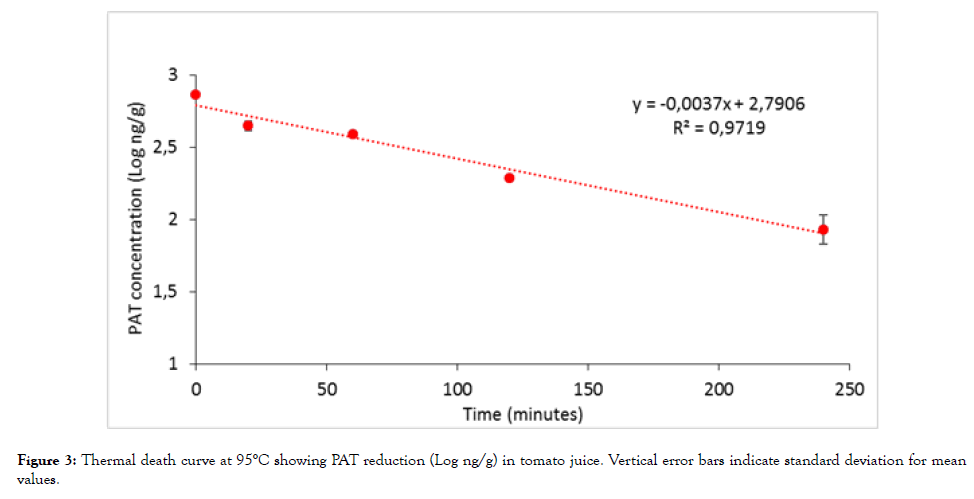
Figure 3: Thermal death curve at 95°C showing PAT reduction (Log ng/g) in tomato juice. Vertical error bars indicate standard deviation for mean values.
A calculation of z-value was not possible since tests were carried out just at the temperature applied in the industrial practice. Despite this, these data seemed to agree with those obtained by Kadakal et al. [22] and Janotová et al. [34], for which a variable reduction in PAT content (12% after 10 min at 90°C, 19% after 20 min at 90°C, and 26% after 20 min at 100°C) was observed in apple products. On the contrary, our D value seemed to be lower than those calculated by Mutti and Quintavalla [17] which found a D94,4=750 min in apple juice, a D96=670,2 min in apricot nectar and a D96,5=720 min in tropical fruit nectar. This difference could be due to the diverse chemical composition of the matrices tested, with higher levels of organic acids such as Lascorbic acid possibly exerting a synergistic effect on PAT degradation. Alternatively, it could be explained by pH effect on PAT, whose heat resistance proved proportional to H+ concentration of the products tested, DT being significantly higher at pH 3.5 rather than at pH 4.5 [35]. In this perspective, and assuming a pH near 4.3 for tomato juice and a pH near 3.5 for fruit juices, our data substantially agree also with Mutti and Quintavalla [17].
Effect of High Pressure Processing (HPP) on PAT
The results of HPP are reported in Figure 4. As the figure shows, no significant reduction in toxin concentration was observed at both treatment times considered, despite the highest power of the apparatus was applied to mimicking industrial conditions.
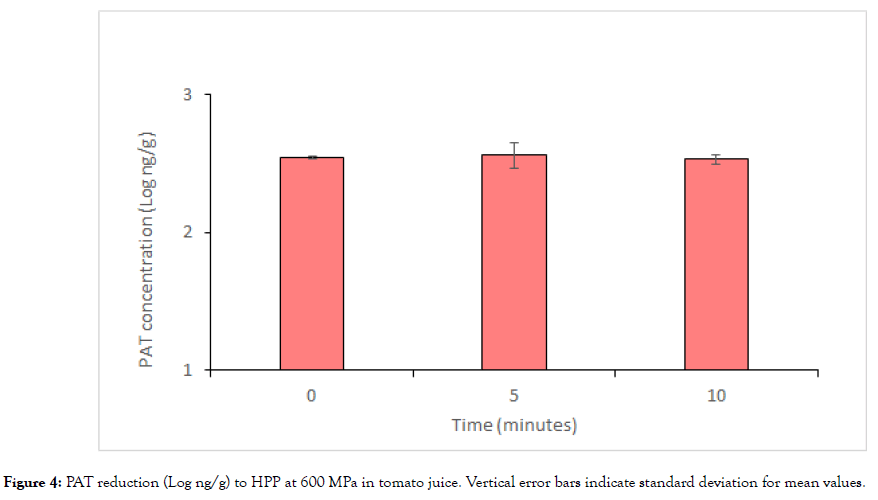
Figure 4: PAT reduction (Log ng/g) to HPP at 600 MPa in tomato juice. Vertical error bars indicate standard deviation for mean values.
Our results substantially agree with those obtained by Maggi et al. [26] which observed no PAT reduction in apricot nectar when 9000 bar for 5 min at 60°C was applied. On the contrary, they diverged from those obtained by Hao et al. [28] which pointed out that an HPP treatment of 600 MPa for 300 sec at 11°C resulted in a partial decrease in PAT contaminated fruit and vegetable juices. This reduction in PAT content was attributed to the binding with sulfhydryl groups such as glutathione or cysteine and to the subsequent formation of adducts that proved 100 times less toxic than PAT itself [36]. Nevertheless, the fact that HPP works on hydrophobic and electrostatic interactions rather than on covalent ones can support to our data.
Conclusion
In this study, the search for an efficient method to detect PAT in tomato products gave different results depending on the matrix considered. For tomato products needing a clarification step (i.e., tomato puree, tomato pulp, and ketchup), SSICA method combined with SPE columns proved the most efficient in terms of recoveries and sample purification, resulting in the absence of interfering substances that could mask PAT. For tomato products that can be analyzed without being clarified (e.g. tomato juice and tomato paste), Mycosep method proved the most efficient in terms of recoveries, time consumption and separation of the chromatographic peaks with a significant reduction in the intensity of HMF, thus allowing PAT detection in thermally over-treated samples. For all tomato products tested, Mycosep®228 AflaPat columns, therefore, proved essential to optimize technique for PAT detection.
Prolonged storages proved effective for PAT reduction just in tomato paste, where the toxin was reduced to undetectable levels just after one month, and only partially effective in tomato puree, where the toxin was reduced to unquantifiable levels (more than 98% reduction) after six months. Analogously, both thermal treatment and HPP did not prove effective in reducing PAT contamination if industrial conditions were considered. The DT value obtained at 95°C on tomato juice was much higher than times usually applied in the industrial practice on non-concentrated tomato products, and the highest time/ pressure combination applicable at an industrial level on tomato juice did not give any decimal reduction to PAT concentration.
This is the first study assessing the fate of PAT in complex matrices such as tomato products, giving useful information to understand how this toxin behaves during tomato products ’ process and commercial life. Unfortunately, the strategies applied did not prove sufficient to inactivate PAT in all products considered. For this reason, tomato products other than pastes could represent a risk for this toxin, in case substantial spoilage by PAT producers occurred on tomato fruits and an insufficient amount of detoxing substances such as L-ascorbic acid was present in the above-mentioned products.
Acknowledgments
The authors are indebted to Dr. Carlo Diaferia for his precious help with the statistical elaboration of PAT levels in different tomato products during storage and to Dr. Luca Sandei for his scientific support. Moreover, the authors would like to thank Dr. Serena Leardini on behalf of ROMER Labs® Inc. for her skilled technical assistance.
REFERENCES
- Andersen B, Frisvad JC. Natural occurrence of fungi and fungal metabolites in moldy tomatoes. J Agric Food Chem. 2004;52:7507-7513.
- Pitt JI, Hocking AD. Fungi and food spoilage. New York: Springer, 2009.
- Samson R, Houbraken J, Thrane U, Frisved J, Andersen B. Food and indoor fungi cbs. Knaw. 2010.
- Somma S, Pose G, Pardo A, Mulè G, Pinto VF, Moretti A, et al. AFLP variability, toxin production, and pathogenicity of Alternaria species from Argentinean tomato fruits and puree. Int J Food Microbiol. 2011;145:414-419.
- Kotzekidou P. Heat resistance of Byssochlamys nivea, Byssochlamys fulva and Neosartorya fischeri isolated from canned tomato paste. J Food Sci. 1997;62:410-412.
- Beuchat LR, Pitt JI. Detection and enumeration of heat-resistant molds. Compendium of methods for the microbiological examination of foods. 2001;3:251-263.
- World Health Organization, International Agency for Research on Cancer. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 1993;56.
- Barkai-Golan R, Paster N. Mouldy fruits and vegetables as a source of mycotoxins: part 1. World Mycotoxin J. 2008;1:147-159.
- Esmailzadeh M, Soleimani MJ, Rouhani H. Exogenous applications of salicylic acid for inducing systemic acquired resistance against tomato stem canker disease. J Biol Sci. 2008;8:1039-1044.
- Perre E, Deschuyffeleer N, Jacxsens L, Vekeman F, Hauwaert WV, Asam S, et al. Screening of moulds and mycotoxins in tomatoes, bell peppers, onions, soft red fruits and derived tomato products. Food Control. 2014;37:165-170.
- Vaquera S, Patriarca A, Pinto VF. Water activity and temperature effects on growth of Alternaria arborescens on tomato medium. Int J Food Microbiol. 2014;185:136-139.
- No OJ. L364 20.12. 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union. 2006.
- European Council. Directive 2012/12/EU of the European Parliament and of the Council of 19 April 2012 amending Council Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption. Official Journal of European Union. 2012;115:25.
- Scott PM, Somers E. Stability of patulin and penicillic acid in fruit juices and flour. J Agric Food Chem. 1968;16:483-485.
- Brackett RE, Marth EH. Ascorbic acid and ascorbate cause disappearance of patulin from buffer solutions and apple juice. J Food Prot. 1979;42:864-866.
- Drusch S, Kopka S, Kaeding J. Stability of patulin in a juice-like aqueous model system in the presence of ascorbic acid. Food Chem. 2007;100:192-197.
- Mutti P, Quintavalla S. Occurence and stability of patulin in fruit products. Industria-Conserve. 1990;64:251-254.
- Perre EV, Jacxsens L, Hauwaert WV, Haesaert I, Meulenaer B. Screening for the presence of patulin in molded fresh produce and evaluation of its stability in the production of tomato products. J Agric Food Chem. 2014;62:304-309.
- Kawashima LM, Soares LM, Massaguer PR. The development of an analytical method for two mycotoxins, patulin and verruculogen, and survey of their presence in commercial tomato pulp. Braz J Microbiol. 2002;33:269-273.
- Cunha SC, Faria MA, Pereira VL, Oliveira TM, Lima AC, Pinto E. Patulin assessment and fungi identification in organic and conventional fruits and derived products. Food control. 2014;44:185-190.
- Sarubbi F, Formisano G, Auriemma G, Arrichiello A, Palomba R. Patulin in homogenized fruit's and tomato products. Food Control. 2016;59:420-423.
- Kadakal C, Nas S. Effect of heat treatment and evaporation on patulin and some other properties of apple juice. J Sci Food Agric. 2003;83:987-990.
- Kubacki SJ. Analysis and occurrence of patulin in apple juice. Bioactive Molecules. 1986;1:293-304.
- Wheeler JL, Harrison MA, Koehler PE. Presence and stability of patulin in pasteurized apple cider. J Food Sci. 1987;52:479-480.
- Avsaroglu MD, Bozoglu F, Alpas H, Largeteau A, Demazeau G. Use of pulsed-high hydrostatic pressure treatment to decrease patulin in apple juice. High Press Res. 2015;35:214-222.
- Maggi A, Gola S, Spotti E, Rovere P, Mutti P. High-pressure treatments of ascospores of heat-resistant moulds and patulin in apricot nectar and water. Industria Conserve. 1994;1:26-29.
- Bruna D, Voldrich M, Marek M, Kamarád J. Effect of high pressure treatment on patulin content in apple concentrate. High Press Res Biosci. 1997:335-338.
- Hao H, Zhou T, Koutchma T, Wu F, Warriner K. High hydrostatic pressure assisted degradation of patulin in fruit and vegetable juice blends. Food control. 2016;62:237-242.
- MacDonald S, Long M, Gilbert J, Felgueiras I. Liquid chromatographic method for determination of patulin in clear and cloudy apple juices and apple puree: collaborative study. J AOAC Int. 2000;83:1387-1394.
- AOAC International. AOAC Official Method 2000.02 Patulin in clear and cloudy apple juices and apple puree. Official methods of analysis of AOAC International (17th edn). 2002.
- Spotti E, Berni E. Patulin determination-by RP-HPLC-in apple and pear purees and concentrated juices. Industria Conserve. 2003;78:429-439.
- Taverniers I, Loose M, Bockstaele E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Analyt Chem. 2004;23:535-552.
- Baert K, Devlieghere F, Flyps H, Oosterlinck M, Ahmed MM, Rajković A, et al. Influence of storage conditions of apples on growth and patulin production by Penicillium expansum. Int J Food Microbiol. 2007;119:170-181.
- Janotová L, Čížková H, Pivoňka J, Voldřich M. Effect of processing of apple puree on patulin content. Food Control. 2011;22:977-981.
- Lovett J, Peeler JT. Effect of pH on the thermal destruction kinetics of patulin in aqueous solution. J Food Sci. 1973;38:1094-1095.
- Lindroth S, Von Wright A. Detoxification of patulin by adduct formation with cysteine. J Environ Pathol Toxicol Oncol. 1990;10:254-259.
Citation: Scaccabarozzi B, Brutti A, Berni E (2020) Effect of Long-Term Storage, Heat and High Pressure Processing on Patulin Reduction in Tomato Products. J Food Process Technol 11:829. doi: 10.35248/2157-7110.2020.11.829
Copyright: © 2020 Scaccabarozzi B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.