Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 4
Effect of Ginger (Zingiber officinale) on Hematological Parameters and Resistant to Aeromonas hydrophila Infection in Nile tilapia, Oreochromis niloticus L.
Alazar Ergena1*, P Natarajan1 and Zufan Bedewi22Department of Biology, Hawassa University, Hawassa, Ethiopia
Received: 20-Oct-2022, Manuscript No. JARD-22-18507; Editor assigned: 24-Oct-2022, Pre QC No. JARD-22-18507 (PQ); Reviewed: 07-Nov-2022, QC No. JARD-22-18507; Revised: 22-Feb-2023, Manuscript No. JARD-22-18507 (R); Published: 01-Mar-2023, DOI: 10.35248/2155-9546.23.14.755
Abstract
Aquaculture is one of the fast growing food industries in the world. However, this industry is hampered by diseases. The present study aimed to determine the effect of ginger powder on the haematology of Oreochromis niloticus, and resistance to Aeromonas hydrophila infection. A completely randomized design was used for the experiment. A total of 300 healthy live experimental fish 20 ± 1.00 g of body weight and 11.06 ± 0.08 cm body length were randomly divided into five treatment glass aquariums. Each in triplicates, representing four treatments (3 g, 5 g, 8 g and 12 g ginger/kg diet) and one control (0.00 g/kg diet). After eight weeks of feeding trial 0.2 ml of A. hydrophila culture containing 1.0 × 107 CFU/ml (LD50) was given by Intra-peritoneal (IP) injection. Blood samples were collected before and after the bacterial infection of the fish for haematological analysis. The result showed that blood parameters of Oreochromis niloticus in all ginger concentrations were significantly higher compared to the control diet before infection (P<0.05). Oreochromis niloticus fed a diet at a concentration of 5 g/kg feed had the highest Red Blood Cell (RBC), haematocrit, haemoglobin, Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC), White Blood Cell (WBC), lymphocytes and monocytes. Erythrocyte Sedimentation Rate (ESR), Mean Corpuscular Volume (MCV), WBC, neutrophils and monocytes show significant increment, while RBC, haemoglobin, haematocrits, MCH, MCHC and lymphocytes decrease significantly after infection (P<0.05). The results also revealed that the fish treated with 5, 8 and 12 g/kg ginger showed a decrease in mortality rates compared to the control diet after infection. The fish fed 5 g/kg ginger had the highest survival rate (81.66%). In conclusion, 5 g/kg of ginger had positive effects on O. niloticus innate immunity system and prevents A. hydrophila infection. Hence, the use of 5g/kg dietary ginger powder in juvenile O. niloticus die is recommended.
Keywords
Aeromonas hydrophila; Haematology; Oreochromis niloticus; Zingiber officinale
Introduction
Rapid population growth, urbanization, and rise in income increase the demand for food [1]. Fish play a significant role in food security and good nutrition [2]. Fish provided 17% of the global population’s intake of animal protein [3]. In freshwater aquaculture, tilapias are the most farmed tropical species of fish in the world after carp because of their suitability for aquaculture, good marketability, and stable prices [4,5]. Niletilapia, Oriochromis niloticus, can be easily cultivated in freshwater [6]. However, the production of aquaculture in general and tilapia culture, in particular, is hampered by diseases causing microorganisms [7].
Bacterial infection is one of the most significant threats to successful Nile tilapia production. Aeromonas spp. has been identified as the major causative bacteria of Nile tilapia [8]. Aeromonas spp. is widespread in untreated and treated water. The occurrence of diseases by Aeromonas spp. is related to stress conditions such as overcrowding and poor water quality. Aeromonas hydrophila, one of Aeromonas spp., is a major fish pathogen known to infect a variety of fishes, predominantly present in freshwaters. A. hydrophila infection in Oreochromis niloticus has caused severe disease outbreaks causing 60% mortality [9]. This decline in fish production and affecting the protein supply of aquaculture and the economy of the world [10]. Stress is frequently considered one of the factors contributing to disease outbreaks by these bacteria [11]. Skin redness, ulcers, swelling of tissues, hemorrhage, and necrosis of the visceral organs are the major symptoms of infected Nile tilapia by A. hydrophila [12].
Antibiotics have been used to prevent and treat bacterial diseases in fish. However, the use of antibiotics in aquaculture for disease control leads to problems with microbial resistance and unacceptable residues in aquaculture products and the environment. The resistant bacterial strains could hurt the therapy of fish diseases or human diseases and the environment of fish farms increases the accumulation of chemicals in fish which is not safe for a human being who is the final consumer [13]. Therefore there is a need to find an alternative to antibiotics to control bacterial diseases of fish including Nile tilapia.
Medicinal plants in aquaculture can be used as an alternative to antibiotics. Out of fifteen medicinal plants tested, ten (66.67%) species showed an antibacterial effect against A. hydrophila [14]. They can be given to fish through intraperitoneal injection to improve their health status of fish and protect against infectious diseases [15-17].
Medicinal Plant such as ginger (Zingiber officinale), garlic (Allium sativum), Oyster mushroom (pleurotus ostreatus), oats (Avena sativa), beetroot (Beta vulgaris), and Moringa (Moringa oleifera) among others have been used as an alternative to antibiotics [18-19]. They produce secondary metabolites. Ginger (Zingiber officinale) for instance produces secondary metabolites such as alkaloids, steroids, flavonoids, gingerols, zingerone, carotenoids, vitamins, and polyphenols which are antibacterial, antifungal, anti-inflammatory, and antioxidant effects on fish [20]. Ginger is also effective as an immunomodulatory agent in fish and helps to reduce the loss in aquaculture by diseases [22]. However, ginger added to fish feed is often made through its ethanolic extract, which may lead to a reduction of some chemical compounds at the filtration stage. Thus, the objective of this study was to determine the effect of ginger powder on the hematology of Nile tilapia and resistance to A. hydrophila infection.
Materials and Methods
Experimental fish and design
A Completely Randomized Design (CRD) was used for the experiment. A total of fifteen experimental glass aquariums (65 cm × 4 cm × 50 cm) were used for the experiment. The water level was maintained at a volume of 40 litters throughout the study period. A total of 300 healthy live Nile tilapia of 20 g ± 1.00 g body weight and 11.06 cm ± 0.08 cm lengths were taken from the research center, Hawassa university, Ethiopia randomly assigned to five treatment glass aquariums in the biology laboratory, Hawassa university. Each in triplicates, representing four treatments and one control. Each replicate contains 20 fish. The fish were acclimatized before the experiment and fed commercial feed for 2 weeks three times daily until apparent satiation. At the end of two weeks, (after acclimatization) fish in each group were fed different concentrations of ginger three times at a level of 3% of body weight daily for 10 weeks (through experimental periods). Control diet fishmeal was free from ginger. Settled fish wastes of aquarium water were siphoned daily. Siphoned water was replaced by clean and aerated water from the storage tank. Water temperature (25.0°C ± 2.0°C), dissolved oxygen concentration (5.6 mg ± 0.15 mg L-1), and pH level (6.8 ± 0.2) were monitored once a week with a YSI 556(r) multi-probe system (YSI Environmental, Yellow Spring, OH, USA.
Preparation of feed
The fresh ginger (Zingiber officinale) rhizome used for the feeding trial was purchased from the local market in Hawassa, Ethiopia. It was dried under shade for one week. The dried ginger was ground to become powder, homogenized, and sieved using a hand sieve. Then different concentrations of ginger (3 g, 5 g, 8 g, and 12 g ginger/kg) and fish meal content were transformed into pellet form by a food grinder and stored at -3°C before feeding.
Challenge test
A. hydrophila that had previously been isolated from Nile tilapia of Lake Hawassa was grown in TSA at 28°C for 18 h. The bacteria suspension was adjusted to 1 CFU/ml × 108 CFU/ml in phosphate buffer saline using the McFarland scale. This concentration was obtained in a previous LD50 trial. In sum, three groups of 30 fish were infected with 0.2 ml of A. hydrophila (1 × 104 CFU/ml, 1 × 106 CFU/ml, and 1 × 107 CFU/ml), and mortality was recorded for 15 days. The LD50 was calculated according to Plumb and Bowser. At the end of 8 weeks feeding trial, fish were challenged with pathogenic A. hydrophila. A 0.2 ml of A. hydrophila culture containing 1.0 × 107 CFU/ml (LD50) was given by intraperitoneal (IP) injection using a 21/gauge sterile needle. Twenty-four hours after injection, fish were fed the same experimental diet as in the feeding trial for 15 days of the challenge period and any clinical symptoms were recorded. The dead fish were removed daily and mortality was confirmed by re-isolating the microorganism from the internal organs of the dead fish. Survival rates of Nile tilapia were computed in percentages.
Survival rate (%)=Nf ×100/Ni
Nf=Number of cultured fish alive at the end of the experiment
Ni=Number of cultured fish stocked at the beginning of the experiment
Blood collection and hematological examination
Blood samples were collected in the early morning hours before and after bacterial infection of the fish for hematological analysis. Three fish from each replicate were sampled and anesthetized with 50 mg/L of tricaine methanesulfonate (MS222, Sigma Chemical Co. St. Louis, MO, USA) and then blood samples were collected from the caudal peduncle with the use of a 5 ml syringe and needle that has been treated with heparin to prevent clotting and transferred sampling bottles that contain ethylene diamine tetra-acetic-acid (EDTA). After the collection, the blood samples were taken to the Veterinary laboratory, at Hawassa University where the hematological analysis was carried out. Blood was analysed with routine methods adopted in fish hematology. The total erythrocyte count (RBC ×106/µl) and total leukocyte count (WBC ×103/µL) were determined manually using a Neubauer's hemocytometer with Hayem solution as a diluent. The hematocrit percentage was determined in duplicate by using microhaematocrit-heparinized capillary tubes of 75 µL volume and a microhaematocrit centrifuge at 15000 g for 5 min. For the determination of hemoglobin concentrations Sahli’s method was used. The values of hemoglobin were expressed as g/dilution. The values of red blood cell indices of mean corpuscular volume (MCV fl) mean corpuscular hemoglobin (MCH pg) and mean corpuscular hemoglobin concentration (MCHC gm/dl), were calculated according to Wintrobe. WBC such as neutrophils, lymphocytes, and monocytes was performed by the diluent/dye direct method outlined by Natt and Herrick in a Neubauer chamber at a dilution of 1:100.
Statistical analysis
Data obtained were expressed as mean ± standard error of the mean. The results were analyzed with a one-way analysis of variance (ANOVA), using SPSS (Statistical Package for Social Science 2006, version 22). Differences at p<0.05 were regarded as statistically significant. Data were presented as mean ± SE before and after the challenge test.
Results and Discussion
The effect of ginger powder on haematological parameters of O. niloticus
Medicinal plants have been used for diseases resistance in fish due to the active phytochemicals such as alkaloids, terpenoids, tannins, saponins, glycosides, flavonoids, phenolics, steroids or essential oils. In the present study, the effect of Z. officinale powder on the hematological parameters of Nile tilapia and its resistance to A. hydrophila infection was determined.
It was observed that all concentrations of the ginger-supplemented diet exhibited significantly higher values of RBC when compared to fish fed the control diet before and after infection (P<0.05). The study by Ferri-Lagneau, et al demonstrated that ginger extract stimulated hematopoiesis in fish, which could explain the present result. Ginger also contains antioxidant that protects the RBC against hemolysis by free radicals. The result also showed that the RBC of Nile tilapia infected with A. hydrophila in the control diet and all ginger-supplemented diets decreased significantly (P<0.05). Similar to the present study, Hardi, et al., and Talpur, et al. reported that the RBC was decreased in tilapia infected with A. hydrophila. Decreased RBC counts indicate that erythrocytes are being affected by the infection. The changes in fish hematology are to be expected for fish infected with diseases (Table 1).
| Variables | Experimental diet | Before infection | After infection |
|---|---|---|---|
| RBC (106/mm3) | 0.0 g Z. officinale/kg feed (control) | 3.0 ± 0.26bB | 1.27 ± 0.06aA |
| 3 g Z. officinale/kg feed | 3.04 ± 0.00cB | 1.43 ± 0.1bA | |
| 5 g Z. officinale/kg feed | 3.87 ± 0.15eB | 3.55 ± 0.11eA | |
| 8 g Z. officinale/kg feed | 3.11 ± 0.06dB | 2.77 ± 0.12dA | |
| 12 g Z. officinale/kg feed | 2.92 ± 0.05aB | 2.10 ± 0.93cA | |
| Haematocrit (%) | 0.0 g Z. officinale/kg feed (control) | 36.71 ± 0.22bB | 20.52 ± 0.53aA |
| 3 g Z. officinale/kg feed | 37.68 ± 0.16cB | 21.77 ± 0.77cA | |
| 5 g Z. officinale/kg feed | 40.70 ± 0.2eB | 37.59 ± 0.21eA | |
| 8 g Z. officinale/kg feed | 38.14 ± 0.08dB | 31.57 ± 0.06dA | |
| 12 g Z. officinale/kg feed | 36.15 ± 0.08aB | 20.96 ± 0.56bA | |
| Haemoglobin (g/dl) | 0.0 g Z. officinale/kg feed (control) | 7.59 ± 0.17bB | 3.69 ± 0.21bA |
| 3 g Z. officinale/kg feed | 7.64 ± 0.2cB | 4.21 ± 0.49cA | |
| 5 g Z. officinale/kg feed | 9.12 ± 0.1eB | 7.29 ± 0.53eA | |
| 8 g Z. officinale/kg feed | 8.05 ± 0.03dB | 5.66 ± 0.11dA | |
| 12 g Z. officinale/kg feed | 7.50 ± 0.04aB | 3.58 ± 0.07aA | |
| ESR (mm/hr) | 0.0 g Z. officinale/kg feed (control) | 5.61 ± 0.12dA | 8.68 ± 0.05dB |
| 3 g Z. officinale/kg feed | 5.19 ± 0.07bA | 8.72 ± 0.13eB | |
| 5 g Z. officinale/kg feed | 4.68 ± 0.08aA | 5.55 ± 0.22aB | |
| 8 g Z. officinale/kg feed | 5. 85 ± 0.09eA | 7.33 ± 0.10bB | |
| 12 g Z. officinale/kg feed | 5. 41 ± 0.12cA | 8.65 ± 0.21cB | |
| MCV (fl) | 0.0 g Z. officinale/kg feed (control) | 121.18 ± 0.98cA | 133.44 ± 7.41aA |
| 3 g Z. officinale/kg feed | 123.69 ± 1.15dA | 130.48 ± 4.39aA | |
| 5 g Z. officinale/kg feed | 105.1 ± 4.52aA | 119.26 ± 1.13aA | |
| 8 g Z. officinale/kg feed | 117 ± 8.26bA | 121.14 ± 4.67aA | |
| 12 g Z. officinale/kg feed | 123.85 ± 2.18eA | 134.29 ±13.05aA | |
| MCH (pg) | 0.0 g Z. officinale/kg feed(control) | 22.97 ± 1.67aA | 25.06 ± 0.78aA |
| 3 g Z. officinale/kg feed | 24.25 ± 0.47dA | 25.09 ± 0.81aA | |
| 5 g Z. officinale/kg feed | 25.52 ± 1.14eA | 23.54 ± 0.67aA | |
| 8 g Z. officinale/kg feed | 23.14 ± 0.64cA | 25.91 ± 0.59aB | |
| 12 g Z. officinale/kg feed | 23.79 ± 0.61bA | 25.69 ± 0.55aA | |
| MCHC (gm/dl) | 0.0 g Z. officinale/kg feed(control) | 20.68 ± 0.57bB | 16.84 ± 0.05aA |
| 3 g Z. officinale/kg feed | 20.30 ± 0.46aB | 18.93 ± 0.64bA | |
| 5 g Z. officinale/kg feed | 22.41 ± 0.34eA | 21.36 ± 0.52eA | |
| 8 g Z. officinale/kg feed | 21.11 ± 0.08dB | 19.04 ± 0.57cA | |
| 12 g Z. officinale/kg feed | 20.74 ± 0.13cA | 19.46 ± 0.62dA | |
| Mean ± standard deviation followed by different superscript letters (a, b, c, d) in the same column in each treatment trial showed significantly different at P<0.05. Capital letters (A, B) in the same row indicated significant difference at P<0.05 before and after infection of Nile tilapia with A. hydrophila. | |||
Table 1: Mean values of hematological parameters (Red Blood Cells (RBCs), hematocrit, Hemoglobin (Hb), Erythrocyte Sedimentation Rate (ESR), Mean Corpuscle Volume (MCV), Mean Corpuscular Hemoglobin (MCH) and Mean Corpuscular Hemoglobin Concentration (MCHC)) of Nile tilapia (O. niloticus) fed different concentration of ginger (0. g 0, 3 g, 5 g, 8 g and 12 g Z. officinale/kg feed).
Hematocrit (or packed cell volume) is the simplest measure of erythrocyte content in blood as a percentage of erythrocytes in blood volume. In the current study the percentage of hematocrit was 36.71% ± 0.22% (0.00g/kg), 37.68% ± 0.16% (3 g/kg), 40.70% ± 0.2% (5 g/kg), 38.14% ± 0.08% (8 g/kg) and 36.15% ± 0.08% (12 g/kg) before infection of Nile tilapia with A. hydrophila, and 20.52% ± 0.53% (0.00 g/kg), 21.77% ± 0.77% (3 g/kg), 37.59% ± 0.21% (5 g/kg), 31.57% ± 0.06% (8 g/kg) and 20.96% ± 0.56% (12 g/kg) after infection, respectively. This indicated that the percentage of hematocrit was significantly declined after Nile tilapia was infected with A. hydrophila (P<0.05). This showed that hematocrit was affected by bacteria infection and developed an anemic state. Brum, et al described anemia for Nile tilapia fed diets supplemented with 10 g/kg of ginger essential oil.
Hemoglobin is directly related to the oxygen-binding capacity of the blood. Therefore it is crucial for the survival of fish. The results of the current study indicated that hemoglobin contents increase significantly in ginger treated Nile tilapia compared to the control diet; these findings came in accordance with the results of previous studies. Ginger showed a significant difference in hemoglobin before and after infection of the fish (P<0.05). There was a significant decrease in hemoglobin after infection of fish with A. hydrophila. This may be due to an increased rate of breakdown of RBC by pathogenic bacteria and/or a reduction in the rate of formation of RBC. According to Lie et al. the hemoglobin content decreases due to RBC swelling and poor Hb mobilization of the spleen and other hematopoiesis organs. Also the reduction of hemoglobin after infection of fish could be the result of severe anemia. The anemic response could be a result of disruption in erythrocyte production, hemodilution, and destruction of intestinal cells involved in the production of vitamin B12 used in the production of the hemoglobin portion of the red cells. The present result disagrees with the result of Brum, et al. who reported no significant effect of ginger oil on the hemoglobin of Nile tilapia (Table 2 and Figure 1).
| Variables | Experimental diet | Before infection | After infection |
|---|---|---|---|
| WBC (103/mm3) | 0.0 g Z. officinale/kg feed(control) | 33.72 ± 0.23aA | 44.37 ± 0.12bB |
| 3 g Z. officinale/kg feed | 40.30 ± 0.12cA | 45.91 ± 0.75dB | |
| 5 g Z. officinale/kg feed | 43.38 ± 0.13eA | 45.51 ± 0.12cB | |
| 8 g Z. officinale/kg feed | 41.37 ± 0.09dA | 53.49 ± 0.06eB | |
| 12 g Z. officinale/kg feed | 38.75 ± 0.07bA | 44.29 ± 0.30aB | |
| Lymphocyte (103/μl) | 0.0 g Z. officinale/kg feed(control) | 27.23 ± 017bB | 23.34 ± 0.90bA |
| 3 g Z. officinale/kg feed | 27.63 ± 0.09cB | 24.56 ± 0.23cA | |
| 5 g Z. officinale/kg feed | 30.19 ± 0.13eB | 27.73 ± 0.15eA | |
| 8 g Z. officinale/kg feed | 28.37 ± 0.04dB | 25.21 ± 0.48dA | |
| 12 g Z. officinale/kg feed | 25.09 ± 0.06aB | 23.18 ± 0.16aA | |
| Neutrophil (103/μl) | 0.0 g Z. officinale/kg feed (control) | 3.44 ± 0.08cA | 5.21 ± 0.08bB |
| 3 g Z. officinale/kg feed | 3.33 ± 0.07bA | 5.48 ± 0.05cB | |
| 5 g Z. officinale/kg feed | 4.54 ± 0.09eA | 7.06 ± 0.23eB | |
| 8 g Z. officinale/kg feed | 4.22 ± 0.10dA | 5.81 ± 0.14dB | |
| 12 g Z. officinale/kg feed | 3.30 ± 0.12aA | 5.22 ± 0.47aB | |
| Monocytes (103/μl) | 0.0 g Z. officinale/kg feed(control) | 1.38 ± 0.03aA | 4.69 ± 0.21aB |
| 3 g Z. officinale/kg feed | 2.86 ± 0.05bA | 5.58 ± 0.05dB | |
| 5 g Z. officinale/kg feed | 3.61 ± 0.04dA | 6.88 ± 0.08cB | |
| 8 g Z. officinale/kg feed | 3.05 ± 0.05cA | 5.66 ± 0.21eB | |
| 12 g Z. officinale/kg feed | 2.85 ± 0.06bA | 4.26 ± 0.16bB | |
| Mean ± standard deviation followed by different superscript letters (a, b, c, d, e) inthe same column in each treatment or prevention trial showed significantly different atP<0.05. Capital letters (A, B) in the same raw indicated significant difference at P<0.05 before and after infection of Nile tilapia with A. hydrophila. | |||
Table 2: Mean values of haematological parameters (White Blood Cells (WBCs), lymphocytes, neutrophils, and monocytes of Nile tilapia (O. niloticus) fed different concentration of ginger (0.0 g, 3 g, 5 g, 8 g and 12 g Z. officinale/kg feed).
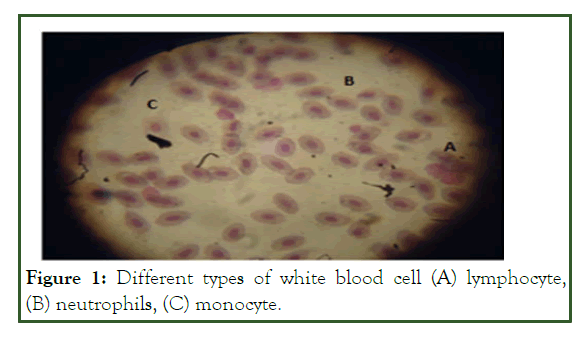
Figure 1: Different types of white blood cell (A) lymphocyte, (B) neutrophils, (C) monocyte.
The Erythrocyte Sedimentation Rate (ESR) is a common hematology test that may indicate and monitor an increase in inflammatory activity within the body caused by one or more conditions such as autoimmune disease, infections, or tumors. The current study showed that the ESR of O. niloticus was significantly increased after the fish were infected with A. hydrophila. Blaxhall and Daisley stated the values are usually raised with increased tissue destruction as in acute infection and heavy metal poisoning among others. In the current study, the maximum value of MCV was recorded at 12 g/kg ginger supplemented diet. The results also showed that the application of powdered ginger at a varying concentration significantly increase MCV and MCH (P<0.05), while significantly decreasing the value of MCHC after infection of Nile tilapia with A. hydrophila, except 5 g/kg and 12 g/kg ginger supplemented diet. Similar to this study, Haniffa and Mydeen demonstrated that catfish (Silurus asotus) exhibited a decrease in MCHC during A. hydrophila infection. But contrarily to the present study, Stanley, et al. reported no significant difference (p>0.05) for MCV, MCH, and MCHC at varying concentrations of powdered ginger.
WBCs, lymphocytes, neutrophils, and monocytes of O. niloticus were significantly higher than that of the control group before infection of O. niloticus with A. hydrophila and their results are presented in WBC was significantly different in all treatments both before and after infection. WBC was dominated by Lymphocytes (23.18 ± 0.16-27.73 ± 0.15 103/μl), followed by neutrophils (5.22 ± 0.47-7.06 ± 0.23 103/μl) and monocytes (4.26 ± 0.16-6.88 ± 0.08 103/μl) after infection of O. niloticus with A. hydrophila.
WBC plays a crucial role in the protection of diseases caused by pathogenic organisms. WBC counts of Nile tilapia fed different concentrations of ginger powder were significantly higher compared to the control group (p<0.05). Similar to the present study, Haghighi and Rohani reported that a fish fed ginger-supplemented diet showed a significant immune-stimulatory effect and increased RBC, Hematocrit, and WBC values when compared to the control (p<0.05). An increase in WBC was believed to be caused by the migration of white blood cells from the spleen to the blood circulation. According to Sutili, et al. feeding fish with ginger-supplemented diets produces immunomodulatory effects. The results also indicated that WBC increased significantly in Nile tilapia after infection with A. hydrophila (P<0.05). This result is similar to those of a previous study, which stated that the WBC increased to tackle the infection in tilapia infected with A. hydrophila.
Three types of WBC, namely neutrophils, lymphocytes, and monocytes were identified in the circulating blood of Nile tilapia. The value of neutrophils was significantly increased in the ginger-supplemented diet compared to the control diet (P<0.05). The maximum value of neutrophils 7.06 ± 0.23 was recorded at the concentration of 5 g Z. official/kg diet after infection of Nile tilapia with A. hydrophila. These results are in line with the results of a previous study, which found that fish treated with immunostimulants usually show enhanced phagocytic cell activities. The results were also supported by Talpur, et al. who reported a beneficial effect of ginger which improve the immunity system of fish. The number of lymphocytes in fish injected with A. hydrophila was significantly lower than those which are not infected (P<0.05). Decreases in lymphocytes after infection of Nile tilapia with A. hydrophila were associated with re-trafficking of cells to lymphoid tissues which consequently leads to clearance of these cells from the bloodstream. Blood monocytes contribute to tissue-resident macrophage populations during inflammatory conditions and the depletion of resident macrophages in their environment. The number of monocytes was significantly increased in the Nile tilapia fed ginger supplemented diet after infection with A. hydrophila (P<0.05), this may be due to intensification of the cell defence mechanism [51]. Also, it was observed that the monocyte value of Nile tilapia fed 5 g/kg ginger was significantly higher than that fed 3, 8 and 12 g/kg (P<0.05). According to Citarasu the choice of herbs, their dose, and time of application was very important for obtaining higher efficiency.
Clinical symptoms
In the present study, before the bacterial challenge, Nile tilapia presented health characteristics within the normal parameters for the species. But after 36 hrs of infection with Aeromonas hydrophila, Nile tilapia showed clinical symptoms like redness of skin, fluid accumulation in the pockets scale, swelling of tissues, ulcers and hemorrhage, necrosis and exophthalmia. Similar to the present result, Noor El–Deen, et al. stated that the bacteria caused acute mortality among infected fishes in which the most visible clinical signs included exophthalmia. The present results also agree with the finding of earlier report (Figure 2).
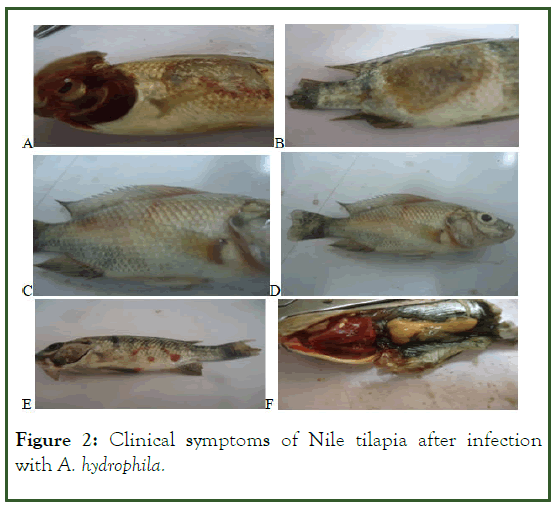
Figure 2: Clinical symptoms of Nile tilapia after infection with A. hydrophila.
Diseases resistant
Survival and mortality rate results of Nile tilapia challenged with A. hydrophilia were presented. Results revealed that the addition of ginger to the O. niloticus diet enhances the body's health and resistance to infection with A. hydrophila. The fish in the treatment trial with 5 g/kg ginger had the highest survival rate (81.66%) compared to the other ginger concentration. There was no mortality to Nile tilapia before infection with A. hydrophila. From this result, it can be suggested that ginger caused no harmful effect on fish, at least in our described experimental condition. Hence, it can be considered safe for use in fish feed. Fish mortality was recorded as early as 36 hrs of an experiment for control fed after infection with A. hydrophila. No mortality was recorded until the 3rd day of the experimental period for a fish fed diet that contains a different concentration of ginger. In all treatments, mortality began to occur on day three post-challenged and continued until day twelve (Figure 3).
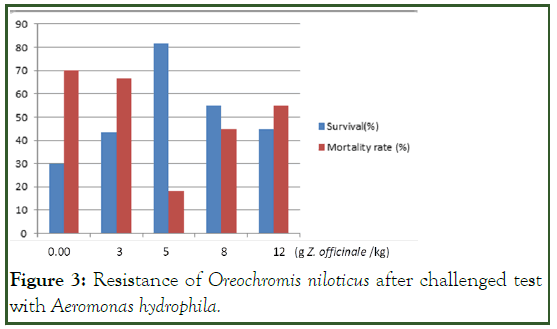
Figure 3: Resistance of Oreochromis niloticus after challenged test with Aeromonas hydrophila.
The highest mortality rate (70%) was recorded for the control group followed by 3 g/kg (66.66%) and 12 g/kg (55%) ginger-supplemented diet, respectively. In line with this study, Payung, et al. reported the highest mortality in the control diet followed by a 3 g/kg ginger-supplemented diet, and the lowest mortality in the 5 g/kg ginger-supplemented diet. The increasing survival rate is related to the increased immune function of the fish. Increased fish immunity will result in increased fish resistance to pathogens. The addition of ginger (Z. officinale) extract in fish feed increases the resistance of fish to pathogens because ginger contains ingredients that can improve the immune system of the fish. According to Nugroho, et al., traditional herbs improve the immune response of fish by increasing granulocytes, macrophages, monocytes, and neutrophils. Maqsood, et al. also reported that immune-stimulants enhanced the general defence system and decreased the mortality against pathogens and increased the viability rate. The results also revealed that the Nile tilapia treated with 5, 8 and 12 g/kg ginger showed a decrease in mortality rates compared to the control diet after A. hydrophila infection. However, as the concentration of Z. officinale in fish feed increases, survival appeared to decrease. This indicates that excessive doses will have an immunosuppression effect that suppresses the immune system of the fish.
Conclusion
The supplementation of ginger (Z. officiale) powder in the fish diet had a significant additive benefit on the immune status of O. niloticus compared to the control diet. Supplementation of ginger at the concentration of 5 g/kg diet provides better protection to O. niloticus against A. hydrophila infection. Hence, the use of 5 g/kg dietary ginger powder in juvenile O. niloticus die is recommended.
Acknowledgments
The authors thank the facility of veterinary, Hawassa University, for the support of the laboratory facility and equipment for the analysis of blood parameters. Department of biotechnology, Hawassa University is acknowledged for providing laboratory space during this experiment. The authors also thank Ashenafi Bekele from Hawassa University, department of biology, for his valuable help during this research.
Declaration of Interest
The author’s report there are is no competing interests to declare.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
- FAO. The State of Food Security and Nutrition in the World 2019. Safeguarding against economic slowdowns and downturns. Food and Agriculture Org. Rome, FAO: 2019;239.
- Beveridge MC, Thilsted SH, Phillips MJ, Metian M, Troell M, Hall SJ. Meeting the food and nutrition needs of the poor: the role of fish and the opportunities and challenges emerging from the rise of aquaculture. J Fish Biol. 2013;83(4):1067-1084.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Lu M. Tilapia polyculture: a global review. Aquac Res. 2016;47(8):2363-2374.
- Waite R, Beveridge M, Brummett R, Castine S, Chaiyawannakarn N, Kaushik S, et al. Improving productivity and environmental performance of aquaculture. World Fish. 2014:59.
- FAO. Tilapia production and the share of the leading producing countries GLOBEFISH-Analysis and information on world fish trade. FAO, 2015.
- van Hai N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquac. 2015;446:88-96.
- Baumgartner WA, Ford L, Hanson L. Lesions caused by virulent Aeromonas hydrophila in farmed catfish (Ictalurus punctatus and I. punctatus× I. furcatus) in Mississippi. J Vet Diagn Invest. 2017;29(5):747-751.
- Hardi EH, Saptiani G, Kusuma IW, Suwinarti W, Nugroho RA. Immunomodulatory and antibacterial effects of Boesenbergia pandurata, Solanum ferox, and Zingiber zerumbet on tilapia, Oreochromis niloticus. Aquac Aquar Conserv Legis. 2017;10(2):182-90. [Crossref]
- Nugroho RA, Manurung H, Nur FM, Prahastika W. Terminalia catappa L. extract improves survival, hematological profile and resistance to Aeromonas hydrophila in Betta sp. Fisheries Aquatic Life. 2017;25(2):103-15..
- Goharrizi LY, Zorriehzahra ME, Adel M. The study on effect of temperature stress on occurrence of clinical signs caused by Aeromonas hydrophila in Capoeta damascina in in vitro condition. Adv Anim Vet Sci. 2015;3(7):406-412.
- Yardimci B, AYDIN Y. Pathological findings of experimental Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Ankara Üniversitesi Veteriner Fakultesi Dergisi. 2011;58(1):47-54.
- Heuer OE, Kruse H, Grave K, Collignon P, Karunasagar I, Angulo FJ. Human health consequences of use of antimicrobial agents in aquaculture. Clin Infect Dis. 2009;49(8):1248-53.
- Chowdhury MB, Rahman T. Efficacy of medicinal plants on microbial fish pathogens. J Bangladesh Agric Univ. 2008;6(452-2018-4023):131-138.
- Awad E, Awaad A. Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol. 2017;67:40-54.
[Crossref] [Google Scholar] [PubMed]
- Reverter M, Tapissier‐Bontemps N, Sasal P, Saulnier D. Use of medicinal plants in aquaculture. Diagnosis and control of diseases of fish and shellfish. 2017:223-261.
- Bulfon C, Volpatti D, Galeotti M. Current research on the use of plant‐derived products in farmed fish. Aquac Res. 2015;46(3):513-551.
- Devi KN, Dhayanithi NB, Kumar TT, Balasundaram C, Harikrishnan R. In vitro and in vivo efficacy of partially purified herbal extracts against bacterial fish pathogens. Aquac. 2016;458:121-133.
- Nile SH, Park SW. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind Crops Prod. 2015;70:238-244.
- Stoner GD. Ginger: is it ready for prime time? Cancer Prevention Research. 2013;6(4):257-262.
Citation: Ergena A, Natarajan P, Bedewi Z (2023) Effect of Ginger (Zingiber officinale) on Hematological Parameters and Resistant to Aeromonas hydrophila Infection in Nile Tilapia, Oreochromis niloticus L. Aquac Res Dev. 14:755.
Copyright: ©2023 Ergena A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

