Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2020) Volume 11, Issue 4
Dual Sword Role of Aspirin as anticancer through induced or inhibition of Autophagy through mTOR pathway
Walaa Fikry Elbossaty*Received: 06-Jun-2020 Published: 13-Jul-2020, DOI: 10.35248/2155-9864.20.11.434
Abstract
Autophagy is a natural physiological lysosomal catabolic process in which many cellular mechanism including degradation, elimination of misfolded protein, and organs damaged under the effect of starvation, stress and tumor suppression. It is like a double-edged sword that can play either a protective or destructive role in cancer, so it becomes the most intensive strategy in treatment of cancer. Autophagy have numerous mechanisms in treatment and controlling of cancer, including up regulation of tumor suppressor genes / Downregulation of oncogenes, and that can lead to inhibition/induction of cancer development. Aspirin is an important drug over the centuries. It is used as an anticoagulant, analgesic, and antipyretic. However, recent studies have shown that aspirin has an effective role in the prevention of cancer.
Introduction
Autophagy
Autophagy is considered one of the most important methods that a person uses to remove damaged cells, which helps to restore healthy cells. This method is one of the methods that a person uses to adapt and maintain survival, as it is the button used by the living organism to clean his body [1]. It is used to reuse damaged protein and it also helps to provide a source of energy during formation of new cells [2]. There are three types of autophagy, macrophage and microphage and auto-macrophage by chaperone (CMA) [3]. In macro phagocytosis, this is one of the most important paths used by the cell to eliminate damaged cells and unused proteins. Mechanism begins to approach the phagophore from unwanted cells and swallow them inside, and by the action of hydrolytic enzymes it begins to analyze and eliminate them [4]. While in microphage, cells reach the cytoplasmic substances inside the lysosome and digest them inside [5].
On the other hand, Chaperone-mediated autophagy, or CMA is one of the most complicated pathways as it depends on the presence of identifiable paths known as hsc70 and in the case of its presence the substance is linked to it and is a CMA-substrate / chaperone complex that is transported through the plasma membrane and elimination of unwanted cells in which [6].
Mechanism action of autophagy
The autophagy process depends on the formation of a double membrane with components of the cytoplasm, and this process is carried out in several stages that can be summarized in the following points [7]:
A. Induction of autophagy
There are various factors responsible for initiation of autophagy including, starvation, stress, hypoxia [8]. The process of autophagy begins with the formation of phosphatidylinositol 3-phosphate in the endoplasmic reticulum (ER) in order to form phagophore [9].
Beclin 1 plays an important role in stimulating the process of autophagy by its indirect effect on the hVps34 rate, where hVps34 plays an important role in the production of phosphatidylinositol 3-phosphate, and stimulating the production of WIPI1 / 2 and DFCP1. The ULK1 / 2 kinase complexes, including ULK1, ULK2, ATG13, and RB1CC1 all have an important role to play in the process of autophagy [10].
TORC1 is protein target of rapamycin has an effective effect on the autophagy process. There are two types of TORC compounds (TORC1 and TORC2) [11]. The effect of TORC on the process of autophagy depends on the feeding process. In the case of good nutrition, TORC works to suppress autophagy through Atg13 is highly phosphorylated, and has a lower affinity for Atg1 and Atg17 and this leads to the suppression of the autophagy process. Conversely, in the event of a nutritional deficiency, TORC acts as a trigger for the autophagy process by regulates the Atg1 – Atg13 – Atg17 kinase complex [12].
B. Phagophore Elongation
Phagophore elongation is performed under the influence of mATG9 and the Ubiquitin-like protein, LC3 [13]. During nutrient starvation, mATG9, which is a semi-membrane protein found in the Golgi network, binds to Phagophore in ULK1-dependent manner [14].
LC3 is one of the most important signs of autophagy, which turns intracellular into LC3-II in two steps [15]:
First: LC3 is divided into LC3-I by ATG4B.
Second: LC3-I then turns into Phagophore with the help of Ubiquitinactivating (E1) -like enzyme ATG7 and the Ubiquitin-conjugating (E2) -like enzyme ATG3, then phosphatidylethanolamine (PE) is bound to form LC3-II.
LC3-II then binds to several adaptor proteins, such as the BNIP3L adapter protein, which is found in the mitochondria, all of which have an effective effect on the Phagophore elongation process.
C. Fusion, Degradation, and Recycling of phagosome
Autophagosome result when Phagophore is fused with lysosome by using lysosomal membrane protein LAMP-2 and the small GTPase Rab7. The phagocytosis process begins by incorporating the double membrane into a sphere, where all the components, especially the amino acids, are transported in to cytoplasm and used in protein synthesis and maintain the cellular function under starvation process [16]. When the double membrane is bound to the lysosome it begins to analyze the inner membrane and the components of the cytoplasm, but the presence of LC3-II on the outer membrane protects it from cracking with the help of ATG4B [17].
Benefits of autophagy
Autophagy has an effective effect on many physiological processes and therefore has an effect on cancer, immune diseases, heart diseases, type II diabetes as shown in Figure 1 [18].
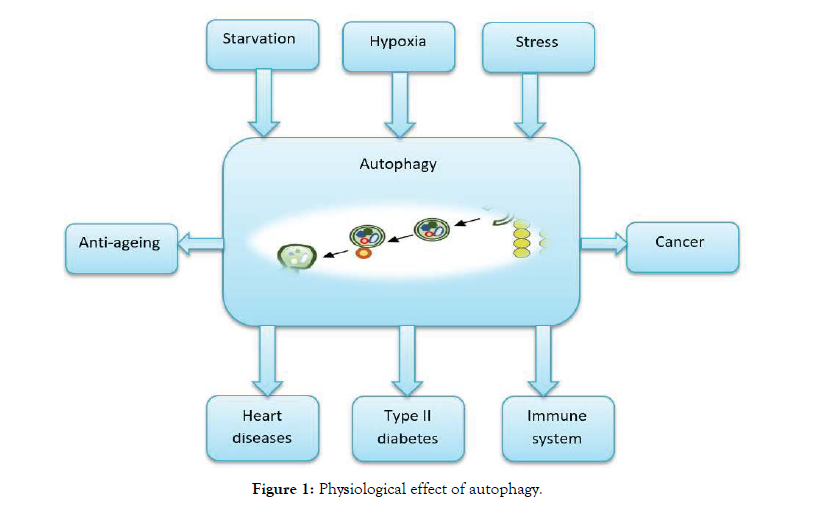
Figure 1: Physiological effect of autophagy.
Autophagy and cancer
There is an indirect relationship between autophagy and cancer through that there are two large groups of genes that affect the course of cancer cells known as Oncogenes and tumor suppressor genes. It was found that there was a direct relationship between the tumor suppressor genes and the process of autophagy [19]. The process of autophagy is the path that the body uses to get rid of cancer cells and works to prevent their proliferation by getting rid of them and encouraging the growth of healthy cells [20]. The scientific research approved that autophagy suppress the development of tumor cells [21].
Aspirin
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID); it is extracted from a plant source where its active substance salicylic acid is extracted from the willow tree. In addition, aspirin is one of the most important non-steroidal anti-inflammatory drugs [22]. It is used as a source of preventive protection in heart disease and protection from heart attacks if used in low doses regularly, and it has also been found to be used in protection against cancer [23]. Aspirin is used as an anti-inflammatory through its inhibitory effect of cyclooxygenase enzymes, which plays an effective role in regulating the production of prostaglandins [24]. It is absorbed in the stomach and is aided by the acidic environment of the stomach. It has been found that the rate of aspirin absorption ranges between 13-19 min. Aspirin is associated with albumin, which is used as a transporter of many substances through the blood stream [25].
After entering the bloodstream, acetate and salicylic acid are decomposed by cholinesterase [26]. In the blood stream, the platelets are absorbed aspirin by concentration-dependent passive diffusion. It was found that intravenous administration of aspirin is more effective than oral use, as its concentration in the blood reaches its highest concentration within 3 minutes [27]. The kidneys are the only way to get rid of the product of the decomposition of aspirin, and this is done within 2 hours of taking the drug [28]. Recent studies have shown that aspirin has an effective effect on many cancers, such as colorectal cancer, colon, digestive system, and lung, after taking aspirin regularly and at low doses [29]. Aspirin has contributed to lowering rates of liver cancer in addition to being used in the treatment of liver cancer as well as the use of chemotherapy [30].
Pharmacokinetics action of Aspirin as anticancer
It has an effective effect on the autophagy process by increasing the rate of AMP-activated protein kinase, c-Jun N-terminal kinase, and Glycogen synthase kinase-3 pathways, aspirin inhibited the mammalian-target-of rapamycin-S6K1 / 4E- BP1 signaling [31]. It works to inhibit Cyclooxygenase and then decrease the production of thromboxane and this contributes to preventing platelet aggregation. Not only this, but aspirin works to change the formation of some proteins that have an effective role in the formation of angiogenesis and increase the tumor [32].
Aspirin plays as an anti-tumor in two ways:
1. By inhibiting the activity of platelet cells that may contribute to tumor proliferation by decreasing angiogenin, RANTES, and growth regulating growth factor (GRO), platelet-derived growth factor (PDGF), and oncostatin M (OSM) [33].
In addition to platelet-derived cytokines (e.g., CXCL4 and CTGF) , Which have an important role in tumor formation, and blood vessels are critical for regulating vascular repair, they also play a role in driving tumorigenesis, angiogenesis, and metastasis [34].
2. By inhibiting the secretion of interleukin 7 from platelet cells by thrombin receptor activating peptide.
There are many chemical signals that play an important role in autophagy, among the most important of which are mTOR pathway. Inhibitor drugs reduce the rates of mTOR and thus stimulate the autophagy process [35].
Adenosine monophosphate ‐ activated protein kinase negatively affects the mTOR rate and thus affects the autophagy process [36].
Scientific research has proven that taking aspirin stimulates the production of adenosine monophosphate ‐ activated protein kinase and thus reduces the rate of mTOR and stimulates autophagy in colorectal cancer, and hepatocellular carcinoma Figure 2 [37]. The effect of aspirin on cancer cells is transmitted between autophagy and the programmed death of cells, depending on the amount of aspirin used in addition to the time of treatment [38]. As the low concentration of aspirin stimulates the process of self-phagocytosis at an early stage while the high concentration of aspirin stimulates the programmed death of cells and inhibits the self-phagocytosis in the later stages [39].
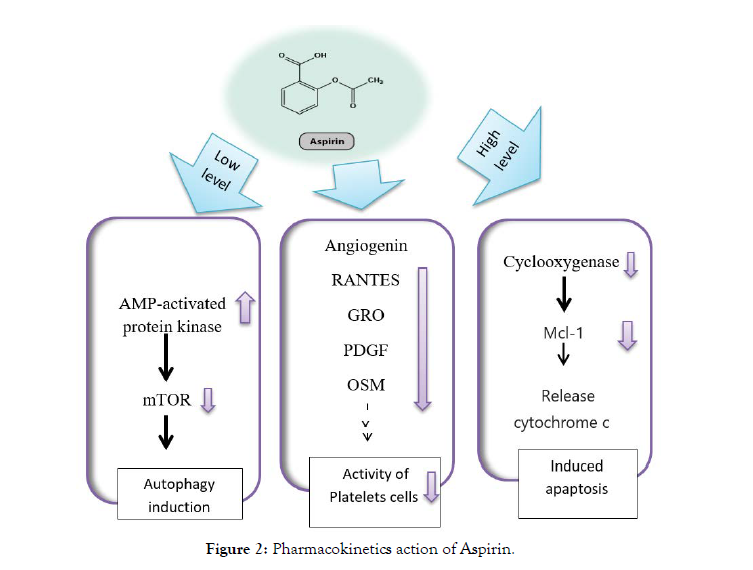
Figure 2: Pharmacokinetics action of Aspirin.
Combining traditional treatment methods that include radiation therapy and chemotherapy and taking aspirin in an organized manner and at low doses reduces the side effects of radiation and chemical treatment and reduces the chances of cancer cells spreading. Aspirin works as an effective prevention of cancer, even if used within treatment protocols in patients with cancer; it improves healing chances and reduces chances of relapse through autophagy stimulation [40].
Conclusion
Autophagy is one of the important processes through which it is possible to get rid of cancer cells, as it is considered one of the most important methods of cleaning the body to give opportunities for healthy cells to grow and fully and beneficially work. Aspirin is one of the most important chemical compounds that have an effective role in protecting the body from cancerous diseases and helps in a speedy recovery in addition to the use of cancer treatment drugs.
It plays an effective role in many vital processes inside the body because it keeps the body from clots because it works to prevent platelets from gathering.
In addition to the fact that the concentration of aspirin affects the process of representation that takes place within the human body, if it is taken in low concentration this encourage autophagy mechanism while in large concentration, it stimulates the apaptosis process. Therefore, aspirin dose is added in the treatment protocol for patients with different cancers.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Tamas M, Viktoria K, Tamas C, Gábor J. On the Fly: Recent Progress on Autophagy and Aging in Drosophila. Front Cell Dev Biol. 2019; 7:1-15.
- Graeme H and Viktor I. Repair, Reuse, Recycle: The Expanding Role of Autophagy in Genome Maintenance. Trends Cell Biol. 2017; 27:340-351.
- Bindi P and Ana M. Methods to study chaperone-mediated autophagy. Methods. 2015; 75:133-140.
- Jeong A, Naresh K, Nina R. Pros and cons of different ways to address dysfunctional autophagy in Pompe disease. Ann Transl Med. 2019;7:279.
- Schmalstieg F, Goldman A. "Ilya Ilich Metchnikoff (1845–1915) and Paul Ehrlich (1854–1915): the centennial of the 1908 Nobel Prize in Physiology or Medicine". J Med Biogr. 2008;16:96-103.
- Guillaume R, Arnaud J, Patrick A. Chaperone-Mediated Autophagy and Its Emerging Role in Hematological Malignancies. Cells. 2019;8:1260.
- Danielle G, Sandra B, Kay F. Autophagy: cellular and molecular mechanisms. J Pathol. 2010; 221:3-12.
- Long H, Jie Z, Jinshan Z, Ning M. Autophagy: The Last Defense against Cellular Nutritional Stress. Adv Nutr. 2018; 9:493-504.
- James H, Lindsey N. Mechanisms of Autophagy Initiation. Annu Rev Biochem. 2017;86:225-244.
- Ji-Man P, Minchul S, Douglas G. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy. 2018; 14:584-597.
- Robbie L, Michael N. Target of Rapamycin (TOR) in Nutrient Signaling and Growth Control. Genetics. 2011; 189:1177-1201.
- Panagiotis T, Ioannis P. Caspase involvement in autophagy. Cell Death & Differentiation. 2017; 24:1369–1379.
- Katherine R. Parzych, Daniel J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid Redox Signal. 2014;20:460-473.
- Thomas J, Andrea G, Sharon A. A molecular perspective of mammalian autophagosome biogenesis. J Biol Chem, 2018; 293:5386-5395.
- Saori R and Noboru M. Monitoring and Measuring Autophagy. Int J Mol Sci, 2017;18:1865.
- Rafah M, Daniel P, Séverine L, Patrice C. Autophagy and microtubules – new story, old players. J Cell Sci. 2013;126:1071-1080.
- Arun K, Jesse T, Wendy V, Jarne P, Ragna S, David A. A novel approach to analyze lysosomal dysfunctions through subcellular proteomics and lipidomics: the case of NPC1 deficiency. Sci Rep. 2017;7:41408.
- Maria C, Evelin P, Michele C, Stefania M. Targeting Autophagy to Overcome Human Diseases. Int J Mol Sci. 2019;20:725.
- Naiara S, Joseph D, Alec C. The Role of Autophagy in Cancer. Annu Rev Cancer Biol. 2017;1:19-39.
- Ramzi M, Irfana M, Leroy L, Clement Y. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35:S78-S103.
- Yenniffer Á, Jimena C, Roberto Bravo-S, Alfredo C. Tumor Suppression and Promotion by Autophagy. Biomed Res Int. 2014;2014:603980.
- Xuning E, Bryan N, Aram S. Targeting Tumor Suppressor Networks for Cancer Therapeutics. Curr Drug Targets. 2014;15:2-16.
- Croce C, Sozzi G, Huebner K. Role of FHIT in human cancer. J Clin Oncol. 1999;17:1618-1624.
- Kay H, Joshua C, Jin S, Hidetaka S. Hits, Fhits and Nits: Beyond enzymatic function. Adv Enzyme Regul. 2011; 51:208-217.
- Tae-Gul L, Eun-Hui J, Seo Yun K, Hye-Ryoun K. Fhit, a tumor suppressor protein, induces autophagy via 14-3-3τ in non-small cell lung cancer cells. Oncotarget. 2017;8:1923-31937.
- Sundberg, Richard J. The Chemical Century: Molecular Manipulation and Its Impact on the 20th Century, 2017; CRC. 491.
- Sunitha V, Jeffrey J, Shereif H. The Role of Aspirin in the Prevention of Cardiovascular Disease. Clin Med Res. 2014;12:147-154.
- Emanuela R, Garret A. FitzGerald. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011;31: 986-1000.
- Hegyi P, Maléth J, Walters J, Hofmann A. Guts and Gall: Bile Acids in Regulation of Intestinal Epithelial Function in Health and Disease. Physiol Rev. 2018;98:1983-2023.
- Akhand P, Arpan B, Aparna S. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. 2019;4:33.
- Argentina O, Niki Z, David G. Beyond COX-1: The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289-303.
- Jennifer B, Anita N, Bertil A. Biowaiver Monograph for Immediate-Release Solid Oral Dosage Forms: Acetylsalicylic Acid. Journal of Pharmaceutical Sciences. 2012;101:2653-2667.
- Miodrag N. Mijac D. General Aspects of Primary Cancer Prevention. Dig Dis. 2019;37:406-415.
- In Cheol H, Jooyoung C, Kyuwoong K. Aspirin Use and Risk of Hepatocellular Carcinoma in a National Cohort Study of Korean Adults. Sci Rep. 2018;8:4968.
- Hua H, Kong Q, Zhang H. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;2:71.
- Isaac T, Guillermo M. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int J Mol Sci. 2018;19:3812.
- Din F, Valanciute A, Houde V, Zibrova D. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504-1515.
- Ting S, Liang M, Yunmeng Y, Yan Z. Beclin 1 acetylation impairs the anticancer effect of aspirin in colorectal cancer cells. Oncotarget. 2017;8:74781-74790.
- Jeong H, Peter M, Rubin M. Phagocytic clearance of apoptotic cells: role in lung disease. Expert Rev Respir Med. 2008;2:753-765.
- Chih-Yang H, Da-Tong J, Chih-Fen C, Muralidhar R. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine (Taipei). 2017;7:23.
Citation: Elbossaty WF (2020) Dual Sword Role of Aspirin as anticancer through induced or inhibition of Autophagy through mTOR pathway. J Blood Disord Transfus 11: 434
Copyright: © 2020 Elbossaty WF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited

