Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2022) Volume 10, Issue 2
Dual LAD Anatomy, Classification and Clinical Significance: A Review
Vijay Shekar P1* and Rajeev Sai Moram22Department of Cardiology, Mediciti Institute of Medical Sciences, Medchal, Telangana, India
Received: 14-Feb-2022, Manuscript No. JVMS-22-15506; Editor assigned: 16-Feb-2022, Pre QC No. JVMS-22-15506 (PQ); Reviewed: 31-Jan-2022, QC No. JVMS-22-15506; Revised: 03-Feb-2022, Manuscript No. JVMS-22-15506 (R); Published: 08-Feb-2022
Abstract
Dual Left Anterior Descending (LAD) anatomy is a rare coronary artery anomaly characterized by presence of two LADs in the anterior inter ventricular groove. Since its first description almost three decades ago, multiple variants of the entity have been reported. This article aims to provide a comprehensive review of the dual LAD variants, their classification and clinical significance.
Keywords
Coronary artery anomalies; Dual LAD anatomy; Long LAD; Short LAD
Introduction
The prevalence of Coronary Artery Anomalies (CAA) varies depending on the definition and imaging modality used. According to the literature, CAA is identified in 0.2%-1.3% of patients undergoing coronary angiography and in 0.3% of autopsy series [1,2]. Classification of CAA anomalies based on anatomical considerations into (a) anomalies of origin and course (b) intrinsic CA anomalies (c) anomalies of termination is widely accepted. A modified embryological-anatomical classification has also been proposed [3].
Dual LAD is a rare congenital coronary anomaly, with its first description almost three decades ago. With increasing number of diagnostic and therapeutic coronary procedures, a higher prevalence of the anomaly is being reported. The use of Computed Tomography Coronary Angiography (CT CAG) allows recognition of the entity and description of variants, in addition to providing prognostic information. With thirteen variants reported to date, few of these variants have important therapeutic implications and hence are important to identify. This article aims to review the definitions, embryologic basis, classification and recognition of clinically significant dual LAD variants.
Dual Lad: Anatomy and Definition
The Left Coronary Artery (LCA) arises from the left sinus of Valsalva, courses behind the pulmonary trunk and bifurcates into the Left Anterior Descending Artery (LAD) and Left Circumflex Artery (LCX). The LAD courses in the Anterior Interventricular Groove (AIG) and gives rise to the diagonal branches (supplying the anterior and lateral wall of LV) and septal perforating branches (runs in the musculature of the interventricular septum). The LAD terminates near the cardiac apex and communicates with ramifications of the posterior descending artery which runs in the posterior interventricular groove.
Dual LAD anatomy, also described as duplication of LAD is defined as presence of two LAD arteries in the AIG. The short LAD usually arises from the left coronary system (in rare instances from right coronary system) and terminates in the proximal portion of AIG without reaching the cardiac apex. The long LAD has a variable origin (either from left or right coronary system), enters the distal portion of AIG reaching up to the cardiac apex. When the long LAD originates from the opposite sinus, it courses pre pulmonic, retro aortic, inter arterial or transeptal to reach the AIG. Transeptal course is the most common, whereas an inter arterial course can be malignant. Septal and diagonal branches may arise from both vessels and is highly variable. Even though this anomaly is seen more often with congenital malformations such as tetralogy of Fallot or transposition of great arteries, its incidence in normal hearts ranges from 0.13%-1.38% [4-7].
Dual Lad: Embryologic Basis
Knowledge of embryologic basis of coronary anomalies is largely derived from animal data. The coronary ostia develop after separation of the common truncus into aorta and pulmonary trunk after 42 days of embryo formation. Animal and human models have demonstrated that cells derived from Peritruncal Capillary Plexus (PCP) penetrate the aortic wall (aided by local apoptosis) resulting in the formation of coronary ostia. Coronary ostia establish connections with capillary plexus (derived from sinus venosus endocardium, ventricular endocardium and epicardial cells) and initiate recruitment of Vascular Smooth Cells (VSMCs) thus establishing coronary circulation. Abnormalities involving Vascular Endothelial Growth Factor (VEGF), Neural Crest Cells (NCCs) and Tbx1 expression (gene implicated in arterial pole development) can result in anomalous connections between coronary ostia and capillary plexus [3,8]. Dual LAD may result from an abnormal development of coronary ostia or due to development of anomalous connections between the ostia and peripheral capillary plexus.
Dual Lad: Morphological Classification
Classification of dual LAD is based on the origin and course of short and long LADs. An early attempt to classify dual LAD anomalies based on angiography was made by Franco et al. [4] in 1983 that lead to the identification of first four types of dual LAD anomalies. Of this type 1 is the most common type while type 4 is the least common. Further studies lead to the addition of several other types based on the origin and course of the two LAD arteries [9-16]. Currently, there are 13 types. Of dual LAD anomalies identified till date. Anatomical classification of dual LAD is depicted in Table 1 (Adapted and modified from [16]) (Figure 1). Though the reported variants are bound to increase with time, the short LAD is more consistent in its origin and course. On the other hand, the long LAD has a variable origin and course and determines the hemodynamic significance in most instances.
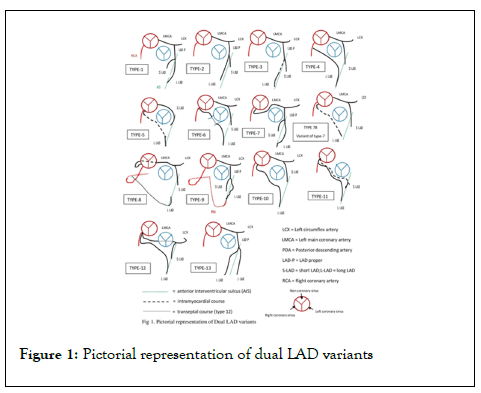
Figure 1: Pictorial representation of dual LAD variants
| Type | Short-LAD origin | Short-LAD course | Long-LAD origin | Long-LAD course |
|---|---|---|---|---|
| 1 | LCS(LAD) | Terminates in the proximal portion of AIG | LAD-Proper | Runs along the LV side of proximal AIG (epicardial) and enters the distal AIG |
| 2 | LCS(LAD) | Terminates in the proximal portion of AIG | LAD-Proper | Runs along the RV side of proximal AIG(epicardial) and enters the distal AIG |
| 3 | LCS(LAD) | Terminates in the proximal portion of AIG | LAD-Proper | Runs intramyocardial course in the emerges epicardial in the distal AIG. |
| 4 | LCS(LMCA) | Terminates in the proximal portion of AIG | Proximal RCA | Anomalous prepulmonic (epicardial) course anterior to RVOT and enters the distal AIG |
| 5 | LCS | Terminates in the proximal portion of AIG | RCS | Anomalous intramyocardial course and tthen emerges epicardial in the distal AIG |
| 6 | LCS(LMCA) | Terminates in the proximal portion of AIG | Proximal RCA | Anomalous epicardial course between RVOT and aortic root, (Interarterial) then enters distal AIG |
| 7 | LMCA that originates in the RCS and courses inter-arterially) | Terminates in the proximal portion of AIG | LMCA that originates in the RCS and courses inter-arterially | Enters distal AIG |
| 7b | LCS(LMCA) | Terminates in the proximal portion of AIG | RCS (separate ostium) | Intramyocardial course and emerges in the distal AIG |
| (Variant of 7) | ||||
| 8 | LMCA (originates from RCS and shows retro-aortic course) | Terminates in the proximal portion of AIG | Mid RCA | Courses the inferior surface of the RV (epicardial), turns around the apex to reach the distal AIG |
| 9 | LCS(LAD) | Terminates in the mid portion of AIG | LCS(LAD) | Runs on LV side of mid AIG(epicardial) then enters the distal AIG, terminating proximal to the apex- |
| (Triple LAD) | ||||
| 10 | LCS(LMCA) | Terminates in the proximal portion of AIG | RCS(separate ostium) | Epicardial prepulmonic course anterior to RVOT and enters the distal AIG |
| 11 | RCS (common ostium with RCA) | Intramyocardial course within proximal septum and emerges in the proximal AIG(where it terminates as the first diagonal branch) | RCS ( common ostium with RCA) | Takes an anomalous prepulmonic course anterior to RVOT and enters the distal AIG |
| 12 | LMCA from RCS-same ostium with RCA | Terminates in the Proximal portion of AIG/LCX arises from LMCA | RCS-common ostium with RCA | Epicardial prepulmonic course to reach distal AIG |
| 13 | No short-LAD | N/A | LCS(LAD) | Two long LADs move out of the AIG and courses to the cardiac apex, one laterally and the other medially to the AIG |
| LCS(LAD) |
Abbreviations: LAD: Left Anterior Descending Artery; RCS: Right Coronary Sinus; LMCA: Left Main Coronary Artery; RCA: Right Coronary Artery; LCS: Left Coronary Sinus; AIG: Anterior Interventricular Groove; LV: Left Ventricle; RV: Right Ventricle; RVOT: Right Ventricular Outflow Tract.
Table 1: Anatomical classification of Dual LAD variants.
Dual Lad Variants and Their Clinical Significance
Dual LAD is commonly an incidental finding during coronary angiography. Most of the dual LAD anomalies are hemodynamically insignificant, except those with inter-arterial or intra-myocardial course. The presence of a short LAD may confer an ischemic protection due to a twin blood supply and potential to form collaterals.
Failure to identify dual LAD anatomy can result in adverse therapeutic implications during bypass artery grafting. Knowledge of anatomy is essential to avoid incorrect placement of arteriotomy. Failure to recognize and graft the short LAD may result in incomplete revascularization. Grafting the short LAD may warrant a more proximal approach, due to its termination in the proximal AIG. A short LAD can be misinterpreted as a Type I LAD, especially when the long LAD arises from the opposite sinus [17]. A short LAD can also mimic a mid-LAD occlusion. On the other hand, occlusion of a short LAD may be overlooked and a potential revascularization benefit may be missed. Detection of hypoplastic or small LAD during angiography and paucity of vessel opacification in the LAD territory should raise the suspicion of a dual LAD anomaly. The long LAD may have an epicardial or intramyocardial course. Type 3 and Type 5 variants of dual LAD are identified by presence of intramyocardial course of the long LAD, which is important to recognize while planning revascularization procedures [18]. Type 6 and 7 variants are characterized by a malignant inter-arterial course and are associated with an elevated risk of myocardial ischemia and sudden cardiac death (SCD). In Type 6 variant, the long LAD has an inter arterial course whereas in type 7 variant, the LMCA arises anomalously from right sinus and courses inter arterially, to divide and give rise to the short and long LAD, along with LCX. Type 10, 11 and 12 variants are more likely to be injured during median sternotomy due to their pre pulmonic course and close proximity with the sternum. Type 11 variant carries additional significance due to intra-myocardial course of short LAD. Dual LAD is also reported to be associated with anomalies involving other coronaries (LCX and RCA) [19].
Though more variants may be added to the classification with time, prompt recognition and treatment of malignant variants carries priority. Long term prognosis of dual LAD variants and their association to increased risk of atherosclerosis have not been established.
Role of CT CAG in Diagnosis
Conventional angiography is considered the gold standard for evaluation of coronary anatomy. But however, multislice CT CAG with 3 dimensional (3D) construction is considered superior for identification and characterization of coronary anomalies. CT CAG has additional advantage of being noninvasive. With respect to dual LAD variants, CT CAG yields valuable information regarding the variable origin (especially of the long LAD) and course of the branches. Conventional angiography may suffice to establish a diagnosis when both short and long LAD arises from common LAD (LAD proper). But however, when the long LAD arises from the opposite sinus, CT CAG is needed to delineate the origin and course. The presence of long LAD arising from opposite sinus can be missed during conventional angiography, if not suspected and searched for. CT CAG studies suggest that prevalence of dual LAD may be much higher than reported using conventional angiography [11]. Intramyocardial or epicardial course is important to recognize in need for revascularization. Identification by CT CAG an inter arterial course between the RVOT and aorta in symptomatic patients demand immediate intervention. In asymptomatic patients with inter arterial course, further evaluation to demonstrate physiological consequences and ischemia is warranted. The risk benefit ratio of surgical procedure in such patients has to be considered. Rare instances where the long LAD was seen arising from the pulmonary artery have also been reported and was identifiable only by CT CAG.
Conclusion
Dual LAD anatomy characterized by presence of short and long LAD is most often a benign coronary anomaly. CT CAG is superior in delineating the origin and the course of short and long LAD compared to conventional angiography and should be considered as the choice of investigation. Failure to identify a dual LAD anatomy may significantly affect the outcome of revascularization procedures. A higher risk of ischemia and sudden cardiac death is associated with few malignant variants and is important to recognize. A high degree of suspicion and judicious use of CT CAG will unmask more dual LADs and their variants.
REFERENCES
- Yildiz A, Okcun B, Peker T, Arslan C, Olcay A, Bulent Vatan M. Prevalence of coronary artery anomalies in adult patients who underwent coronary angiography. Clin Cardiol. 2010; 33:60-64.
- Zhang LJ, Yang GF, Huang W, Zhou CS, Chen P, Lu GM. Incidence of anomalous origin of coronary artery in 1879 Chinese adults on dual-source CT angiography. Neth Heart J. 2010; 18:466-470.
[PubMed] [Google Scholar]
- Pérez-Pomares JM, Pompa JL, Franco D, Henderson D, Yen Ho S, Houyel L, et al. Congenital coronary artery anomalies: A bridge from embryology to anatomy and pathophysiology: A position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc. Res. 2016; 109: 204–216.
[Cross ref] [PubMed] [Google Scholar]
- Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: Angiographic description of important variants and surgical implications. Am Heart J. 1983; 105:445-455.
[Cross ref] [PubMed] [Google Scholar]
- Baskan, Ozdil, Erol C, & Paksoy Y. "Dual (type IV) left anterior descending artery." S Afr J. Radiol. 2013; 21:145-146.
- Sidhu NS, Wander GS. Prevalence and characteristics of dual left anterior descending artery in adult patients undergoing coronary angiography. Future Cardiol. 2019; 15:425-435.
[Cross ref] [PubMed] [Google Scholar]
- Şeker, M. Prevalence and morphologic features of dual left anterior descending artery subtypes in coronary CT angiography. Radiol med. 2020; 125: 247–256.
[Cross ref] [PubMed] [Google Scholar]
- Sharma B, Chang A, Red-Horse K. Coronary artery development: Progenitor cells and differentiation pathways. Annu Rev Physiol. 2017; 79:1-19.
[Cross ref] [PubMed] [Google Scholar]
- Manchanda A, Qureshi A, Brofferio A, Go D, Shirani J. Novel variant of dual left anterior descending coronary artery. J Cardiovasc Comput Tomogr. 2010; 4:139-141.
[Cross ref] [PubMed] [Google Scholar]
- Maroney J, Klein LW. Report of a new anomaly of the left anterior descending artery: type VI dual LAD. Catheter Cardiovasc Interv. 2012; 80:626-629.
- Bozlar U, Uğurel MŞ, Sarı S, Akgün V, Örs F, Taşar M. Prevalence of dual left anterior descending artery variations in CT angiography. Diagn Interv Radiol. 2015; 21:34-41.
[Cross ref] [PubMed] [Google Scholar]
- Saglam M, Ozturk E, Kara K, Kardesoglu E, Mutlu H. A new variation in coronary artery anatomy: type VII dual left anterior descending artery. Kardiol Pol. 2015; 73:217.
[Cross ref] [PubMed] [Google Scholar]
- Celik T, Bozlar U, Ozturk C, Balta S, Verim S, Demir M, et al. A new anomaly of the left anterior descending artery: Type X dual LAD. Indian Heart J. 2015; 67:14-17.
[Cross ref] [PubMed] [Google Scholar]
- Al-Umairi RS, Al-Kindi FA, Al-Tai SA. A new variant of dual left anterior descending artery anomaly: Type XI. Sultan Qaboos Univ Med J. 2018; 18:386-388.
[Cross ref] [PubMed] [Google Scholar]
- Pandey NN, Shaw M, Sharma A, Ganga KP, Gulati GS. Yet another novel variant of dual left anterior descending artery: type XII. Heart Lung Circ. 2020, 29:33-35.
[Cross ref] [PubMed] [Google Scholar]
- Pellegrini JR, Munshi R, Alvarez Betancourt A, Tokhi B, Makaryus AN. "Two for One", Novel dual left anterior descending artery (LAD) Variant: Type XIII. Cureus. 2021; 13:14717.
[Cross ref] [PubMed] [Google Scholar]
- Bhargav, A., Otaal, P.S. & Singhal, M.K. Type X dual left anterior descending (LAD) artery masquerading as type 1 LAD: A case report. Egypt J Intern Med.2021.
- Wróbel G, Spałek M, Spałek J, Kuder T. Dual left anterior descending coronary artery (type III) and the presence of myocardial bridges: A post-mortem examination. Folia Morphol (Warsz). 2020; 79:634-639.
[Cross ref] [PubMed] [Google Scholar]
- AravindaKumar S, VijayShekar P. Rare combination of coronary anomaly: Type IV dual LAD with anomalous LCX origin IHJ. Cardiovascular Case Reports (CVCR) 5. 2021.
Citation: Shekar PV (2022) Dual LAD Anatomy, Classification and Clinical Significance: A Review. J Vasc Surg. 10:441.
Copyright: © 2022 Shekar PV. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.