Indexed In
- Open J Gate
- Academic Keys
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- CABI full text
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2023) Volume 11, Issue 1
Does Nebulized Unfractionated Heparin Cause Changes in Derived Neutrophil to Lymphocyte Ratios in Severe COVID-19 Pneumonitis?
Cecily W Thompson1*, Stacey-Ann M Robinson1, Jevonne J McIntosh1, Jodian S Risden1, Dwayne R White2, Keri S Morgan1 and Tamara S Beecher12Department of Anaesthetics, Harrogate District Hospital, Harrogate, United Kingdom
Received: 24-Mar-2023, Manuscript No. JTD-23-20239; Editor assigned: 27-Mar-2023, Pre QC No. JTD-23-20239 (PQ); Reviewed: 11-Apr-2023, QC No. JTD-23-20239; Revised: 19-Apr-2023, Manuscript No. JTD-23-20239 (R); Published: 28-Apr-2023, DOI: 10.35241/2329-891X.23.11.372
Abstract
Background: COVID-19 a multi systemic disorder caused by the SARS-CoV-2 virus with deleterious and often fatal effects on respiratory function. Many therapeutic but expensive options have been presented. Unfractionated heparin is widely available and has been shown to have antiviral properties in vitro (by inhibiting the interaction of the spike protein of the coronavirus with ACE2), as well as anti-inflammatory, mucolytic and anticoagulant effects.
Objective: To conduct a retrospective cohort study on COVID-19 patients admitted to an isolation ward in Jamaica to determine if nebulized unfractionated heparin delivered to patients with severe COVID-19 pneumonitis would influence the derived Neutrophil to Lymphocyte Ratio (dNLR), a predictor of infection severity that was routinely measured.
Methods: Study participants were hospitalized with COVID-19 pneumonitis, confirmed by polymerase chain reaction, between August 4, 2021 and November 13, 2021 and managed by anaesthesiologists. All had SpO2<92% on room air at admission. Patients received a standard COVID-19 care management protocol, as per the national guidelines of Jamaica. Seventeen patients with median (range) age 53 (35-67) years received nebulized unfractionated heparin (the study group); and seventeen patients with median (range) age 53 (38-67) years were not given nebulized unfractionated heparin (the control group). Derived neutrophil to lymphocyte ratios were observed daily over a 15- day period and tabulated and charted using stacked line graphs and box plots to compare changes in the variable between the groups.
Results: The demographics and illness severity were comparable for the groups. Stacked line graphs of the dNLR in each group indicated a more rapid decline in the group treated with nebulized unfractionated heparin than in the control group. Univariate analysis using box plots of serial dNLR changes showed larger interquartile ranges, longer whiskers, higher means and medians in the control group than in the study group.
Keywords
Nebulized-unfractionated-heparin; COVID-19-pneumonitis; Oxygen; Anaesthetics-department; Neutrophil-to-lymphocyte-ratio; Delta-wave; High flow nasal oxygen
Introduction
COVID-19 has killed more than six million people worldwide and more than three thousand Jamaicans since the start of the pandemic. The period in which there was the greatest toll on the health services in Jamaica was during the period when the B.1.614.2 (delta) variant of COVID-19 was dominant.
The coronavirus has emerged as a significant cause of morbidity before: (SARS-CoV in 2002 MERS CoV in 2012) [1]. The Sars- CoV-2 virus uses ACE2 to enter type II pneumocytes in the respiratory tract and other areas where ACE2 is found, such as the endothelial cells of capillaries [2].
Unfractionated heparin has been shown to be antiviral in vitro (inhibiting the interaction of the spike protein of the coronavirus with ACE2, anti-inflammatory, mucolytic and anticoagulant. These properties exceed those of low molecular weight heparins [3]. Unfractionated heparin causes a dose dependent inhibition of SARS-CoV-2 virus in vitro [4-7].
The SARS-CoV-2 virus uses ACE2 to enter alveolar cells and endothelial cells, causing apoptosis. Macrophages then react by producing cytokines and chemokines, attracting neutrophils. There is then diapedesis of these macrophages into alveolar sacs which produces several problems: Neutrophil extracellular traps, extrusion of cytotoxic histones, neutrophil elastase and release ofextra-cellular DNA. All of this leads to destruction of alveoli, mucus production and hyper coagulation with hyaline membrane deposition in the alveoli and micro thrombi in capillaries. The virus thus causes diffuse alveolar damage and hyper inflammation, leading to hypoxia and possible organ failure [8].
Could inhaled unfractionated heparin possibly attenuate the pathology of COVID-19 pneumonitis? As a therapeutic agent, it is not new: inhaled unfractionated heparin along with inhaled N-acetylcysteine has been used to treat inhalation injury in burn patients [9]. Nebulized unfractionated heparin has been shown to be safe in normal lungs and in COVID-19 and might help to inhibit the dysfunctional hyper inflammation and hyper coagulation, without causing systemic coagulopathy [10].
In acknowledging the molecular biology outlined above and the resultant hyper inflammation of severe COVID-19 pneumonitis, this study sought to explore the effect of nebulised unfractionated heparin on an inflammatory marker. The derived neutrophil to lymphocyte ratio has been shown to be a more specific inflammatory marker of COVID-19 than it is of influenza and respiratory syncytial virus [11]. In this study, we sought to discern the following: if there was any significant difference between the study and control groups such as age, sex and indices of severity (e.g. ISARIC 4C); and using an acknowledged index of severity, the derived Neutrophil to Lymphocyte Ratio (dNLR), if there was a difference when nebulised unfractionated heparin was administered. We thus compared the effect of nebulised unfractionated heparin to usual care in hospitalized patients on serially observed derived Neutrophil to Lymphocyte Ratios (dNLR).
Materials and Methods
Study design
We received permission from the Ethics Committee of the Southern Regional Health Authority (SRHA) in Jamaica to conduct this study. We complied with our governing ethics committee’s requirement consent for use of data [12].
On the COVID-19 isolation ward at May Pen Hospital, Clarendon, Jamaica, the anaesthetics department became involved with all patients displaying signs of respiratory failure (increasing Respiratory Rate (RR) and low (<92%) SpO2 on room air). We adjusted oxygen therapy and respiratory care plans. We also monitored their laboratory and radiological investigations as well as participated in and recommended therapeutic interventions [13]. It was a period of severe morbidity, rapid deterioration and high mortality (Figure 1), seen throughout Jamaica and other regions of the world during the pandemic wave most closely associated with emergence of the delta virus variant [14]. Our involvement ceased when the patients discontinued oxygen, and the internists assigned to the COVID ward continued care. All study participants were admitted to this ward and at inclusion of the study, had moderately high to very high O2 needs from 10 to 60, 1 min (many times adding l min via face mask and reservoir bag) (Figure 1). Oxygen therapy included nasal cannula; face masks with or without nonrebreather attachments, and HFNO devices (Figure 2).
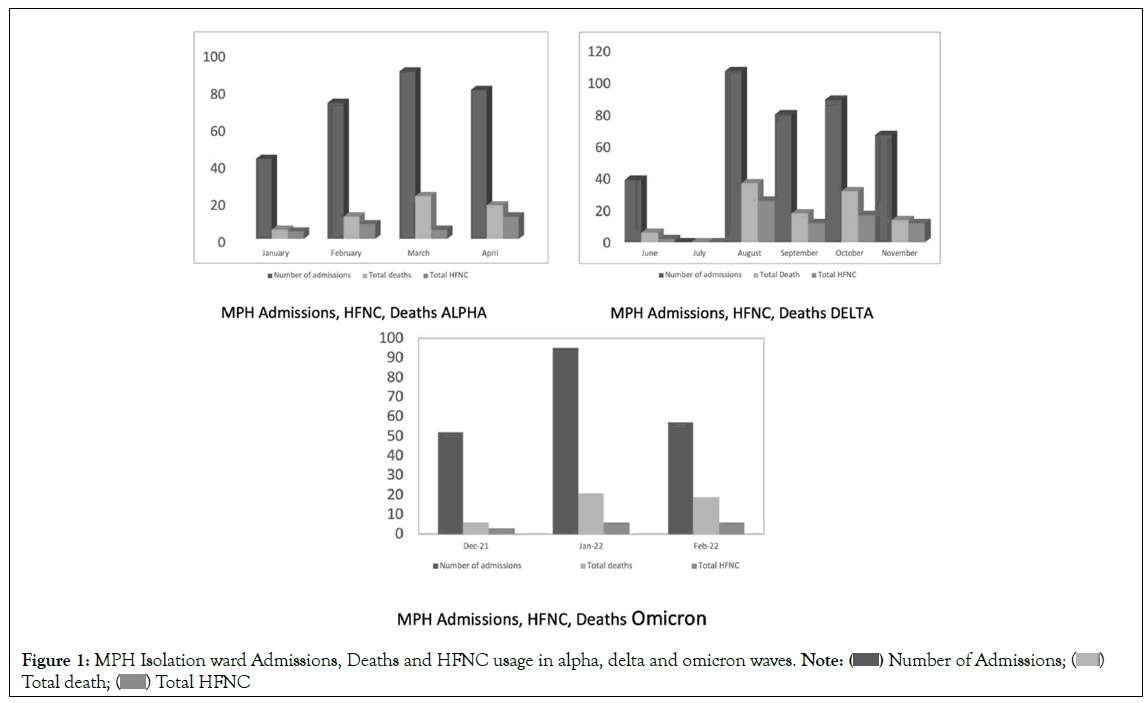
Figure 1: MPH Isolation ward Admissions, Deaths and HFNC usage in alpha, delta and omicron waves. Note: ( ) Number of Admissions; (
) Number of Admissions; ( )
Total death; (
)
Total death; (  ) Total HFNC
) Total HFNC
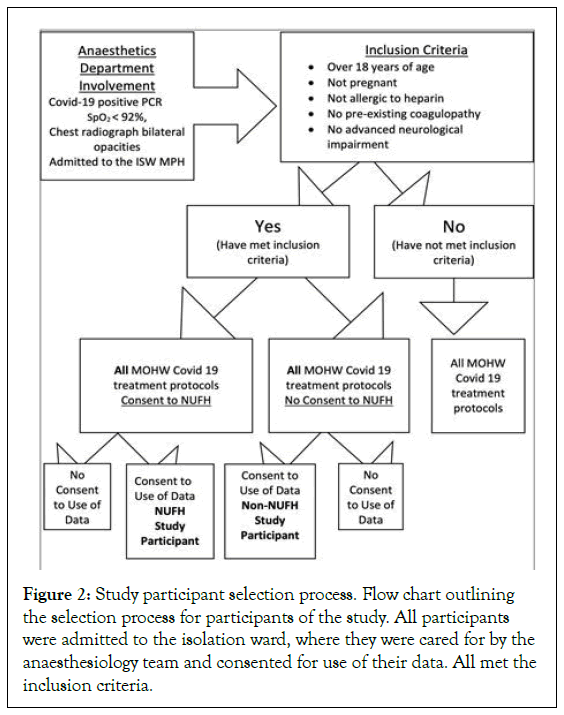
Figure 2: Study participant selection process. Flow chart outlining the selection process for participants of the study. All participants were admitted to the isolation ward, where they were cared for by the anaesthesiology team and consented for use of their data. All met the inclusion criteria.
Bar charts showing admissions, High Flow Nasal Oxygen (HFNO) requirements and deaths on the isolation ward during the alpha, delta and omicron waves of the COVID-19 pandemic at May Pen Hospital. The deaths/admissions during the alpha wave (dominant variant B.1.1.7) was 20%; that of the delta wave (dominant variant B.1.614.2) was 29% and the first omicron wave (dominant variant BA.2.12.1), 22%. There were difficulties retrieving the July 2021 data [1].
Setting and participants
The 26-bed COVID-19 ward in a hospital in rural Jamaica. Conducted during the SARS-Cov-2 pandemic when the delta variant was the dominant COVID-19 variant in Jamaica: From August 4, 2021, to November 13, 2021.
All patients were confirmed to have SARS-CoV-2 infection (using PCR). All patients had radiological confirmation of a pneumonitis with bilateral lung involvement from the referring ward or hospital. Seventeen patients aged 35 to 67 years (median age 53 years), 8 females and 9 males, were given nebulized unfractionated heparin (nebulized unfractionated heparin, or study group); and 17 patients aged 38 to 67 years (median age 53 years), 9 females and 8 males, were not given the nebulized unfractionated heparin (non-nebulized unfractionated heparin, or control group) (Table 1). All patients were given the standard COVID care management protocol, as per the Ministry of Health and Wellness (MOHW) guidelines see study inclusion and exclusion process (Figure 2) [15].
| Variable | Nebulized unfractionated heparin (n= 17) | Non-nebulized unfractionated heparin (n=17) | P-value (Mann Whitney U) |
|---|---|---|---|
| Median/Mean age | 53/52.1765 | 53/53.1176 | 0.7066 |
| Males | 9 (53%) | 8 (47%) | |
| Females | 8 (47%) | 9 (53%) | |
| Chronic illnesses | 12 (71%) | 12 (71%) | |
| Mean starting ISARIC 4C scores | 7.1176 (SD 2.97) | 8.0588 (SD 2.63) | 0.2673 |
| No. of ISARIC 4C low (0 to 3) | 0 | 0 | |
| No. of ISARIC 4C Intermediate (4 to 8) | 12 (71%) | 11 (65%) | |
| No. of ISARIC 4C High (9 to 14) | 5 (29%) | 7 (35%) | |
| No. of ISARIC 4C Very High (>14) | 0 | 0 | |
| Mean Starting DNLR | 7.22 (SD 2.42) | 7.03 (SD 5.08) | 0.377 |
| Patients treated with HFNO | 6 (35%) | 9 (53%) | |
| HFNO deaths | 1/6 = 17% | 4/9=44% | |
| Tocilizumab | 0 | 4 | |
| Total Deaths | 1/17=5.88% | 5/17=29% | |
| Note: Number of patients with comorbidities was similar. Neither the mean age, nor severity scoring systems at admission such as the mean ISARIC 4C score or the mean dNLR values (p=0.76, p=0.27, and p=0.38, respectively as per Mann-Whitney U test) showed a significant difference between the groups. Deaths: 29% (5/17) of the non-nebulized unfractionated heparin patients died compared to 5.88% (1/17) of the nebulized unfractionated heparin patients. Four of the non-nebulized unfractionated heparin patients had received TCZ as part of their therapy and they all survived. Three of these four patients were severe enough to need HFNO. None of the nebulized unfractionated heparin patients in the study received TCZ. ISARIC4C: International Severe Acute Respiratory and Emerging Infection Consortium Coronavirus Clinical Characterization Consortium HFNO: High Frequency Nasal Oxygen. |
|||
Table 1: Summarizing demographics, 4C severity scores, interventions and deaths.
Patients who received nebulized heparin generally received 25000 IU of heparin (Ryvis Unfractionated Heparin 5 000 iu.ml) and 1 ml of salbutamol for inhalation in a jet nebulizer chamber. Jet nebulizers are an efficient method of drug delivery for inhaled drugs [16]. Salbutamol was used as a carrier to help ensure that the heparin was delivered to the lower parts of the respiratory tree. This was delivered every 6 hours. Nebulized unfractionated heparin was typically prescribed for a period of 7 days.
The MOHW COVID-19 protocol included the administration of the following drugs/therapies to all patients, barring contraindications:
1. Low molecular weight heparin at 1 mg.kg twice daily
2. Dexamethasone 6 mg once daily
3. Vitamin C 1 g once daily
4. Vitamin D3 5000 IU once daily
5. Remdesivir was used until, guided by studies, its use was discontinued
6. Prophylactic antibiotics for community acquired pneumonia
7. Nebulized salbutamol and/or ipratropium bromide as needed and tolerated
8. ALL patients would have received physiotherapy daily or at least on alternate days.
Data sources and measurement
All participants in this study were admitted to the COVID ward during the time specified and all 34 patients consented to the use of their data for the study, of this group, all patients receiving treatment with nebulized unfractionated heparin also provided informed consent.
All study participants had preadmission plain chest radiographs with evidence of bilateral, often panlobular, pneumonitis. Variables such as SpO2, respiratory rate, oxygen flow and FiO2 were measured several times a day and were recorded from admission to the unit until the patient was either discharged, died or came off oxygen. The degree of dyspnoea and hypoxemia was determined by oxygen saturation, respiratory rate and other clinical signs such as nasal flaring, use of accessory muscles and speech interference.
Derived neutrophil to lymphocyte ratios were calculated from haematological investigations performed on alternate days. The main outcome measure was to determine if the serially measured derived Neutrophil to Lymphocyte Ratio (dNLR) in patients with severe COVID-19 pneumonitis who were given nebulized unfractionated heparin differed from that in patients who did not receive nebulized unfractionated heparin. The derived neutrophil to lymphocyte ratio has been proven to be a reliable marker of severity in COVID-19 [17,18].
Other investigations included urea and electrolytes, liver function tests and proteins; some of these variables were also needed to calculate the International Severe Acute Respiratory Infection Consortium Clinical Characterisation Protocol (ISARIC 4C) score (an easily computed score developed in 2020 during the first COVID-19 wave using age, oxygen saturation, respiratory rate, sex, the presence of comorbidities, Glasgow coma score, C-reactive protein and BUN as variables) [19]. Clotting indices were also measured occasionally. At no time was any coagulopathy or heparin induced thrombocytopenia seen in laboratory measurements. All other adverse events were recorded when brought to our attention.
Statistical analysis
All statistical analyses were conducted in the Stat Pl us Version 7.51 statistics and analysis software:
1. The Mann Whitney U test was used to determine p-value for statistical significance in differences between the demographic and some starting clinical variables between the groups. The comparison of the cohort was internal: same ward, same wave, similar chronological period.
2. Stacked line graphs and boxplots for each group were used to chart the serial changes in dNLR values for each patient the line graphs and the box plots were compared.
Results
Two groups of patients with severe COVID-19 pneumonitis were compared: One group received nebulized unfractionated heparin and the other did not.
The groups were similar in demographics and illness severity. The mean age of study group was 52.1 and that of the control group was 53.11 (p=0.7066); there were 9 males and 8 females in the study group and 8 males and 9 females in the control group; 12 participants in each group had one or more chronic illnesses; the mean starting ISARIC 4C score for the study group was 7.12 and that of the control group was 8.06 (p=0.27); No patient was in the low or very high risk category ISARIC 4C, and they were either intermediate or high risk; the mean initial dNLR for the study group was 7.22 and that of the control group was 7.03 (p=0.38). Six patients in the study group were on HFNO, of which 1 died (17%) and 9 patients in the control group had HFNO therapy of which 4 died (44%) (Table 1).
The stacked line graphs show the changes in dNLR over 15 days of records. The graphs show the trends in the data, with each line trend belonging to a participant. The rate of change in dNLR was clearly different with gradients of the stacked lines being steeper in descent in the nebulized unfractionated heparin group, compared to the non-nebulized unfractionated heparin group (Figure 3).
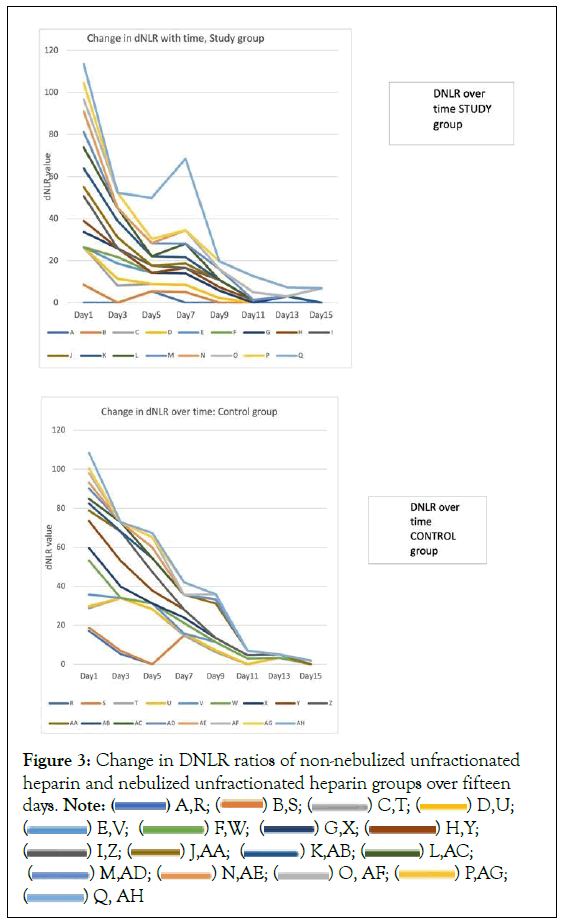
Figure 3: Change in DNLR ratios of non-nebulized unfractionated
heparin and nebulized unfractionated heparin groups over fifteen
days. Note: ( ) A,R; (
) A,R; ( ) B,S; (
) B,S; ( ) C,T; (
) C,T; ( ) D,U;
(
) D,U;
( ) E,V; (
) E,V; ( ) F,W; (
) F,W; ( ) G,X; (
) G,X; (  ) H,Y;
(
) H,Y;
( ) I,Z; (
) I,Z; ( ) J,AA; (
) J,AA; ( ) K,AB; (
) K,AB; (  ) L,AC;
(
) L,AC;
( ) M,AD; (
) M,AD; ( ) N,AE; (
) N,AE; ( ) O, AF; (
) O, AF; ( ) P,AG;
(
) P,AG;
( ) Q, AH
) Q, AH
These stacked line graphs showed that the DNLR ratios fell more rapidly in the nebulized unfractionated heparin group than the non-nebulized unfractionated heparin group. Nebulized unfractionated heparin group had a more rapid fall over 5 days, flattened or slightly increased between 5 to seven days and rapidly fell again after 7 days. At day 9, most survivors (5 out of 6) in the nebulized unfractionated heparin group had DNLR‘s of less than 4. The patient with the steep rise on day 7 was the patient who eventually died in the nebulized unfractionated heparin group. By day 9 in the non-nebulized unfractionated heparin group, 4 out of 7 in this group had DNLR‘s of less than 4.
In the nebulized unfractionated heparin group, the decrease in dNLR was more rapid over 3 days, less rapid from 3 to 5 days and flattening slightly between 5 and 7 days. By day 9, five out of six or 83% of survivors in this group had a dNLR of less than 4. By day 9 in the non-Nebulized Unfractionated Heparin group, four out of seven or 57% of patients had DNLRs of less than 4. The patient with the steep rise on day 7 was the patient who eventually died in the nebulized unfractionated heparin group.
Univariate analysis using box plots of the dNLR changes in the two groups over time showed a widened interquartile range in the control group compared to the flatter boxes of the study group. The ‘whiskers’ of the boxes in the control group were also longer than those of the study group. Generally, the medians and the means were lower in the study group. The differences in the means were more obvious in this observation. The medians of the control group also tended to be further from the centre of the boxes than those of the study group. There was one mild and two extreme outliers seen in each group (Figure 4).
Neither distribution is normal, the difference in groups can be seen in the inter quartile ranges: Larger ranges for the control group compared with the relatively tighter ranges in the study group, larger spread of data longer ‘whiskers’. The ‘means’ in the study group lower than those of the control group, generally, the medians are slightly lower in the study group especially before day 9. Nebulized heparin treatments were discontinued in the study group after 7 days.
Cox proportional hazard regression analysis
This was done to see if Terminal Charging Zone (TCZ) influenced the time spent on oxygen in the patients who received it (Figure 3). If TCZ had a clinical benefit with this variable it was not statistically significant (HR=0.892, p=0.35), CI (95%).
Adverse events
The use of inhaled unfractionated heparin has been deemed safe and appeared to be well tolerated. No patient in the study group was discontinued because of unacceptable side effects.
1. Headache: 5 study participants
2. Chest pain: 3 study participants
3. Tachycardia (usually reversed by discontinuing the nebulized salbutamol): General complaint
4. Bloody sputum: Seen especially on initial nebulized unfractionated heparin treatment 10/17 participants
Challenges
These included staff compliance and acceptance, especially among the nursing staff, which were reluctant to commence something ‘new’. Anaesthesiology staff had to medicate the first few study patients to demonstrate to the staff.
Patient acceptance
Patients who were overwhelmed with the disease and its toll on them were also sometimes doubtful about therapies, and this was not offered throughout the health service.
Drug availability
There were one or two days when salbutamol was not available on the ward. Increase responsibilities for staff. Administration of the heparin nebulizations was yet another layer of duties for the staff. They had to chart it and observe the patients and record any concerns.
Discussion
Key results
This small retrospective cohort study was performed during the COVID-19 pandemic when the dominant COVID-19 variant was delta. On the COVID-19 ward, nebulized unfractionated heparin was administered to 17 patients with severe COVID-19 pneumonitis and they were compared with 17 similarly afflicted patients who did not receive the treatment. One of the variables measured regularly was the derived Neutrophil to Lymphocyte Ratio (dNLR) (Table 2).
| Patient ID | Day1 | Day3 | Day5 | Day7 | Day9 | Day11 | Day13 | Day15 | Survived |
|---|---|---|---|---|---|---|---|---|---|
| A | - | - | 5.5 | - | - | - | - | - | YES |
| B | 8.5 | - | - | 5.11 | - | - | 3.05 | - | YES |
| C | 17.94 | 8.12 | 3.39 | 3.39 | 2.25 | - | - | - | YES |
| D | - | 3.23 | - | - | - | - | - | - | YES |
| E | - | 7.27 | 5.45 | 5.45 | 3.58 | - | - | - | YES |
| F | - | 3.05 | - | - | - | - | - | - | YES |
| G | 7.17 | 4.22 | - | - | - | - | - | - | YES |
| H | 5.33 | - | - | 2.62 | 1.52 | 1.33 | - | - | YES |
| I | 11.78 | - | 3.29 | - | 3.46 | - | - | - | YES |
| J | 4.33 | 5.15 | - | 2.05 | - | - | - | - | YES |
| K | 8.87 | 7.79 | 4.42 | 2.99 | - | - | - | - | YES |
| L | 9.9 | 6.08 | - | 6.48 | - | - | - | 6.88 | YES |
| M | 7.38 | - | 6.17 | - | 5.2 | - | - | - | YES |
| N | 9.83 | - | - | 6.34 | - | 3.66 | - | - | YES |
| O | 5.77 | 7.32 | 2.1 | - | - | - | - | - | YES |
| P | 7.69 | - | - | - | 3.61 | 7.61 | 4.24 | - | YES |
| Q | 9.07 | - | 19.5 | 34 | - | - | - | - | NO |
| Patient ID | Day1 | Day3 | Day5 | Day7 | Day9 | Day11 | Day13 | Day15 | SURVIVED |
| R | 17.11 | 5.26 | - | 14.81 | 6.23 | - | 3.323 | - | YES |
| S | 1.65 | 1.62 | - | - | - | - | - | - | NO |
| T | 10 | 27.05 | 28.21 | - | - | - | - | - | NO |
| U | 1.01 | - | - | 1.01 | 1.02 | - | - | YES | |
| V | 5.98 | - | 3.01 | - | 4.16 | 3.02 | - | - | YES |
| W | 17.4 | - | - | 5.35 | - | - | - | - | YES |
| X | 6.46 | 5.96 | - | 2.77 | 2.08 | 1.61 | 1.72 | - | YES |
| Y | 13.79 | 13.17 | 6.6 | 4.19 | - | - | - | - | YES |
| Z | 5.41 | 14.94 | 9.45 | - | - | - | - | - | NO |
| AA | - | - | 7.05 | 7.6 | 17.74 | 2.4 | - | - | NO |
| AB | 3.56 | - | - | - | 1.98 | - | - | 1.87 | YES |
| AC | 2.44 | 4.8 | - | - | - | - | - | - | YES |
| AD | 5.35 | - | 5.6 | - | - | - | - | - | YES |
| AE | 2.88 | - | - | - | 2.72 | - | - | - | YES |
| AF | 4.85 | - | 5.2 | - | - | - | - | - | YES |
| AG | 2.46 | - | - | 6.28 | - | - | - | - | NO |
| AH | 8 | - | 2.24 | - | - | - | - | - | YES |
Table 2: Nebulized unfractionated heparin and non-nebulized unfractionated heparin participants’, change in dNLR over time.
Stacked line graphs Figure 3 derived from Table 2 indicate a more rapid decline in dNLR in the study group than in the control group. In the study group, there was a steep decrease in dNLR over 5 days, with a gentler downslope of the curves in the next few days. At day nine, 83% of patients in the Nebulized unfractionated heparin group had dNLR’s of less than 4. By day 9 in the non-nebulized unfractionated heparin group, 57% of patients had a dNLR of less than 4.
Univariate analysis using box plots (Figure 4) to compare serial changes in the same variable generally indicated a wider dispersion of dNLR in the control group (taller boxes, wider interquartile ranges, longer whiskers) and higher means and medians. Generally, the medians of the boxes in the stud group appeared more centred in the boxes than those of the control group. This could indicate a more predictable response of dNLR to the nebulized heparin therapy compared to usual care.
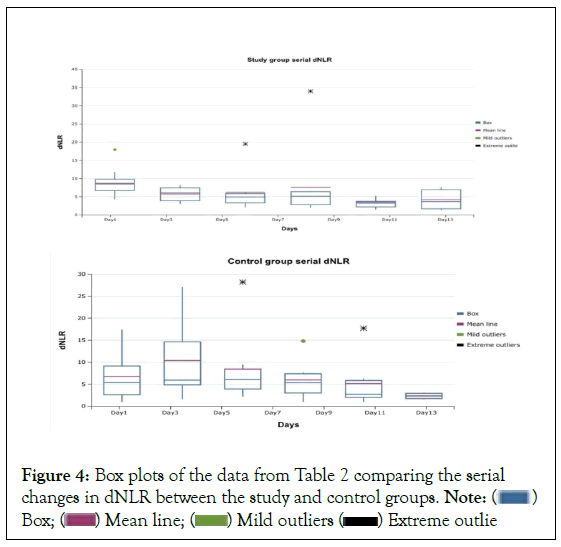
Figure 4:Box plots of the data from Table 2 comparing the serial
changes in dNLR between the study and control groups. Note: ( )
Box; (
)
Box; ( ) Mean line; (
) Mean line; ( ) Mild outliers (
) Mild outliers ( ) Extreme outlie
) Extreme outlie
It should be noted that the mean of the starting dNLR ratios for both groups (Table 1) were considered in the ‘severe’ COVID 19 prognostic category (7.22 for the study group and 7.03 for the control group) [20].
TCZ was administered (one dose of 8 mg.kg each) to four of the twelve non-nebulized unfractionated heparin surviving participants. Although this probably had some clinical significance on the outcomes of the non-nebulized unfractionated heparin group, Cox regression analysis did not show this benefit to be of statistical significance (Figure 5).
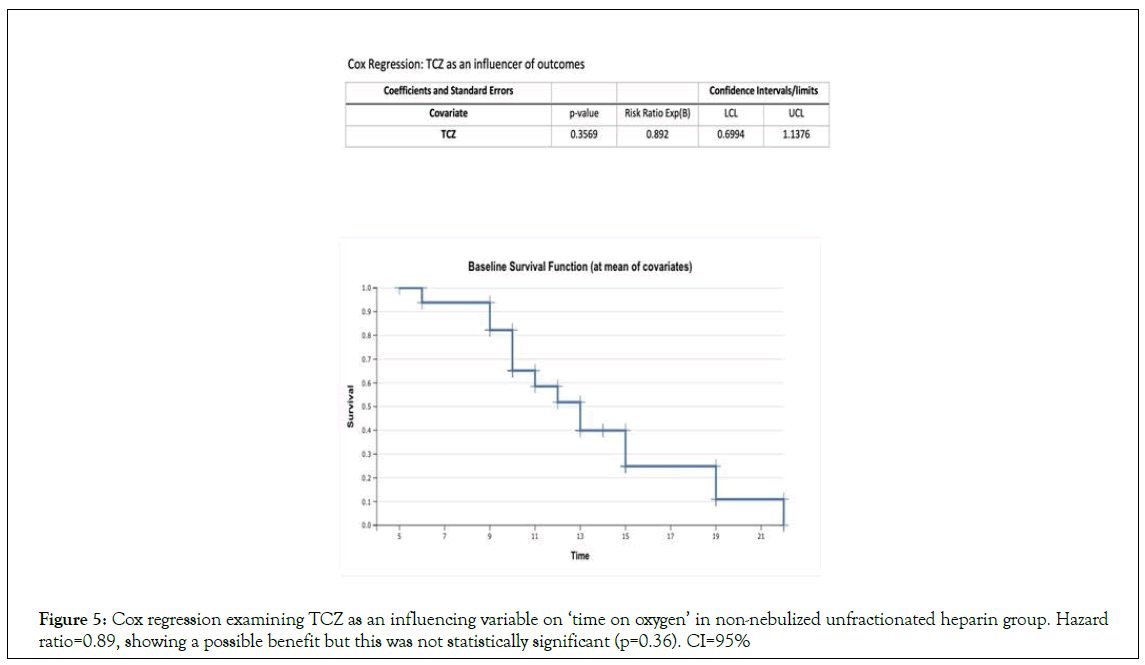
Figure 5: Cox regression examining TCZ as an influencing variable on ‘time on oxygen’ in non-nebulized unfractionated heparin group. Hazard ratio=0.89, showing a possible benefit but this was not statistically significant (p=0.36). CI=95%
The literature is replete with studies identifying dNLR as a useful inflammatory marker and indicator of COVID-19 severity even more than it is in influenza and respiratory syncytial virus [21]. This study was aimed at using these replicated findings to determine if the administration of nebulized unfractionated heparin to severely affected COVID-19 patients produced any changes in dNLR. This study suggests that it might help to accelerate a reduction in dNLR.
As indicated before, inhaled unfractionated heparin has been shown to be quite safe and used with other lung pathologies such as burn inhalation [22]. Unfractionated heparin has been shown in vitro to attack the Covid-19 virus. Because of this, it has been proposed that locally delivered unfractionated heparin be used as a therapy for COVID-19 pneumonitis [23]. Case reports have been published suggesting its advantage in Covid-19 Pneumonitis [24]. In fact, F.M.P van Haren et al are conducting a large multicentre study to evaluate the use of inhaled unfractionated heparin in severe COVID-19 pneumonitis [25-27]. These studies have looked at clinical outcomes such as reduction of ventilator time or reducing the need for ventilation. Our study, however, looks primarily at dNLR and its changes with nebulized unfractionated heparin (Figures 3 and 4).
Study limitations include the following
1. Study size criteria were not applied. The sample is a relatively small one mainly because the cohort presented literally in a snapshot in time: the period of study was from late July 2021 to mid-November, 2021. [28], when the delta variant was dominant. Cases were in a limited space, with limitation of presentations of disease. There are many instances when sample size justification is difficult if not impossible [29].
2. There was neither randomization nor blinding, an issue sometimes seen with retrospective studies; nevertheless, there is recognition that such studies add value to research [30].
3. This was a single-centre study, but the study population was disease specific.
4. The time for following the participants, we limited it to 15 days because most patients had definitive outcomes by then.
5. The same team involved in patient selection using the inclusion and exclusion criteria recorded the data. We did not have a dedicated team to observe outcomes and adverse reactions. Notations were made as part of the care of patients.
6. There was no available blood gas analysis at the time of the study (we have that now, happily): we could not calculate the ratio of arterial oxygen partial pressure to fractional inspired oxygen (P/F ratio) ratios, for example, to determine the severity of ARDS, or acute lung injury. By extrapolation, however, many of the patients had oxygen saturations determined by pulse oximetry as low as the high eighties. In fact, all the patients needing HFNO had this. This would be equivalent to an arterial oxygen tension of 55 mmHg. With a FiO2 setting of 90% on the HFNO device, the P/F ratio for these patients would be 61. For example, a P/F ratio of less than 300 indicates acute respiratory failure [31].
7. No CT, high resolution or otherwise were used to support assessment of disease severity, just plain chest radiographs. This might not have been a significant limitation [32].
8. Human resources restrictions
9. The nursing staffs were asked to take on the additional burden of yet another therapeutic option. The perception that it was doing some good might have kept them motivated, however.
10. There were a few (not many) instances when patients missed their therapies due to staffing issues.
11. Monoclonal antibodies such as Tocilizumab (TCZ), which was often available at private pharmacies in Jamaica, were not available to the majority of patients due to the tremendous cost: No study participants were resourceful enough to purchase TCZ, whilst a total of four non-nebulized unfractionated heparin patients had it administered during the course of the study.
12. One millilitre of salbutamol was administered to the study group in the heparin mix. However, many patients requiring some respiratory support were also administered nebulized salbutamol, which became a standard adjuvant therapy for COVID-19 pneumonitis in the hospital. It is doubtful therefore that the results shared here were the result of salbutamol administered
13. Sometimes therapies were inconsistent: e.g., the hospital pharmacy running out of inhalation salbutamol.
The strengths of this study include the following
1. Independent researcher: Even though this trial was neither randomized nor blinded, the researcher who consent the participants for use of their data in this manuscript was not employed by our department during the administration of the unfractionated heparin to patients. That researcher was simply given a list of all patients who received the therapy and asked to find controls who matched for age, severity, comorbidities etc. and try to consent as many numbers of participants as would agree. Some amount of bias was therefore removed in the selection of study and control group participants.
2. Demographic similarities between the groups: There was no significant statistical difference between the groups with regard to severity of illness, comorbidities, age (median/mean 53/52.1765 for study group and 53/53.1176 for the control group, p=0.706), initial dNLR (p=0.37) and ISARIC 4C scores (p=0.267), as determined by the Mann-Whitney U test (Table 1)
3. The derived Neutrophil to Lymphocyte Ratio (dNLR) is a very accessible measurement at our institution, where there is strong laboratory support for haematological investigations. The derived neutrophil to lymphocyte ratio has been found to have some amount of specificity as an inflammatory marker of COVID-19 and has also been shown to have significant predictive value in the disease. A normal dNLR range is 1 to 3. We were able to demonstrate a difference between the groups with regard to the serial decline of dNLR.
4. During the time of the study, when the dominant COVID-19 variant was B.1.614.2 (delta), the COVID-19 ward at MPH had a relatively high death rate, compared to other waves in the pandemic (Figure 2). While the sample size was small, we note that the death rate on the Covid-19 of 29% was similar to that of the control group, while that of the study group was lower (5.88%) (Table 1).
5. Tocilizumab as an influencer of outcomes: This might have helped some of our control patients, we do not know. However, tocilizumab has not been shown to influence survival in patients who were not receiving mechanical ventilation (those similar to our cohort), but was noted to reduce the likelihood of progression [32].
Interpretation
Nebulized unfractionated heparin appeared to induce faster rates decline in dNLR values (Figure 3), as well as a less varied and perhaps a more homogenous, more predictable effect on dNLR compared with usual care (control group) (Figure 4). A randomized, larger study with fewer limitations would be necessary to validate this decline in dNLR was more obvious in the first five days of treatment. All were nonventilated patients. All were started on therapy, both conventional (in both groups) and nebulized unfractionated heparin (in study group), when they presented to the COVID ward. Many therapies for COVID-19 have been associated with better outcomes when started early. It remains to be seen if this could be one of them.
Conclusion
Participants treated with nebulized unfractionated heparin had a sharper decline in dNLR than patients who did not receive this therapy. There was a less varied, more predictable serial dNLR response in the study group than in the control group. Larger studies should explore the clinical implications of this finding.
Acknowledgment
To the staff of the isolation ward at May Pen Hospital, Clarendon, Jamaica: nurses, physiotherapist and internists for your bravery, guidance, care and encouragement. For the invaluable guidance and editorial input, Professors Neill Adhikari and Rob Fowler of the Department of Critical Care Medicine at the University of Toronto. For providing a path, Assistant Professor in the Department of Anaesthesia and Pain Medicine, Neville Burke Dalhousie University, Canada.
Contribution of this Study
Investigating the effect of a new but old therapeutic option on Covid-19 pneumonitis using, a known inflammatory marker. The coronavirus was responsible for the SARS-CoV2 (in 2002) and Mers-CoV (in 2012) outbreaks. Lessons from each of these outbreaks has built experience and knowledge about the pathology, options for therapies and creation of vaccines. Studies on therapies for SARS-CoV2 can only contribute to this. There has been a short list of expensive therapeutics for severe COVID-19, yet there is no cure. This resulted in astronomical costs, both human and economic. It seemed prudent to look for inexpensive, accessible effective and safe therapies. In a retrospective cohort study, using the dNLR as a marker of illness severity, nebulized unfractionated heparin appeared to promote a faster decline of this marker, with perhaps less variability than usual care. Whether or not nebulized unfractionated heparin and its local anti-inflammatory, antiviral and anticoagulation properties could improve the course of severe COVID-19 pneumonitis remains to be seen. Similar to SARSCoV- 2, the SARS-CoV-1 and MERS-CoV viruses used ACE2 for viral entry into cells. Could locally applied unfractionated heparin be part of a solution in any future SARS-CoV outbreak? The question should be answered.
Funding
The authors of this manuscript did not receive any specific grant funding from agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
References
- Marik PE, Iglesias J, Varon J, Kory P. A scoping review of the pathophysiology of COVID-19. Int J Immunopharmacol. 2021;35:20587384211048026.
[Crossref] [Google Scholar] [PubMed]
- Su D, Elli S, Li Y, Guimond S, Miller G. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv. 2020:2020.
- Paiardi G, Richter S, Oreste P, Urbinati C, Rusnati M, Wade RC. The binding of heparin to spike glycoprotein inhibits SARS-CoV-2 infection by three mechanisms. J Biol Chem. 2022;298(2):101507.
[Crossref] [Google Scholar] [PubMed]
- Partridge LJ, Urwin L, Nicklin MJ, James DC, Green LR, Monk PN. ACE2-independent interaction of SARS-CoV-2 spike protein with human epithelial cells is inhibited by unfractionated heparin. Cells. 2021;10(6):1419.
[Crossref] [Google Scholar] [PubMed]
- Mycroft-West C, Su D, Elli S, Li Y, Guimond S, Miller G, et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv. 2020:2021.
- Conzelmann C, Müller JA, Perkhofer L, Sparrer KM, Zelikin AN, Münch J, et al. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin Med (Lond). 2020:e218-e221.
[Crossref] [Google Scholar] [PubMed]
- Ashraf U, Bajantri B, Roa-Gomez G, Venkatram S, Cantin A, Diaz-Fuentes G. Nebulized heparin and N-acetylcysteine for smoke inhalational injury: A case report. Medicine. 2018;97(19):e0638.
[Crossref] [Google Scholar] [Pubmed]
- Bendstrup KE, Gram J, Jensen JI. Effect of inhaled heparin on lung function and coagulation in healthy volunteers. Eur Respir J. 2002;19(4):606-610.
[Crossref] [Google Scholar] [PubMed]
- Prozan L, Shusterman E, Ablin J, Mitelpunkt A, Weiss-Meilik A, Adler A, et al. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Sci Rep. 2021;11(1):21519.
[Crossref] [Google Scholar] [PubMed]
- Tassé AM, Budin-Ljøsne I, Knoppers BM, Harris JR. Retrospective access to data: the ENGAGE consent experience. Eur. J Hum Genet. 2010;18(7):741-745.
[Crossref] [Google Scholar] [PubMed]
- Bilinski A, Thompson K, Emanuel E. COVID-19 and Excess All-Cause Mortality in the US and 20 Comparison Countries, June 2021-March 2022. JAMA. 2023;329(1):92-94.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization. 2020.
- Bendstrup KE, Newhouse MT, Pedersen OF, Jensen JI. Characterization of heparin aerosols generated in jet and ultrasonic nebulizers. J Aerosol Sci.1999;12(1):17-25.
[Crossref] [Google Scholar] [PubMed]
- Ardestani SK, Salehi MR, Attaran B, Hashemi SM, Sadeghi S, Ghaffarpour S, et al. Neutrophil to Lymphocyte Ratio (NLR) and Derived NLR Combination: A Cost-effective Predictor of Moderate to Severe COVID-19 Progression. Iran J Allergy Asthma Immunol.2022;21(3):241.
[Crossref] [Google Scholar] [PubMed]
- Asghar MS, Akram M, Yasmin F, Najeeb H, Naeem U, Gaddam M, et al. Comparative analysis of neutrophil to lymphocyte ratio and derived neutrophil to lymphocyte ratio with respect to outcomes of in-hospital coronavirus disease 2019 patients: A retrospective study. Front Med (Lausanne).2022;9.
[Crossref] [Google Scholar] [PubMed]
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ: British Medical Journal (Online). 2020;369.
[Crossref] [Google Scholar] [PubMed]
- Bonten TN, Plaizier CE, Snoep JJ, Stijnen T, Dekkers OM, van der Bom JG. Effect of β‐blockers on platelet aggregation: a systematic review and meta‐analysis. Br J clin. pharmacol. 2014;78(5):940-949.
[Crossref] [Google Scholar] [PubMed]
- Yang AP, Liu JP, Tao WQ, Li HM. El papel diagnostico y predictivo de NLR, d-NLR y PLR en pacientes con COVID-19. Int Inmunofarmaceutico. 2020;84:106504.
[Crossref] [Google Scholar] [PubMed]
- Citu C, Gorun F, Motoc A, Sas I, Gorun OM, Burlea B, et al. The predictive role of NLR, d-NLR, MLR, and SIRI in COVID-19 mortality. Diagnostics. 2022;12(1):122.
[Crossref] [Google Scholar] [PubMed]
- Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. International immunopharmacology. 2020;84:106504.
[Crossref] [Google Scholar] [PubMed]
- Lan X, Huang Z, Tan Z, Huang Z, Wang D, Huang Y. Nebulized heparin for inhalation injury in burn patients: a systematic review and meta-analysis. Burns and trauma. 2020;8.
[Crossref] [Google Scholar] [PubMed]
- Conzelmann C, Müller JA, Perkhofer L, Sparrer KM, Zelikin AN, Münch J, et al. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin Med (Lond). 2020:e218-221.
[Crossref] [Google Scholar] [PubMed]
- Does Nebulized Unfractionated Heparin Cause changes in derived neutrophil to lymphocyte ratios in severe COVID-19 pneumonitis. 2023
- Van Haren FM, Page C, Laffey JG, Artigas A, Camprubi-Rimblas M, Nunes Q, Smith R, et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit Care. 2020;24(1):1-1.
[Crossref] [Google Scholar] [PubMed]
- Lakens D. Sample size justification. Collabra Psychol. 2022;8(1):33267.
- Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together?. BMC Anesthesiol. 2016;16.
[Crossref] [Google Scholar] [PubMed]
- P/F Ratio Calculations – Supplement to CDI Pocket Guide
- Sverzellati N, Ryerson CJ, Milanese G, Renzoni EA, Volpi A, Spagnolo P, et al. Chest radiography or computed tomography for COVID-19 pneumonia? Comparative study in a simulated triage setting. Eur Respir J. 2021;58(3).
[Crossref] [Google Scholar] [PubMed]
- Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. International immunopharmacology. 2020;84:106504.
[Crossref] [Google Scholar] [PubMed]
- Lozano M, Iftimi A, Briz-Redon A, Peiró J, Manyes L, Otero M, et al. Clinical characteristics of COVID-19 hospitalized patients associated with mortality: A cohort study in Spain. Infectious Medicine. 2022;1(2):81-87.
- Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20-30.
[Crossref] [Google Scholar] [ PubMed]
- Goyal DK, Mansab F, Iqbal A, Bhatti S. Early intervention likely improves mortality in COVID-19 infection. Clin Medic. 2020;20(3):248.
[Crossref] [Google Scholar] [PubMed]
Citation: Thompson CW, Robinson CA, McIntosh JJ, Risden JS, White DR, Morgan KS, et al. (2023) Does Nebulized Unfractionated Heparin Cause changes in derived neutrophil to lymphocyte ratios in severe COVID-19 pneumonitis?. J Trop Dis. 11:372.
Copyright: © 2023 Thompson CW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

