Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 12
Distribution, Virulence and Diversity of Leptosphaeria maculans and Leptoshaeria biglobusa at Major Brassica Growing Areas of Ethiopia
Belachew Bekele* and Habtewold KifelwReceived: 30-Oct-2020 Published: 08-Dec-2020, DOI: 10.35248/2157-7471.20.12.230
Abstract
A study was conducted to investigate the virulence and diversity of blackleg in major brassica growing areas of Ethiopia. The highest blackleg severity was recorded at Holeta on station canola type cultivar with severity range of 3.5% to 25.6%. The rest visited fields were free from black leg disease. Most of the fields were found covered with Brassica carinata which is under species has BB genome which confers resistance to blackleg. A total of 48 fungal isolates were recovered from leaves and steam of Brassica species. 52% of the isolate goes to L. biglobusa followed by L. maculans 31.25%. Morphological characteristics of the isolates were studied on a PDA medium at 25 ± 1°C: Colonies were found circular in shape after 5 days, and were observed in isolates: BLHH-1, BLHH-2, BLHH-3, BHLL-4, LM-1, LM-2, LB-1, and LB-2. Mycelia were loose, colored white to white smoke. Some of them form colonies with irregular round shape and lobular edges. The pycnidia of the fungus were black, globose to subglobose in shape, the single-celled conidia, hyaline and fusiform with diameters of 4–5 × 1.5–2 μm. From the result slow growth was observed on L. maculans isolate with high sporulation, whereas faster growth rate was observed on L. biglobosa with low sporulation. For the purpose of isolates separation, based on pigment formation on liquid Czapek agar, it was observed that after 30 days isolates LM-1, LM-2, LB-1 and LB-2 where produce yellow-brown pigment which indicate places isolates in a group of non-aggressive strains in conformity with the L. biglobosa. Isolate BLHH-1, BLHH-2, BLHH-3 and BLHH-4 which did not produce pigment; the situation indicates the aggressiveness of the isolate and which is under group L. maculans. Blackleg were found less distributed in major growing areas of Ethiopia, however L. maculans and L. biglobosa were confirmed their presence based on morphological and cultural characteristics. So in line with resistance variety development other management options need to be address to make rapeseed return to production.
Keywords
Blackleg; Leptosphaeria biglobosa; Leptoshperia maculans; Morphological characteristics
Introduction
Ethiopia is the country with large diversity and history of using Brassica species as vegetable and oilseed. The research system start introducing Brassica napus in 1970 from Australia and Europe the variety is known for production of edible oil [1]. Since then production of the crop have been continued in Bale and Arsi zones large state farms till the black leg epidemics occur in 1983.
Effort in crop protection research on oilseed has been very low compared to that of vegetable, legumes and cereals, and the record showing diseases as being among production constraints in oil crop in Ethiopia [2]. Blackleg caused by the fungus Leptospharia maculans (anamorph Phoma lingam), is a devastating diseases present most of rapeseed growing areas. The pathogen can infect all part of the plant, but stem canker is the most serious symptom as it causes plant lodging and yield loss [3].
Protecting crops from catastrophic yield losses caused by plant pathogens is a major goal of agriculture, to safeguard global food security in response to growing concerns about food shortages and climate change [4]. Among the bacterial, fungal, viral and phytoplasmic-like diseases, blackleg is the most important global disease of B. napus and causes annual yield losses of more than $900 million in Europe, North America and Australia [5]. Under epiphytotic conditions, this disease can cause yield losses of up to 90 per cent [6], in Ethiopia due to blackleg epidemics in 1983 up to 100% yield loss were recorded and make the crop out of production [1]. Currently Ethiopia has emerged as one of the largest importer of edible oil in the world market and more than 85% of domestic demand is met through imports even if the country has the potential of producing this crop.
Epidemics of the disease initiated by air-borne L. maculans ascospores released from pseudothecia produced on stem debris of previous crops [7]. The symptomatology of the diseases starts with the appearance of gray-green to ash-gray lesions on the lower leaves, when the diseases advances basal stem cankers, small grey oval lesions on the leaf tissue and root rot were observed.
Today is more than 37 years since the disease is reported from Ethiopia; however its distribution, current status and pathogen virulence and diversity have not yet known and determined. Since population of blackleg pathogen are complex of at least two genetically distinct groups, now referred to as two different species, L. maculans (highly virulent, A-group) and L. biglobosa (weakly virulent, B-group) [3] and according to the interaction phenotype (IP), isolates can be classified in to at least five pathogenicity groups (PGs): PG-1, PG-2, PG-3, PG-4 and PGT, there is no research done in Ethiopia to see the virulence and diversity of blackleg pathogen complex. The present study have been conducted to see the distribution and status of blackleg disease and to investigate the virulence and diversity of isolate obtaining from major growing areas.
Materials and Methods
Description of the study area
Field survey and sample collection were done during the main season of 2018 major brassica growing areas of (North Shewa, South West Shewa and West Shewa and Gurage zone some places) of Ethiopia (Figure 1). The zone has a bimodal rainfall distribution and is a typical sub-humid, high altitude agro-climatic zone.
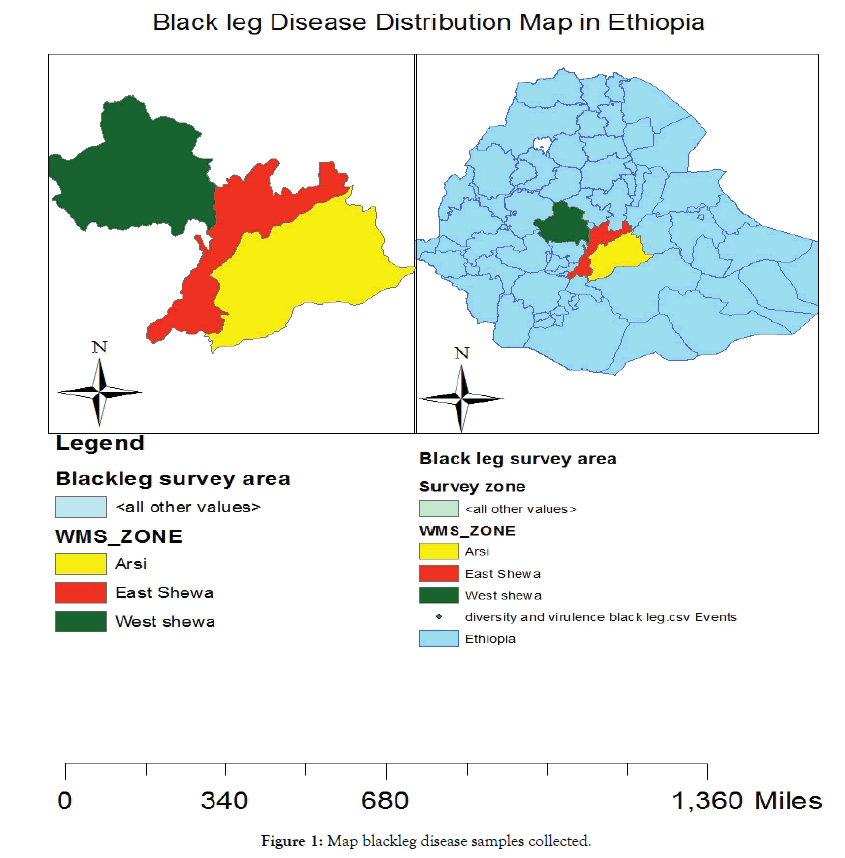
Figure 1: Map blackleg disease samples collected.
Assessment of Black leg disease
Purposive sampling was applied to select fields following the main roads and accessible routes. In the selected fields, black leg disease assessment was made along the two diagonals in an (‘’X’’ fashion). Up to five spots per field were assessed. The disease were rated using a 0-6 scoring system whereby ratings of 0, 1, 2, 3, 4 and 5 respectively are assigned to plants with 0, 1-5, 6-25, 26-50, 51-75 and 76-100% of the cross section infected, and a rating of 6 for dead plants. Disease incidence was determined as percentage of plants showing blackleg symptoms out of the plants sampled.
Data on geographical information (latitude, longitude and altitude) of each field was recorded using a GPS (Legend GPS system, Garmin). The GPS data (latitude and longitude) were used to plot surveyed fields on a map using computer programmed Arc View 3.0. During the survey, growers were asked information on locality name, field history, varieties grown, cropping systems, planting date, weeding practices and disease control practices employed to determine relationship with the disease. Stem and leaf samples showing typical symptoms of blackleg were collected.
Isolation of the pathogen
Samples of infected brassica plants with black leg symptom were collected during the survey. They were analyzed in Holetta Agricultural Research Center pathology laboratory. Isolation was done from plant parts (root crown, stem, leaf,) with clear symptoms of the disease. Fragments of infested tissue were submerged in a 3% sodium hypochlorite solution for 3 to 5 minutes, washed with distilled water and dried naturally. After drying, fragments of infested tissue were placed on a Potato Dextrose Agar (PDA). Petri dishes with PDA were placed in incubator at 25 ± 1°C.
Morphological characteristics
Macroscopic traits of fungal isolates (growth, appearance and color of mycelia, appearance of colony edges, the presence of fruiting bodies, and pigment secretion) were examined on a PDA at 25 ± 1°C (for all 8 isolates tested), as well as microscopic traits - mycelium texture, appearance, size, shape and color of pycnidiospores and pycnidia [8]. The trial was set up in four replications. Mycelia growth of the isolates was measured in cm on days 5, 10 and 15.
Pigment formation on a liquid Czapek agar plate
For the purpose of dividing isolates and affiliation to their respective groups of the tested fungi (L. maculans and L. biglobosa), pigment formation was analyzed on a liquid Czapek agar. Agar was placed into the test tubes (10 ml per tube) and sterilized in the autoclave at 120°C for 20 minutes. After cooling, fungal mycelium of all tested isolates was placed onto the agar and under the photoperiod of 12 h at 20°C. Pigment formation was monitored for 4 weeks, based on the changes in color of the agar.
Results and Discussion
Disease distribution
During the survey, the highest blackleg severity was recorded at Holeta on station canola type cultivar with severity range of 3.5% to 25.6% (Table 1). The rest visited fields were free from black leg disease. During the survey most of the fields were covered with Brassica carinata variety (Yellow Dodola) which is under species has BB genome which confers resistance of blackleg. The epidemiology of blackleg differs between location and regions because of differences in climate, growing season, cultivars and especially fungal populations [9].
| Location | N | E | Altitude (m.a.s.l.) | Black leg severity (%) | Variety |
|---|---|---|---|---|---|
| Kulumsa | 08°01’09.169 | 039°09’18.921 | 2176 | 0 | B. carinata |
| Eteya 1 | 08°01’06.439 | 039°09’17.466 | 2174 | 0 | B. carinata |
| Kulumsa seed enterprise | 08°02’28.772 | 039011’49.197 | 2262 | 0 | B. carinata |
| Eteya 2 | 08°05’45.400 | 039°13’25.284 | 2200 | 0 | B. carinata |
| Eteya 3 | 08°06’31.492 | 039°13’38.069 | 2207 | 0 | B. carinata |
| Robe gebeya | 09°12’48.318 | 038°27’36.295 | 2898 | 0 | Dual |
| Cheri | 09021’20.700 | 038046’15.450 | 2892 | 0 | Dual |
| Robe gebeya | 090210957° | 038046’21.920 | 2855 | 0 | Dual |
| Robe gebeya (Agota) | 090209597° | 038046’13.180 | 2853 | 0 | Dual |
| Holeta on station 1 | 09°03’23.441 | 038°30’07.492 | 2366 | 25.6 | Rape seed |
| Holeta zuria 1 | 09°07’52.076 | 038°26’18.706 | 2614 | 3.5 | B. carinata |
| Holeta zuria 2 | 09°05’37.760 | 038°27’14.420 | 2536 | 5.5 | B. carinata |
Table 1: Survey location and blackleg disease distribution.
Symptomatology
During the diseases on set, emergence of brown irregular spots on stem base of rapeseed was observed during the second week of August 2018 (Figure 2). Although the increase in the number of spots occurred over time, involving a larger tissue area, delayed growth or decay of the infested plants was not observed. Based on the visual observation, only the necrosis of surface tissue was detected during August to October [10-12].
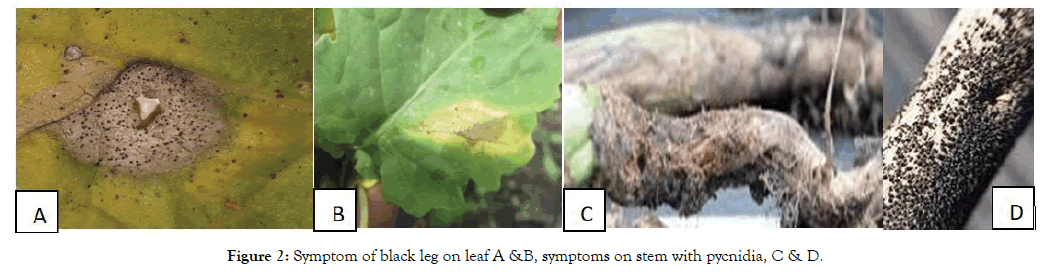
Figure 2: Symptom of black leg on leaf A &B, symptoms on stem with pycnidia, C & D.
Leptosphaeria maculans were found attacking nearly all parts of the plant, including cotyledons, leaves, stems, roots and pods, and cause leaf lesions and stem cankers (Figure 2). The first obvious symptoms of blackleg on leaves were the appearance of gray-green to ash-gray lesions on the lower leaves (Figure 2). The presence of small, black pycnidia at the edge or scattered across the blackleg lesions distinguishes them from lesions caused by another common foliar pathogen, Alternari brassicae. Basal stem lesions are the most damaging. When these occur in the seedling or rosette phases of growth, symptoms resemble damping-of or cut-worm damage. In older plants, the more canker symptom leads to premature ripening or lodging (falling over) of the crop. Based on the cross-section and longitudinal section of infested plants during the second half of the growing period, further decay of infested tissue was detected. Besides delayed growth, infested plants had fewer side stems and flowers during inflorescence, which resulted in yield decrease.
Morphological characteristics
From samples collected, L. biglobusa (B group isolate) were frequently isolated majorly from leaf 52.08%; whereas L. maculans were from cankered stem and leaf (31.25%) and Alternaria were also isolated (16.7%) from leaf. From the study we confirm that, the presence of the aggressive pathogen L. maculans and weekly virulent pathogen L. biglobusain major producing areas and different brassica species. The result supported by Williams and Fitt [13], Strains of L. maculanswere classified into two pathotypes: the highly virulent, aggressive ‘A’ group strains that cause stem cankers, and the nonaggressive, weakly virulent, ‘B’ group strains that do not cause stem cankers. According to Johnson & Lewis, A group isolates caused damaging brown, cortical lesions but B group isolates penetrated the leaf gap to enter the stem pith, rarely causing externally visible phoma stem canker (Table 2).
| S. No | Species | No. of isolate | Total | (%) age | |
|---|---|---|---|---|---|
| Leaf | Stem | ||||
| 1 | L. maculans | 10 | 5 | 15 | 31.25 |
| 2 | L. biglobusa | 25 | 0 | 25 | 52.08 |
| 3 | Alternaria | 8 | 0 | 8 | 16.70 |
| Total | 43 | 5 | 48 | 100 | |
Table 2: Isolation frequency.
Cultural characteristics
Colonies were found circular in shape grew on a PDA medium after 5 days (at 25°C ± 1°C), and were observed in isolates: BLHH- 1, BLHH-2, BLHH-3, BHLL-4, LM-1, LM-2, LB-1, and LB-2. Mycelia were loose, colored white to white smoke. Some of them form colonies with irregular round shape and lobular edges after 5 days (Figure 3).
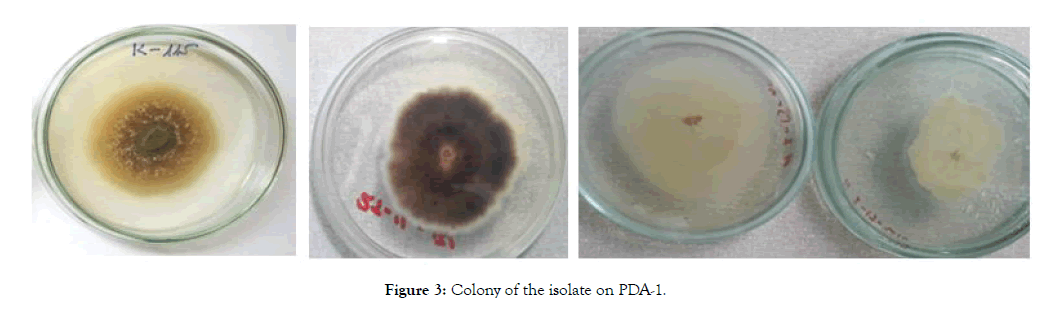
Figure 3: Colony of the isolate on PDA-1.
The pycnidia of the fungus were black, globose to subglobose in shape, the single-celled conidia, hyaline and fusiform with diameters of 4–5 × 1.5–2 μm. Most of the isolates were produced pigment in the liquid culture in variable color brown to black (Figure 4).
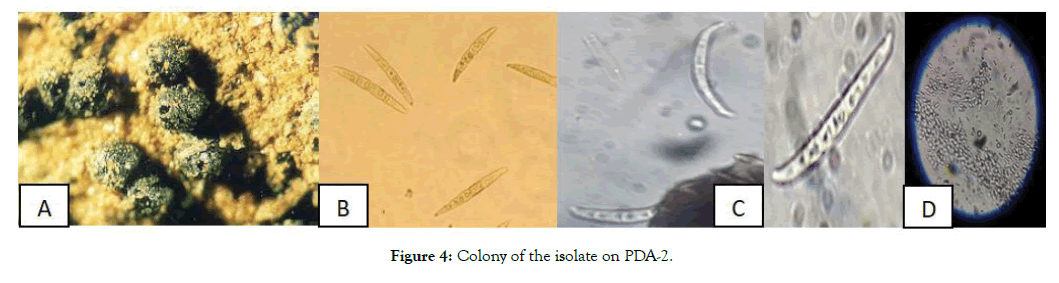
Figure 4: Colony of the isolate on PDA-2.
Growth rate
From the result slow growth was observed on Isolate (BLHH- 1, BLHH-2, BLHH-3, BLHH-4, LM-1 AND LM-2) with high sporulation, which is a typical characteristic of L. maculans of A group isolate, whereas faster growth rate was observed on isolate (LB-1 AND LB-2) with low sporulation which is the characteristics of L. biglobosa (Table 3).
| Isolates | After 5 days | Sporulation | After 10 days | Sporulation | After 15 days | Sporulation |
|---|---|---|---|---|---|---|
| BLHH-1 | 0.57 | - | 1.27 | ++ | 2.07 | +++ |
| BLHH-2 | 0.95 | - | 1.97 | ++ | 2.6 | +++ |
| BLHH-3 | 2.03 | - | 2.87 | - | 3.23 | - |
| BLHH-4 | 1.53 | - | 2.47 | - | 2.80 | - |
| LM-1 | 1.53 | - | 3.37 | ++ | 5.21 | +++ |
| LM-2 | 1.49 | - | 3.59 | ++ | 4.96 | +++ |
| LB-1 | 1.61 | - | 3.94 | - | 8.9 | - |
| LB-2 | 1.56 | - | 4.32 | - | 9 | - |
Table 3: Diameter (cm) and sporulation of tested isolates on a PDA at 25 ± 1°C.
Pigment formation on liquid Czapek agar
For the purpose of isolates separation, based on pigment formation on liquid Czapek agar, it was observed that after 30 days isolates LM-1, LM-2, LB-1 AND LB-2 where produce yellow-brown pigment which indicate places isolates in a group of non-aggressive strains in conformity with the L. biglobosa. Isolate BLHH-1, BLHH-2, BLHH-3 and BLHH-4 which did not produce pigment; the situation indicates the aggressiveness of the isolate and which is under group L. maculans (Figure 5). The pathotypes are visually indistinguishable in culture but can be differentiated on their ability to produce pigment in liquid culture, colony growth rate, and disease reactions on B. napus [14].
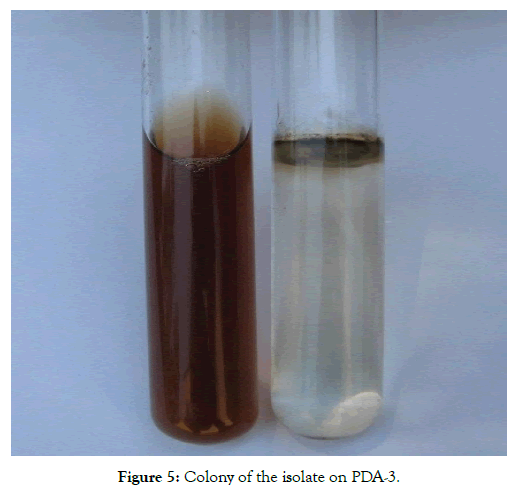
Figure 5: Colony of the isolate on PDA-3.
Summary and Recommendations
From the survey based on morphological and cultural characterization of the isolates two types of phytopathogenic fungi of Leptosphaeria complex: Leptosphaeria maculans and Leptospaeria biglobosa were found. As a result we recommend the breeding program need to consider both isolates in their breeding program. The isolation relied on different pigment production in media to distinguish A group and B group isolates. This study also needs to support by molecular means of characterization. The study covers and includes isolates from only few producing areas, in order to know the status of the pathogen in the country, major rapeseed producing areas is required further assessment. Where L. maculans populations are a mixture of A group and B group, the proportions of the different isolates appear to be influenced by a complex interaction between pathogen, host, climatic and cultural factors. Even within a region, there can be large differences in the population structure of the pathogen.
REFERENCES
- Teklemariam W, Asfaw T, Mesfin T. Review of research on oil crop diseases in Ethiopia. P. 292-312. In: Tsedeke Abate (edn). A Review of Crop Protection Research in Ethiopia, Proceedings of the First Ethiopian Crop Protection Symposium, IAR, Addis Ababa, Ethiopia. 1985.
- Simeane Y. Pathological research on Niger, linseed, Gomenzer and rapeseed in Ethiopia. In: Oilseeds Research and Development in Ethiopia. Proceedings of the First National Oilseed Workshop, Addis Ababa. 1991:151-162.
- Brun H, Levivier S, Somda I, Ruer D, Renard M, Chèvre AM. A field method for evaluating the potential durability of new resistance sources: application to the Leptosphaeria maculans-Brassica napus pathosystem. Phytopathol. 2000;90(9):961-966.
- Bebber DP, Ramotowski MA, Gurr SJ. Crop pests and pathogens move polewards in a warming world. Nature Climate Change. 2013;3(11):985-988.
- Fitt BD, Brun H, Barbetti MJ, Rimmer SR. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). In Sustainable strategies for managing Brassica napus (oilseed rape) resistance to Leptosphaeria maculans (phoma stem canker). Springer. 2006:3-15.
- Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury PA, Howlett BJ. Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern Australia. Aus J Exp Agri. 2004;44(6):601-606.
- Brun H, Chèvre AM, Fitt BDL, Powers S, Besnard AL, Ermel M, et al. Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytologist. 2010;185(1):285–99.
- Muntanjola-Cvetković M. Opšta mikologija (General mycology). Belgrade, Serbia: NIRO Književne novine. 1987.
- West JS, Kharbanda PD, Barbetti MJ, Fitt BD. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant pathol. 2001;50(1):10-27.
- Johnson RD, Lewis BG. Variation in host range, systemic infection and epidemiology of Leptosphaeria maculans. Plant Pathol. 1994;43:269-277.
- Thürwächter F, Garbe V, Hoppe HH. Ascospore discharge, leaf infestation and variations in pathogenicity as criteria to predict impact of Leptosphaeria maculans on oilseed rape. J Phytopathol. 1999;147(4):215-222..
- Wang G. Evaluation of Brassica napus Seed Infection by Leptosphaeria maculans/Phoma lingam. Poznan, Poland: University of Agriculture (Doctoral dissertation, MSc thesis).
- Williams RH, Fitt BD. Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker (blackleg) of oilseed rape. Plant Pathol. 1999;48(2):161-175..
- Williams PH. Biology of Leptosphaeria maculans. Can J Plant Pathol. 1992;14(1):30-35.
Citation: Bekele B, Kifelw H (2020) Distribution, Virulence and Diversity of Leptosphaeria maculans and Leptoshaeria biglobusa at Major Brassica Growing Areas of Ethiopia. J Plant Pathol Microbiol 11:530.
Copyright: © 2020 Bekele B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

