Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 6
Distribution and Physiological Races of Wheat Stem Rust (Puccinia graminis F. Sp tritici) in North and East Shoa Zones of Ethiopia
Kitessa Gutu1*, Girma Adugna2 and Netsanet Bacha32Department of Horticulture and Plant Sciences, Jimma University College of Agriculture and Veterinary Medicine, Jimma, Ethiopia
3Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia
Received: 07-Apr-2021 Published: 24-Jun-2021, DOI: 10.35248/2157-7471.21.12.557
Abstract
Wheat (Triticum aestivumL.) is the most important staple crop in temperate zones and is in increasing demand in countries undergoing urbanization and industrialization. However, its production is affected by many biotic and abiotic factors. Among biotic production constraints; wheat stem rust ( Puccinia graminis f.sp tritici) is the most important one. This study was (i) to assess the importance of wheat stem rust in North and East Shoa zones of central Ethiopia and (ii) to identify physiological races. Purposive multistage sampling was used to select major wheat growing districts and farmers associations from each zone. Wheat stem rust race identification was carried out via inoculation of isolates on susceptible line (McNair); single pustule isolation; inoculation on standard differential sets and infection type evaluation of each line fourteen days after inoculation. One hundred fifty wheat fields (75 from each zone) were assessed. Wheat stem rust was observed in 71 (94.7%) and 52 (73.3%) of wheat fields in East and North Shoa zones, respectively. Disease incidence and severity were significantly different (p < 0.0001) between the two zones. Six physiological races of Puccinia graminis f.sp tritici (pgt) namely; TKTTF, TTTTF, TKKTF, TTKTT, TTKTF and TTTTT were identified. TKKTF was the dominant race which was detected from 40 (48.2%) samples followed by TKTTF (Digelu race) which was identified from 28 (33.7%) samples. But, TTTTT, TTKTT and TTTTF were less frequent races. They were identified from 1 (1.2%), 2 (2.4%) and 4 (4.8%) samples, respectively. The majority of resistance genes in differential host lines (80-100%) were defeated with the races. Resistance genes Sr24 and Sr31 were effective to majority of races identified. Hence, they can be used as source of resistance in breeding program.
Keywords
Incidence; Prevalence; Race; Severity; Stem rust
Introduction
Wheat (Triticum aestivum L.) is the most important staple crop in temperate zones and is in increasing demand in countries undergoing urbanization and industrialization. It is a major source of starch, energy and provides substantial amounts of several components that are essential or beneficial for health like protein, vitamins B, dietary fiber, and phytochemicals that have health benefits for humans [1]. Wheat represents approximately 19% of global major cereal crop production. East African countries, North Africa, and the Middle East consume over 150% of their wheat production and are heavily dependent on imports to meet their food security [2]. Ethiopia is the largest wheat producer in sub- Saharan Africa [3]. In Ethiopia, wheat ranks fourth inland coverage and total production after tef, maize, and sorghum. It is grown on around 1.7 million ha of land [4] and is grown primarily as a rainfed crop by subsistence farmers in mid to high land areas. The low productivity is principally because of abiotic and biotic stresses which are increasing in intensity and frequency associated with climate change [3]. The average yield of wheat in Ethiopia is 2.8 t/ ha [4] is lower than global wheat average yield which is 3.43t/ha [5].
Stem rust caused by Puccinia graminis f.sp. tritici (Pgt) is one of the major production constraints in most wheat growing areas of the country, causing yield losses of up to 100% during epidemic years [6]. Wheat stem rust populations in Ethiopia were reported to be highly variable. Hailu, et al. [7] identified 9 races in 2013 from 80 samples collected from Oromia, Amhara, and Tigray regions of the country. Besides, Hei, et al. [8] reported six races out of 179 samples collected from Oromia, Amhara, Tigray and Southern Nations and Nationalities Peoples’ regions in 2014 cropping season and seven races from 168 stem rust samples analyzed in 2015 main cropping seasons. Races prevalent in the central highlands of Ethiopia are among the most virulent in the world [9]. The study also revealed that the wheat stem rust pathogen consists quite complex pathotypes in central high lands of Ethiopia. Lemma, et al. [10] identified 16 races from samples collected from major durum and bread wheat growing areas of East Shoa zone in 2009 main cropping season. Nevertheless, recent information is limited about intensity and virulence distribution of wheat stem rust disease in North and East shoa zones of central Ethiopia. Because of quick alteration of genetic makeup of wheat stem rust pathogen, it has to be surveyed regularly to know the status of the disease and to monitor shift in virulence. In addition, the evolution of new stem rust races with broad virulence on many of the resistance genes deployed in wheat breeding is a driving force to search for new sources of resistance. Therefore, regular virulence analysis of the pathogen is mandatory to monitor shift in virulence. This in turn helps to get ready and combat in case any new strains of the pathogen have occurred. Thus, knowledge of resistance genes in cultivars is important for deploying cultivars with different genes to reduce damage. The study was therefore undertaken to assess the distribution and physiological races of stem rust (Pgt) in North and East Shoa zones of Ethiopia.
Materials and Methods
Survey of wheat stem rust in major wheat producing districts of East and North Shoa zones
Surveys were conducted using purposive multistage sampling that was used to select major wheat growing districts and peasant associations from each zone. Ten districts (five from each zone) were selected depending on area coverage. Farmers associations where wheat is predominantly grown were selected from all districts and three wheat fields were assessed from each peasant associations at 5-10 km interval. The map of the study area where the surveys were carried out is indicated below (Figure 1).
Figure 1: Map of the study area where the surveys were carried out.
Disease assessments
Disease assessment was made at five points along the two diagonals (in an ‘’X’’ pattern) of each field using 0.5 m × 0.5 m (0.25 m2) quadrate. In each field, wheat plants within the quadrant were counted and recorded as diseased/infected and healthy/noninfected and the intensity of stem rust was calculated. Accordingly, the Incidence of stem rust was calculated by using the number of infected plants and expressed as a percentage of the total number of plants assessed.
Disease prevalence
Disease prevalence was calculated as number of fields affected by stem rust over the total fields assessed and expressed in percentages. Disease severity was examined visually on the whole plants within the quadrates as the percentage of plant tissue affected and recorded according to modified Cobb's scale [11]. In order to take into account the different sizes of pustules and their distribution, this scale provides four series of diagrams (each series containing twelve actual diagrams) covering a wide range of combinations of pustule size and distribution. According to modified Cobb’s scale, severity (percentage of the plant infected) and response (type of disease reaction) was recorded together. Intervals (Trace, 5, 10, 20, 40, 60, and 100) was used to estimate percentage of plant tissue infected. The host plant response/infection type to infection was scored using the description of Roelfs et al. [12]. The coefficient of infection (CI) was calculated by multiplying the level of disease severity and the constant value of infection type. The constant values for infection types were R = 0.2, MR = 0.4, MR-MS = 0.6, MS = 0.8, MS-S = 0.9, S = 1 [13]. Besides, data on variety, field history, crop growth stage using Zadok’s scale [13] and geographical information (latitude, longitude, and elevation) using GPS were recorded for each field.
Physiological races and Virulence Spectrum of Puccinia graminis f.sp. tritici
Collection of infected tissue samples: Samples of infected stems (one sample per field) were collected at 5-10 km interval from wheat fields from North and East Shoa zones, Ethiopia. Wheat fields along the main and feeder (accessible) roadsides were assessed in the surveyed areas. Stem and/or leaf sheath of wheat plants infected with stem rust was cut into small pieces of 5 to 10 cm in length using scissors and placed in paper bags after the leaf sheath separated from the core tissue (stem) to dry the samples. This technique helps the samples not to deteriorate before analysis [14]. Thereafter, samples collected in the paper bags were labeled with the name of the zone, district, variety, GPS information (altitude, latitude and longitude) and date of collection and was taken to Ambo Agricultural Research Center (AARC) laboratory for analysis.
Isolation and multiplication of single-pustule isolates: Five seeds of universally susceptible wheat variety (McNair) were sown in 10 cm diameter pots filled with a mixture of sterilized soil, sand, and manure in 2:1:1 by volume, respectively. Seedlings were grown in the greenhouse with a temperature and relative humidity of 18-25°C and 98-100% relative humidity. Urediniospores from each field was suspended in lightweight mineral oil, Soltrol 170 (Chevron Phillips Chemical Company, The woodlands, Texsas, United States) and sprayed onto 7-day-old seedlings of variety McNair [12]. Seven days after inoculation (when the flecks/ symptoms become clearly visible) leaves containing a single fleck that produces a single pustule was selected from the base of the leaves and the remaining seedlings within the pots were removed using scissors. Only leaves containing single pustules from each location were covered with cellophane bags and tied up at the base with a rubber band to avoid cross-contamination [15]. Two weeks later, spores from each pustule was collected to prepare the suspension by mixing urediospores with Soltrol 170. Then after, it was inoculated on seven-day-old seedlings of the susceptible variety McNair for multiplication purpose for each of the single pustules in separate pots.
After inoculation, seedlings were placed in incubation chamber in dark condition at 18- 22ºC for 18 hours and were exposed to light for four hours and transferred to the greenhouse. After 14 days, the spores of every single pustule were collected separately in gelatin capsules and inoculated on the standard differential lines.
Inoculation of wheat stem rust differential lines
The seedlings of 20 wheat differential host lines with known stem rust resistance genes that and a susceptible variety McNair were grown in 10 cm diameter pots. Differential lines were originally brought to Ambo Agricultural Research Center from Cereal Disease Laboratory (CDL), Minnesota, USA. Each rust isolate was suspended in Soltrol 170. The suspension was sprayed onto seven-day old seedlings of the differentials using spore inoculators. Inoculated seedlings were put in a dew chamber for 18 hours at 18- 25°C and 98-100% relative humidity. Then, plants were exposed to fluorescent light for four hours to provide a conducive condition for infection and were allowed to dry for about 1-2 hours. Finally, inoculated plants were transferred to greenhouse benches where the temperature and relative humidity is adjusted at 18 – 25°C and 98-100% [13], respectively.
Determination of races
Seedling infection type was scored 14 days after inoculation using a 0 to 4 scale [16]. The IT readings of 3 (medium-size uredia with/without chlorosis) and 4 (large uredia without chlorosis or necrosis) were regarded as susceptible. Other readings, i.e. 0 (immune or fleck), 1 (small uredia with necrosis), and 2 (small to medium uredia with chlorosis or necrosis) were regarded as low infection type or resistance reaction. The variations were refined by modifying characters like -, uredinia slightly smaller than normal for the infection type; +, uredinia slightly larger than normal for the infection type [16].
Race designation was done by grouping the differential lines into five subsets as indicated in Table 1. Each isolate was assigned using a five-letter designation based on its reaction on the differential lines [17,18].
| Infection types produced on near-isogenic Sr lines | |||||
|---|---|---|---|---|---|
| Pgt- code | Set 1 | 5 | 21 | 9e | 7b |
| Set 2 | 11 | 6 | 8a | 9g | |
| Set 3 | 36 | 9b | 30 | 17 | |
| Set 4 | 9a | 9d | 10 | Tmp | |
| Set 5 | 24 | 31 | 38 | McN | |
| B | Lowa | Low | Low | Low | |
| C | Low | Low | Low | Highb | |
| D | Low | Low | High | Low | |
| F | Low | Low | High | High | |
| G | Low | High | Low | Low | |
| H | Low | High | Low | High | |
| J | Low | High | High | Low | |
| K | Low | High | High | High | |
| L | High | Low | Low | Low | |
| M | High | Low | Low | High | |
| N | High | Low | High | Low | |
| P | High | Low | High | High | |
| Q | High | High | Low | Low | |
| R | High | High | Low | High | |
| S | High | High | High | Low | |
| T | High | High | High | High | |
aLow = Infection types 0, ;, 1, and 2 and combinations of these values.
bHigh = Infection types 3 and 4 and a combination of these values.
Table 1: Nomenclature of Puccinia graminis f. sp. tritici based on 20 differential wheat host lines.
Data analysis
Survey data was arranged using three stage nested design with the model:
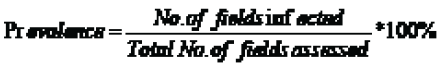
Where: γijk is the wheat stem rust disease intensity whereas peasant association k is nested within district J nested within Zone i, μ is the overall mean, τi is the effect of the ith zone, βj(i) is the effect of the jth district within the ith zone, and γk(ij) is the effect of the kth peasant association within the jth district and ith zone, and εl(ijk) is the error term. Analysis of variance (ANOVA) was performed using SAS version 9.4 Software package (SAS, 2012). Means were separated using LSD test at the alpha level of 5%. The associations of disease incidence and severity with independent variables viz. altitude, variety and growth stage was computed by Pearson’s correlation using SAS version 9.4 Software package. Each of the independent variables were tested with the incidence and severity of stem rust as the dependent variable. Linear regression analysis was done by plotting disease severity against altitude. Determination of regression intercept, slope and coefficient of determination were computed using SAS version 9.4 Software package. Race analysis data were analyzed by using Microsoft Spread Sheet software.
Results and Discussion
Distribution and intensity of wheat stem rust on North and East Shoa Zones
A total 150 wheat fields were assessed in North (75 fields) and East Shoa (75 fields) zones during the 2019 cropping season. Wheat stem rust disease was prevalent all over surveyed districts of North and East Shoa zones. Out of 150 fields assessed, 110 (73.3%) of the fields were infected with wheat stem rust disease. The disease was observed in 71 (94.7%) and 52 (73.3%) of wheat fields in East and North Shoa zones, respectively. The highest (100%) disease prevalence was recorded in Akaki district followed by Adea, Gimbichu, Liben and Liben where the disease was equally (93.3%) prevalent (Table 2 ).
| Zone | Districts | Number of fields assessed | Number of fields  infected | Prevalence (%) |
|---|---|---|---|---|
| East Shoa | Adea | 15 | 14 | 93.3 |
| Gimbichu | 15 | 14 | 93.3 | |
| Liben | 15 | 14 | 93.3 | |
| Lume | 15 | 14 | 93.3 | |
| Akaki | 15 | 15 | 100 | |
| Sub total | 75 | 71 | 94.7 | |
| North Shoa | Siyadebrina Wayu | 15 | 7 | 46.7 |
| Basoana Warana | 15 | 5 | 33.3 | |
| Kimbibit | 15 | 8 | 53.3 | |
| Abichuna Gna’a | 15 | 8 | 53.3 | |
| Aleltu | 15 | 11 | 73.3 | |
| Sub total | 75 | 39 | 52 | |
| Total/mean | 150 | 110 | 73.3 |
Table 2: Prevalence of wheat stem rust disease across districts of North and East Shoa Zones in 2019 main cropping season.
Disease incidence and severity were significantly different (p < 0.0001) between the two zones. The overall mean incidence and severity in the zones were 23.1 and 10.3, respectively. Mean disease incidence of 41.6 and 5.7 were recorded in East and North Shoa zones, respectively. Moreover, mean disease severity of 17.9 and 3.3 were recorded from East and North Shoa zones, respectively.
Wheat stem rust incidence was significantly different (p < 0.0001) across districts of North and East Shoa zones. The highest mean incidence was recorded in Liben and Lume districts 50.7 and 47.5, respectively with no significant difference between the two districts. Likewise, the highest mean severity (21.3 and 20.7) was recorded in Liben and Lume districts with a significant difference recoded between them. However, the lowest mean disease intensity (2.5, 2.5, 2.7, 3.3) and severity 1.5, 2, 2, 2.2) was recorded in Kimbibit, Abichuna Gna’a, Basona werana, Siyadebrina Wayu districts, respectively with no significant difference among the districts (Table 3). Furthermore, the highest range of wheat stem rust incidence was recorded in Lume, Adea, Liben and Akaki districts with incidence range of 0-100, 0-90, and 0-80, respectively. On the other side, the lowest incidence range of wheat stem rust disease was recorded in Siyadebrina Wayu and Abichuna Gna’a districts with a similar incidence range value of 0-20 followed by Kimbibit district with an incidence range of 0-30. Besides, the maximum wheat stem rust severity range (0-70S) was recorded in Kimbibit district followed by Liben district (0-60 MSS). This study revealed that there was high disease pressure in East Shoa than Northern Shoa. The higher disease intensity in East Shoa might be due to mid-altitude ranges (1593 m-2289 m) in East Shoa zone that is suitable for stem rust pathogen [12]. However, the higher altitudes range (2306 m-3034 m) and cold weather conditions in North shoa which was less favorable for the pathogen. Ismail, et al. [19] reported relatively lower wheat stem rust severity in high altitude areas of more than 2200 m.a.s.l. than lower altitude areas of less than 1900 m.a.s.l in North rift regions of Kenya. The variation in crop disease intensity between and within specific locations could be due to diversity in wheat variety grown, time of disease onset, the virulence of the pathogen and favorable environmental conditions [20].
| Zones | Districts | Disease Incidence | Disease Severity | ||
|---|---|---|---|---|---|
| Range | Mean | Range | Mean | ||
| East Shoa | Liben | 0-80 | 50.7a | 0-60MSS | 21.3a |
| Lume | 0-100 | 47.5a | 0-70S | 20.7a | |
| Akaki | 10-80 | 40b | 5MS-70S | 14.5b | |
| Adea | 0-90 | 39.3b | 0-40MS | 16.9b | |
| Gimbichu | 0-60 | 27.3c | 0-70S | 14.8b | |
| North Shoa | Aleltu | 0-60 | 14.6d | 0-40MS | 7.3c |
| Siyadebrina Wayu | 0-20 | 3.3e | 0-20MS | 2.2d | |
| Basona Werana | 0-40 | 2.7e | 0-40MS | 2d | |
| Abichuna Gna’a | 0-20 | 2.5e | 0-20MSS | 2d | |
| Kimbibit | 0-30 | 2.5e | 0-30MSS | 1.5d | |
| Overall mean | 23.1 | 10.3 | |||
| LSD (0.05) | 6.3781 | 3.0433 | |||
| CV % | 26.02 | 28.01 | |||
Table 3: Mean incidence and severity of wheat stem rust across wheat producing districts of East and North shoa zones.
Intensity of wheat stem rust across altitude ranges
The survey was carried out in an area with altitude range of (1593- 3034 m.a.s.l) which fall under two altitude classes namely midaltitude (1593 m-2289 m) and high altitudes (2306-3034 m.a.s.l) [21]. Wheat stem rust disease prevalence and intensity varied with altitude ranges. Accordingly, wheat stem rust was more prevalent in mid altitudes than higher altitudes. Out of 62 wheat fields assessed in the mid-altitude area, 58 (93.5%) fields where infected. But, Out of 88 wheat fields assessed from highland areas; 52 (59.1) fields where infected with wheat stem rust disease. In the same manner, wheat stem rust intensity was significantly different (p < 0.0001) between mid and high altitude ranges. The maximum mean disease incidence and severity of 44.4 and 18.7 were recorded in mid and high altitudes, respectively. Meanwhile, the lower mean disease incidence and severity of 8.9 and 4.9 were recorded in mid and high altitudes, respectively (Table 4).
| Altitude range | Class name | Number of fields inspected | Number of fields infected | Prevalence (%) | Mean Incidence | Mean Severity |
|---|---|---|---|---|---|---|
| 1593 m-2289 m | Mid-altitude | 62 | 58 | 93.5 | 44.4a | 18.7a |
| 2306 m-3034 m | High altitude | 88 | 52 | 59.1 | 8.9b | 4.9b |
| Total/overall mean | 150 | 110 | 76.2 | 26.7 | 11.8 | |
| LSD (0.05) | 2.718 | 1.2969 | ||||
| CV | 27.8 | 29 |
Table 4: Prevalence and intensity of wheat stem rust across altitude ranges.
This study showed that the higher the altitude the lower wheat stem rust prevalence and intensity, and vice versa. Wheat stem rust is quite important at low and mid altitudes (<2400 m). However, it could be important at higher altitudes on late sown and/or latematuring wheat varieties, specially grown on vertisols [22].
The correlation analysis between altitude and incidence (r = -0.76) and severity (r= -0.7) of wheat stem rust disease were also highly significant (p < 0.001) and negative in the present study (Table 5). The same scenario was also reported by different authors. Abebe, et al. [23] and Hailu, et al. [7] reported higher wheat stem rust disease intensity at a lower altitude than at higher altitude ranges. Ismail, et al. [19] also reported a highly significant negative correlation between stem rust severity and altitude in Kenya. Various studies also showed that wheat stem rust disease is more important in low and mid-altitude areas than high land areas in Ethiopia. Hailu, et al. [7] reported that higher wheat stem rust disease intensity (incidence and severity) in low land (1500-200 m.a.s.l) and mid-altitude (2001- 2500 m.a.s.l) than high land (2501-3560 m.a.s.l) areas of West and South West Shoa zones of Ethiopia. Likewise, Regasa et al. [24] reported higher wheat stem rust incidence and severity in the midattitude range of (1568 m-2300 m) than high altitude range (2301 m-3008 m) in the Southern Tigray region of Ethiopia.
| Variables | DS | DI | ALT | GS |
|---|---|---|---|---|
| DS | 1 | -0.7*** | 0.43*** | |
| DI | 1 | -0.76*** | 0.49*** | |
| 1 | -0.58*** | |||
| 1 |
*Significant level at p<0.05; **Significant level at p<0.01; ***Significant level at  p<0.001
Table 5: Pearson’s correlation coefficients between wheat stem rust intensity with altitude, growth stage and weed status.
A negative relationship was observed between stem rust disease severity and altitude ranges with regression analysis. As elevation increases (in meter), stem rust disease severity is reduced by 0.02 (Figure 2). The negative relationship between altitude and disease severity implied that the disease was more important at lower and mid altitudes resulting in decreased intensity at higher altitudes [25].
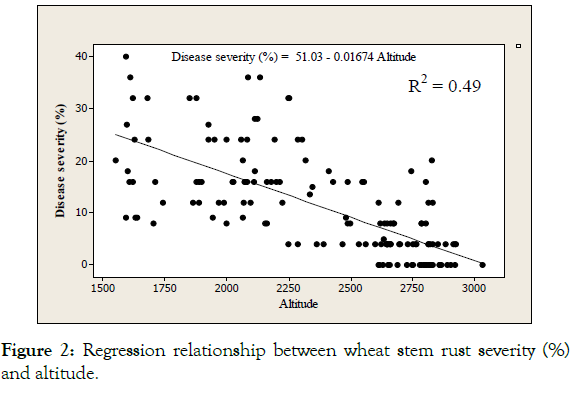
Figure 2: Regression relationship between wheat stem rust severity (%) and altitude.
Wheat stem rust prevalence and intensity across wheat varieties grown in the study area
A total of 9 wheat varieties (Utuba, Dashen, Kakaba, Mangudo, Kubsa, Kingbird, Hidase, Danda’a, Digelu and ET-13) and Unknown varieties were encountered during the survey. Wheat stem rust prevalence was varied among varieties. Accordingly wide range wheat stem rust sverity was observed on different varieties during the survey. The disease was 100% prevalent on varieties Utuba, Dashen, Mangudo, Kingbird and Digelu. Moreover, disease prevalences of 82.6%, 68.8%, 66.7%, 66.7% and 50% were recorded from Kakaba, Danda’a, Kubsa, Hidase and ET-13, respectively. The disease was 68% prevalent in unknown varieties grown in the study area (Table 6). Likewise, there was a significant difference in wheat stem rust disease incidence and severity among varieties grown in North and East Shoa zones. The highest mean disease incidence (70%) was recorded from Utuba variety. The second highest mean disease incidence (50%) was recorded on Dashen variety followed by Kakaba, Mangudo, Kubsa, and kingbird with a mean incidence value of 40%. However, the lowest mean disease incidence (7.5%) was recorded from a variety ET-13. The maximum mean disease severity (36%) was recorded from Utuba variety followed by Kubsa, Kakaba and Dashen with mean incidence values of 17.3, 16.6 and 16%, respectively. However, the lowest mean disease severity (2%) was recorded on ET-13 variety followed by Digelu, Danda’a, Hidase and Kingbird varieties with mean disesse severity value of 8, 9.4, 12.5 and 14%, respectively. The mean disease severity recorded from Unknown varieties was 11.1%. Wide ranges of recently released commercial wheat varieties released in Ethiopia are vulnerable to rust disease shortly after their release. This might be due to virulence variability in the pathogen population and deployment of major gene resistance in the majority of wheat cultivars [26].
| Variety | No. of fields inspected | No. of fields infected | Prevalence (%) | Mean incidence | Mean severity |
|---|---|---|---|---|---|
| Utuba | 2 | 2 | 100 | 70a | 36a |
| Dashen | 1 | 1 | 100 | 50b | 16cb |
| Kakaba | 23 | 4 | 82.6 | 40.3b | 16.6b |
| Mangudo | 1 | 1 | 100 | 40b | 13.5bcd |
| Kubsa | 3 | 1 | 66.7 | 40b | 17.3b |
| Kingbird | 4 | 4 | 100 | 40b | 14bcd |
| Hidase | 6 | 4 | 66.7 | 21cd | 12.5bcd |
| Unknown | 25 | 17 | 68 | 24c | 11.1bcd |
| Danda’a | 80 | 55 | 68.8 | 18.7cde | 9.4cd |
| Digelu | 3 | 3 | 100 | 11.7de | 8d |
| ET-13 | 2 | 1 | 50 | 7.5e | 2e |
| LSD (0.05) | 11.59 | 5.59 | |||
| CV | 26.2 | 27.85 |
Table 6: Wheat stem rust prevalence and intensity across wheat varieties grown in the study area.
Wheat stem rust prevalence and intensity by growth stage of wheat varieties grown in the study area
Different growth stages (flowering to dough) were observed during the survey time. Out of 150 fields assessed, 73 fields were at dough stage, 40 at soft Dough stage, 32 at milk stage and 5 fields were at the flowering stage. Significantly different wheat stem rust intensity was recorded at different stages of the crop in wheat growing areas of North and East Shoa zones of Ethiopia in 2019 main cropping season. The highest mean disease incidence (35.7) was recorded at Dough growth stage followed by Soft Dough growth stage with a mean incidence value of (16.6). However, the lowest mean disease incidence (6) was scored at the flowering stage followed by milk stage with a mean disease incidence value of 10.6. In the same way, the maximum mean disease severity of 15.4 was recorded at Dough followed by and Soft Dough (8.3) growth stages. The lowest mean disease severities of 4.8 and 5.4 were scored at flowering and milk stage, respectively. However, there was no significant difference between the two growth stages (Table 7). On the other hand, the correlation analysis between growth stage and disease intensity indicated that there was a significant (p < 0.001) and positive correlation between the wheat growth stage and stem rust severity (r = 0.43) and incidence (r = 0.49). This showed that wheat stem rust intensity increased with increasing stage of crop from flowering to dough stage. Roelfs et al. [12] also stated that, the late growth stages of the crop are a crucial time for stem rust disease to reach its maximum severity level. Similarly, Regasa, et al. [24] also reported the positive correlation of wheat stem rust intensity and crop growth stage.
| Growth stage | Number of fields | Mean incidence | Mean severity |
|---|---|---|---|
| Flowering | 5 | 6.7c | 5.5c |
| Milk | 32 | 10.6c | 5.24c |
| Â Soft Dough | 40 | 16.6b | 8.33b |
| Dough | 73 | 35.7a | 15.4a |
| LSD (0.05) | 4.64 | 2.13 | |
| CV | 28.15 | 28.42 |
Table 7: Wheat stem rust intensity across wheat growth stages in North and East Shoa Zones, 2019.
Physiological races and Virulence spectrum of Puccinia gramminis f.sp tritici
A total of 110 stem rust samples (39 from North and 71 from East Shoa zones) were collected in 2019 main cropping season. Of these, 83 live samples were analyzed following the procedure of Roelfs and Marthens to identify the physiological races of the stem rust pathogen. However, the remaining samples were not viable because no visible symptoms were produced after being inoculated on susceptible wheat line (McNair). Six pgt races namely TKTTF, TTTTF, TKKTF, TTKTT, TTKTF and TTTTT were identified. TKKTF was the dominant race being identified from 40 (48.2%) samples followed by TKTTF (Digelu race) that was identified from 28 (33.7%) samples. However, TTTTT and TTKTT race were the least dominant races. They were identified from 1 (1.2%) and 2 (2.4%) samples, respectively (Table 8). TKKTF was reported in Germany and was among the races that caused an unusual wheat stem rust outbreak in 2013/14 cropping season [27]. TKTTF (Digelu race) was the dominant race in major wheat producing districts of East and North Shoa zones of Ethiopia.
| Zone | Varieties | Race | Number of samples | Frequency (%) |
|---|---|---|---|---|
| East Shoa | Danda’a | TKKTF | 10 | 12.04 |
| TTTTF | 3 | 3.6 | ||
| TKTTF | 6 | 7.2 | ||
| TTKTF | 2 | 2.4 | ||
| Kakaba | TKTTF | 9 | 10.8 | |
| TTTTF | 2 | 2.4 | ||
| TKKTF | 2 | 2.4 | ||
| TTKTT | 1 | 1.2 | ||
| Mangudo | TKKTF | 1 | 1.2 | |
| Hidase | TKKTF | 2 | 2.4 | |
| Dashen | TTTTF | 1 | 1.2 | |
| Kubsa | TKKTF | 3 | 3.6 | |
| TTTTF | 1 | 1.2 | ||
| TKTTF | 1 | 1.2 | ||
| Utuba | TKKTF | 2 | 2.4 | |
| Unknown | TKKTF | 7 | 8.4 | |
| TTTTF | 1 | 1.2 | ||
| TTKTF | 2 | 2.4 | ||
| TKTTF | 2 | 2.4 | ||
| North Shoa | Danda’a | TKKTF | 11 | 13.3 |
| TKTTF | 6 | 7.2 | ||
| TTKTT | 1 | 1.2 | ||
| TTTTT | 1 | 1.2 | ||
| ET-13Â | TKTTF | 1 | 1.2 | |
| Digelu | TKKTF | 1 | 1.2 | |
| Hidase | TKTTF | 1 | 1.2 | |
| Unknown | TKTTF | 1 | 1.2 | |
| TKKTF | 1 | 1.2 | Total | 6 | 83 |
Table 8: Wheat stem rust races identified from different wheat varieties and their frequencies.
TKTTF was first detected in 2012 in Southeastern parts of Ethiopia and caused wheat stem rust epidemics in 2013/14 cropping season by attacking the popular variety Digelu which was resistant to TTKSK (Ug99 race) [6]. It was bad news for Ethiopian wheat growers because stem rust resistance gene SrTmp that is available in popular bread wheat variety (Digelu) was defeated by this virulent stem rust strain [28]. TTKTT is virulent on all resistance genes in differential lines except Sr36. The spatial distribution of the pgt races was different among the two zones. Five races including TKKTF, TTTTF, TTKTF, TKTTF and TTKTT were distributed in East Shoa zone. Lemma et al., [10] also reported a wider variability of pgt populations in central Ethiopia. They identified 16 races from samples collected from major wheat producing districts of East Shoa zone. Four races namely TKKTF, TKTTF, TTKTT and TTTTT races were distributed in North Shoa zone. Out of the six races identified in this study; TTTTT was detected only in North Shoa zone while TTKTF and TTTTF were identified only in East Shoa zone.
Wheat varieties are grown North and East Shoa zones were infected with one or more of pgt races. Danda’a variety was infected with four pgt races namely TKKTF, TTTTF, TKTTF and TTKTF in East Shoa zone. Similarly, TKKTF, TTTTF, TTKTT and TTTTT races were identified from this variety in North Shoa zone. Besides, Kubsa variety was infected with multiple races (TKKTF, TTTTF and TKTTF) in East Shoa zone. Similarly, Hidase was infected with TKKTF race in East Shoa zone and TKTTF in North Shoa zone. However, Mangudo variety was infected with a single race (TKKTF). TKKTF race was the most frequently appeared in North and East shoa zones being identified from 11 (13.3%) and 10 (12.04%) in East Shoa zones, respectively. The second most dominant race detected from Danda’a variety was TKTTF (Digelu race) which was identified from 6 (7.2%) in each zones. On the other hand, TTKTF and TTTTF races appeared less frequently on Danda’a variety in East Shoa zone being identified from 2 (2.4%) and 3 (3.6%) stem rust samples, respectively. The least frequent races on Danda’a variety were TTKTT and TTTTT each identified from 1 (1.2%) samples. Furthermore, Kakaba (bread wheat variety) was infected with four pgt races (TKTTF, TTTTF, TKKTF and TTKTT) in East Shoa zone. Among these races, TKTTF (Digelu race) had a high frequency being identified from 9 (10.8%) samples. However, TTKTT, TKKTF and TTTTF were less frequent races on Kakaba variety. TTKTT was identified from 1 (1.2%) while TKKTF and TTTTF races were each identified from 2 (2.4%) samples (Table 8). TTKTT race was first reported in Ethiopia in 2018 from commercial wheat cultivars Shorima, Huluka, Ogolcho, Hidase, and Danda’a [29]. It has the most virulence combination of all Ug99 (TTKSK) race groups. This race was first reported in Kenya in 2014 and its spread to different wheat growing areas of the world was highly significant [30]. TTTTT pgt race was identified from a sample collected from Danda’a (bread wheat variety) in North Shoa zone. TTTTF race was reported from samples collected in 2009 from the Eastern Shoa zone of central Ethiopia [10]. It was also detected in Iran in 2010 [31]. Moreover, TTTTF race caused a wheat stem rust outbreak in Italy hitting several thousands of hectares of durum wheat [32] (Table 9).
|  Race | Identified from number of samples  | Frequency (%) |
|---|---|---|
| TKTTF | 28 | 33.7 |
| TKKTF | 40 | 48.2 |
| TTTTF | 8 | 9.6 |
| TTKTT | 2 | 2.4 |
| TTKTF | 4 | 4.8 |
| TTTTT | 1 | 1.2 |
Table 9: Frequency of pgt races identified from samples collected from the study area.
Wheat stem rust is considered as a reemerging disease, having outbreaks and epidemics in East Africa, Europe, and Central Asia. Severe epidemics occurred in Ethiopia (2013-2014), Kazakhstan and South Siberia (2015-2016), outbreaks in Germany (2013), Italy (2016), and Sweden (2017) [33-35]. After the occurrence and spread of Ug99, new races with critical virulence have been occurring that have been posing a threat to both bread and durum wheat in many countries including Ethiopia. The epidemic of stem rust has occurred in Ethiopia in 2013/14 on popular variety Digelu have caused up to 100% yield loss. It was caused by a new strain of the pathogen called TKTTF or Digelu race during the main cropping season [6].
Virulence spectrum of Puccinia graminis f.sp tritici isolates
Wheat stem rust races identified in the study area have a variable virulence spectrum on stem rust resistance genes. The majority of resistance genes in differential host lines (80%-100%) were defeated with stem rust races identified in the study area. Unusual virulence was noted by race TTTTT which defeated 100% Sr genes in differential lines. Likewise, TTKTT race was virulent on 95% Sr genes except Sr36. Similarly, TTTTF, TKTTF and TKKTF races were virulent on 90%, 85% and 80% of Sr genes. TTTTF was virulent on all Sr genes except Sr24 and Sr31. Unlike to present study, Lemma et al., [10] reported that differential host line carrying Sr24 was effective to all races identified in central Ethiopia. TKTTF and TKKTTF races have almost similar virulence pattern since both are virulent on Sr genes Sr11, Sr24 and Sr31. TKKTF race have a similar virulence profile to race TKTTF. Nevertheless, low infection type was displayed on Sr36 in a former race (Table 10).
| Races | Virulence/ineffective Sr genes | Avirulence/effective Sr genes |
|---|---|---|
| TKTTF | 5, 21, 9e, 7b, 6, 8a, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 11, 24, 31 |
| TKKTF | 5, 21, 9e, 7b, 6, 8a, 9g, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 11, 36, 24, 31 |
| TTTTF | 5, 21, 9e, 7b, 11, 6, 8a, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 24, 31 |
| TTKTT | 5, 21, 9e, 7b, 11, 6, 8a, 9g, 9b, 30, 17, 9a, 9d, 10, Tmp, 24, 31, 38, McN | 36 |
| TTKTF | 5, 21, 9e, 7b, 11, 6, 8a, 9g, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 36, 24, 31 |
| TTTTT | 5, 21, 9e, 7b, 11, 6, 8a, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 24, 31, 38, McN | - |
Table 10: Virulence/Avirulence spectra of the Pgt races identified from North and East Shoa Zones.
The majority of resistance genes in differential host lines were ineffective against races identified in this study. Differential hosts carrying Sr5, Sr21, Sr9e, Sr7b, Sr6, Sr8a, Sr9g, Sr9b, Sr30, Sr17, Sr9a, Sr9d, Sr10, SrTmp, Sr38 and SrMcN were ineffective to all races identified from stem rust samples collected in the season. Sr11 and Sr36 were ineffective to 66.7% and 50% of races, respectively. While Sr24 and Sr31 were ineffective to 33.3% of races identified from North and East Shoa zones of Ethiopia in 2019 main cropping season. The result of the present study is in agreement with previous findings. Admasu et al., [9] reported that Sr7a, Sr7b, Sr8b, Sr9a, Sr9b, Sr9d, Sr9g, Sr10 and Sr17 were susceptible to the majority of stem rust races identified from Shewa, Arsi and Bale zones, Ethiopia. Abebe et al., [23] also reported that most of the resistance genes possessed by differential lines were ineffective against one or more of stem rust isolates collected from Tigray region of Ethiopia. Moreover, various studies showed that virulence to Sr6, Sr8b, Sr9a, Sr9d and Sr11 is common worldwide [12].
Stem rust resistance gene Sr24 is effective against most races of Puccinia graminis f. sptritici and is used widely in commercial wheat cultivars worldwide [18]. Sr24 became ineffective for the first time in 2006 with TTKST race. Susceptible infection type response was observed on wheat lines and cultivars carrying Sr24 in a field stem rust screening nursery at Njoro, Kenya [18]. Besides, virulence to this effective stem rust resistance gene had been detected in different countries including South Africa [36], Eritrea [37] and Ethiopia [29]. Durable Stem rust resistance gene Sr31 since 1980 was overwhelmed due to a highly virulent race arisen in eastern Africa (Uganda) in 1999. The race was known as TTKSK or (Ug99) and is virulent to the majority of the world’s wheat cultivars [38]. It has spread from Uganda throughout eastern Africa, Yemen, and Iran [39-41].
On the other hand, differential host lines that caries Sr24 and Sr31 were effective to the majority of stem rust races detected in this study. Both of the lines were effective against 4 (66.7%) races (TKTTF, TTTTF, TKKTF and TTKTF) except TTKTT and TTTTT. Likewise, the differential line that caries Sr36 was effective to 3 (50%) of races identified in the study area. It was resistant against TKKTF, TTKTT and TTKTF. The lowest resistance spectra were recorded on the differential lines that caries Sr11. It was resistant to only 2 (33.3%) including TKTTF and TKKTF. Unlike to present study, Lemma et al. [10] reported that differential host line carrying Sr24 was effective to all races identified in central Ethiopia. However, there was no effective Sr gene to all races identified in this study (Table 11). All/majority of stem rust resistance genes in differential host lines became susceptible to stem rust races identified in this study. Therefore, searching for novel sources of resistance is pertinent to develop durable rust-resistant wheat cultivars.
| Stem rust resistance gene (Sr gene) | Virulence frequency (%) | Stem rust resistance gene (Sr gene) | Virulence frequency (%) |
|---|---|---|---|
| Sr5 | 100 | Sr30 | 100 |
| Sr21 | 100 | Sr17 | 100 |
| Sr9e | 100 | Sr9a | 100 |
| Sr7b | 100 | Sr9d | 100 |
| Sr11 | 66.7 | Sr10 | 100 |
| Sr6 | 100 | SrTmp | 100 |
| Sr8a | 100 | Sr24 | 33.3 |
| Sr9g | 100 | Sr31 | 33.3 |
| Sr36 | 50 | Sr38 | 100 |
| Sr9b | 100 | SrMcN | 100 |
Table 11: Virulence frequency of pgt isolates on 20 stem rust resistance genes.
The detection of six races in the study area is an indicator of the great variability of pgt populations. The result of this finding is in agreement with previous studies conducted in different parts of wheat producing areas of the country [42]. Also, some pathotypes identified in this study have more virulence combinations than preexisting races in the country. For instance, TTKTT and TTTTT races have 95% and 100% virulence spectra to stem rust resistance genes within differential lines. Resistance genes (Sr24) that is available in most of the commercial varieties worldwide became ineffective with these races. Roelfs et al., [12] also stated that wheat stem rust is continued to be the main challenge of wheat production worldwide because of the great variability in the pathogen population. This could be created by different mechanisms like mutation and sexual recombination that enable the pathogen to overcome resistance genes within wheat genotypes.
Summary and Conclusion
Wheat is one of the major cereal crops with tremendous nutritional value and is one of the staple crops in many parts of the world. African countries are heavily dependent on imports to meet their food security. Ethiopia is a leading country in wheat production from sub-Saharan countries. Never the less, its production is constrained by many biotic and abiotic factors. Of these, wheat stem rust disease is the most important biotic constraint since a long time ago. Present study showed that wheat stem rust was prevalent all over surveyed districts and different level of disease intensity was notified. Disease incidence and severity were significantly different (p < 0.0001) between the two zones. Mean disease incidence of 41.6 and 5.7 were recorded in East and North Shoa zones, respectively. Moreover, mean disease severity of 17.9 and 3.3 were recorded from East and North Shoa zones, respectively. Moreover, wheat stem rust disease intensity was significantly varied among varieties grown, altitudes ranges and weed infestation levels. Eighty three stem rust isolates were analyzed using 20 standard differential lines. Six races namely TKTTF, TTTTF, TKKTF, TTKTT, TTKTF and TTTTT were identified. TKKTF was the dominant race being detected from 40 samples followed by TKTTF (Digelu race) which was identified from 28 samples. However, TTTTT and TTKTT races were the least dominant races. The detection of six races in the study area is an indicator of the great variability of pgt populations. In addition, some of pathotypes identified in this study have more virulence combinations than pre-existing races in the country. For instance, TTKTT and TTTTT races have 95% and 100% virulence spectra to stem rust resistance genes within differential lines. Sr24 resistance gene that is available in most of the commercial varieties worldwide became ineffective to these races. Hence, it needs high emphasis to reduce the potential spread of this virulent pgt strains to another wheat producing regions of the country and beyond. On the other hand, stem rust resistance gene Sr24 and Sr31 were effective against majority of races identified in present study. Therefore, they can be used as source of resistance in breeding program. The rapid evolution of new races within the rust population is a bottleneck to rust management worldwide. Therefore, regular surveillance work and identification of physiological races is mandatory to know the importance of the disease and to monitor shift in virulence pattern.
Acknowledgement
I am grateful to the Ethiopian Institute of Agricultural Research (EIAR) for financial support and Delivering Genetic Gains in Wheat (DGGW) project for partial financial support.
REFERENCES
- Shewry PR, Hey SJ. The contribution of wheat to human diet and health. Food Energy Secur. 2015;4(3):178-202.
- FAOSTAT. Agriculture Organization of the United Nations. Statistical Database. 2018.
- Tadesse W, Bishaw Z, Assefa S. Wheat production and breeding in Sub-Saharan Africa: Challenges and opportunities in the face of climate change. Int J Clim Chang Strateg Manag. 2018;11(5):696-715.
- CSA (Central Statistics Authority). Report on area and production of major grain crops. Addis Ababa, Ethiopia. 2019.
- FAO. Food Outlook: Biannual Report on Global Food Markets, Food and Agricultural Organization of the United Nations. 2018.
- Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, Gale S, et al. Phenotypic and Genotypic Characterization of Race TKTTF of Puccinia graminis f . sp . tritici that Caused a Wheat Stem Rust Epidemic in Southern Ethiopia in 2013 – 14. Phythopathol. 2015;105(7):917–928.
- Hailu A, Woldeab G, Dawit W, Hailu E. distribution of wheat stem rust (Puccinia graminis F. Sp. Tritici) in West and Southwest Shewa Zones and identification of its phsiological races. ACST. 2015;3(4):189.
- Hei NB, Tesfaye T, Woldeab G, Hailu E, Hundie B, Kassa D, et al. Distribution and frequency of wheat stem rust races (Puccinia graminis f. sp. tritici) in Ethiopia. JACR. 2018;6(5):88-96.
- Admassu B, Lind V, Friedt W, Ordon F. Virulence analysis of Puccinia graminis f. sp. tritici populations in Ethiopia with special consideration of Ug99. Plant Pathol. 2009;58(2):362-369.
- Lemma A, Woldeab G, Semahegn Y, Dilnesaw Z. Survey and virulence distribution of wheat stem rust (Puccinia graminis f. sp. tritici) in the major wheat growing areas of central Ethiopia. Sci-Afric J Sci Res Essays. 2014;2(10):474-478.
- Peterson RF, Campbell AB, Hannah AE. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res. 1948;26(5):496-500.
- Roelfs AP. Rust diseases of wheat: concepts and methods of disease management. Cimmyt; 1992.
- Stubbs RW, Prescott JM, Saari EE, Dubin HJ. Cereal disease methodology manual. CIMMYT. 1986:51.
- Woldeab G, Hailu E, Bacha N. Protocols for Race Analysis of Wheat Stem Rust (Puccinia graminis f. sp. tritici). Ethiopian Institute of Agricultural Research, Ambo Plant Protection Research Center, Ethiopia. 2017:27.
- Fetch Jr TG, Dunsmore KM. Physiologic specialization of Puccinia graminis on wheat, barley, and oat in Canada in 2001. Can J Plant Pathol. 2004;26(2):148-55.
- Stakman EC, Stewart DM, Loegering WQ. Identification of physiologic races of Puccinia graminis var. tritici. Washington: USDA; 1962:617.
- Roelfs AP, Martens JW. An International System of Nomenclature for Puccinia graminis f. sp. tritici. Phytopathol. 1988;78(5):526-33.
- Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch Jr T. Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Disease. 2008;92(6):923-926.
- Ismail SG, Kinyua MG, Kibe AM, Wagara IN. Wheat stem rust severity and physiological races in North Rift region of Kenya. Asian J Plant Pathol. 2012;6(2):25-32.
- Letta T, Tilahun A. Stability analysis for selecting stem rust resistance in some Ethiopian durum wheat varieties. Afr Crop Sci J. 2007:853-856.
- Ferede T, Ayenew AB, Hanjra MA, Hanjra M. Agroecology matters: Impacts of climate change on agriculture and its implications for food security in Ethiopia. Global food security: Emerging issues and economic implications. 2013:71-112.
- Badebo A, Hundie B. Incidence and Challenges of Rust Diseases in Wheat Production. Containing the Menace of Wheat Rusts. Ethiopian Institute of Agricultural Research. 2016:153.
- Abebe T, Woldeab G, Dawit W. Distribution and physiologic races of wheat stem rust in Tigray, Ethiopia. J Plant Pathol Microbiol. 2012;3:1-6.
- Regasa GH, Senbeta GA, Hei NB. Distribution and Occurrence of Wheat Stem Rust (Puccinia graminis f. sp. tritici) in Tigray Region, Northern Ethiopia. IJRAFS. 2019;5(7):23-46.
- Badebo A, Bekele E, Bekele B, Hundie B, Degefa M, Tekalign A, et al. 2008. Review of two decades of research on diseases of small cereal crops. Increasing crop production through improved plant protection. 2008:375-429.
- Hei N, Shimelis HA, Laing M. Appraisal of farmers wheat production constraints and breeding priorities in rust prone agro-ecologies of Ethiopia. Afr J Agric.Res. 2017;12(12):944-952.
- Olivera Firpo PD, Newcomb M, Flath K, Sommerfeldt-Impe N, Szabo LJ, Carter M, et al. Characterization of Puccinia graminis f. sp. tritici isolates derived from an unusual wheat stem rust outbreak in Germany in 2013. Plant Pathol. 2017;66(8):1258-1266.
- Hodson D. Summary of Ethiopia 2014/15 rust situation. Re-current, localized stem rust epidemics caused by race TKTTF (“Digalu” race) in Ethiopia. Extreme caution & vigilance needed in East Africa. Rust tracker. org, Global wheat rust monitoring system. 2015.
- Hei NB, Tsegaab T, Getaneh W, Girma T, Obsa C, Seyoum A, et al. First Report of Puccinia graminis f. sp. tritici Race TTKTT in Ethiopia. Plant Disease. 2020;104(3):982.
- Patpour M, Hovmøller MS, Justesen AF, Newcomb M, Olivera P, Jin Y, et al. Emergence of virulence to SrTmp in the Ug99 race group of wheat stem rust, Puccinia graminis f. sp. tritici, in Africa. Frontiers in Plant Science. 2016.
- Patpour M, Afshari F, Hasan Bayat Z, Nazari K. Pathotype identification of Puccinia graminis f. sp. tritici, the Causal Agent of Wheat Stem Rust under Greenhouse Condition. Seed and Plant Improvement Institute-SPII. 2014:26.
- Patpour M, Hovmøller MS, Hansen JG, Justesen AF, Thach T, Rodriguez-Algab J, et al. Epidemics of yellow and stem rust in Southern Italy 2016-2017. BGRI.2018.
- Shamanin V, Salina E, Wanyera R, Zelenskiy Y, Olivera P, Morgounov A. Genetic diversity of spring wheat from Kazakhstan and Russia for resistance to stem rust Ug99. Euphytica. 2016;212(2):287-296.
- Bhattacharya S. Deadly new wheat disease threatens Europe’s crops. Nature News. 2017;542(7640):145-146.
- Singh RP, Hodson DP, Jin Y, Lagudah ES, Ayliffe MA, Bhavani S, et al. Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology. 2015;105(7):872-884.
- Pretorius ZA, Bender CM, Visser B, Terefe T. First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant Dis. 2010;94(6):784.
- Wolday A, Fetch T, Hodson DP, Cao W, Briere S. First report of Puccinia graminis f. sp. tritici races with virulence to wheat stem rust resistance genes Sr31 and Sr24 in Eritrea. Plant Dis. 2011;95(12):1591.
- Pretorius ZA, Singh RP, Wagoire WW, Payne TS. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Dis. 2000;84(2):203.
- Nazari K, Mafi M, Yahyaoui A, Singh RP, Park RF. Detection of wheat stem rust (Puccinia graminis f. sp. tritici) race TTKSK (Ug99) in Iran. Plant Dis. 2009;93(3):317.
- Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, et al. Will stem rust destroy the world's wheat crop?. Adv Agron. 2008;98:271-309.
- Rouse MN, Wanyera R, Njau P, Jin Y. Sources of resistance to stem rust race Ug99 in spring wheat germplasm. Plant Dis. 2011;95(6):762-766.
- Admassu B, Fekadu E. Physiological races and virulence diversity of Puccinia graminis f. sp. tritici on wheat in Ethiopia. Phytopathologia Mediterranea. 2005;44(3):313-318.
Citation: Kitessa G, Adugna G, Bacha N (2021) Distribution and Physiological Races of Wheat Stem Rust ( Puccinia graminis F. Sp tritici) in North and East Shoa Zones of Ethiopia. J Plant Pathol Microbiol 12:557.
Copyright: © 2021 Kitessa G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

