Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- SWB online catalog
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 9, Issue 3
DILI Causality Assessment Methods Comparison: CIOMS Scale Versus Non-Specific Methods
Amina Berradia*, Fznmekaouche, Fetati H and Toumi HReceived: 20-Jul-2020 Published: 23-Oct-2020, DOI: 10.35248/2167-1052.20.9.232
Abstract
The Pharmacovigilance Department of the University Hospital establishment Oran (UHEO) collects, evaluates and monitors adverse drug reactions (ADRs) with corrective or preventive measures taken within UHEO.
Faced with the divergence of causality assessment results in drug induced liver injury (DILI) cases during our practice, we conducted a study to compare the results of non-specific and specific causality assessment method (CAM).
A comparative study spanning June 2011 to August 2017 was conducted on the archived statements of ADRs at the pharmacovigilance department or on new declarations from various UHEO departments. A special sheet has been designed to collect the information needed to assess causality.
After collecting informations, the causality was assessed by combining non-specific CAMs (Naranjo et al. /Bégaud et al.) with a DILI-specific CAM (the CIOMS scale).
While comparing CAMs results, we found that non-specific methods often over-notify cases of DILI compared to the CIOMS scale. The use of the specific method is, then, recommended. However, it has certain limitations that should be perfected for a better use.
Introduction
DILI is considered as the leading cause of acute liver failure in most countries [1]. Pharmacovigilance assess causality through algorithmic causality assessment methods (CAMs), specific or not to DILI. These methods have not always convergent results for a same analyzed case [2]. Since 2011, the Pharmacovigilance Department of the University Hospital of Oran (UHEO) ensured the safety of the drug and contributed to their proper use. Therefore, we conducted a study to compare the results of specific DILI CAM, with those that are non-specific for each reported case.
Methods and Materials
Our study was conducted in the Pharmacovigilance department of UHEO from June 2011 to August 2017, on archived declarations sent from various UHEO departments.
The members of the Pharmacovigilance unit meet to discuss and analyze each statement as follows:
1- Analysis of drug interactions that may have caused DILI using Vidal software®, Prescrire® magazine and Drugs.com.
2- Study of DILI: its chronology, its risk- factors, its none-drug causes, the pathogenic mechanism by which the drug induce DILI, if it’s labelled, widely, rarely or never published.
3- Assessment of the drug-DILI causality (degree of causality) using two non-specific methods (the French method and the Naranjo et al. method) and a specific method (the CIOMS scale) [3-5].
Afterwards, we have compared the results of these CAMs:
Comparison by nature and number of drugs involved by considering:
• "Match": the case where both methods incriminated the same drugs in kind and in number.
• "Mismatch": the case where the two methods did not incriminate the same drugs in kind or in number.
Comparison of causality grading results:
We have compared causality grading, between the CIOMS scale and the Naranjo et al method, using the kappa parameter [6].
Results
During our study period, DILI represented 15.13% among collected ADRs. In 85% of the cases, only 1 drug was incriminated. In 15% of cases, more than one drug was implicated. According to the CIOMS scale, causality was possible in 43% of cases, probable in 12% of cases and very likely in only 3% of cases. The most implicated drugs in DILIs were anti-TB drugs (44%), followed by other types of antibiotics (15%) (Figure 1).
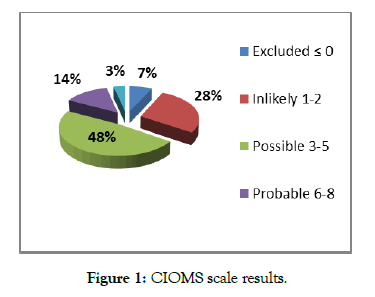
Figure 1: CIOMS scale results.
According to Naranjo et al. method:
The degree of causality was mainly possible (73% of cases) and probable in 17% of cases (Figure 2).
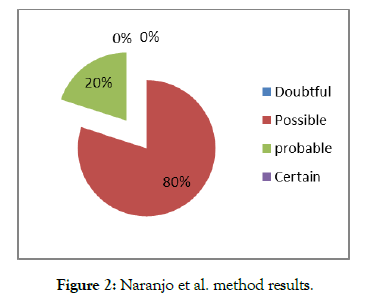
Figure 2: Naranjo et al. method results.
According to the French method: In 70% of cases, intrinsic imputability was I4 or I5 while I6 and I1 score were lowest with a percentage of 3% each. Most incriminated drugs (93%) had a bibliographical score of B4 (Figure 3).
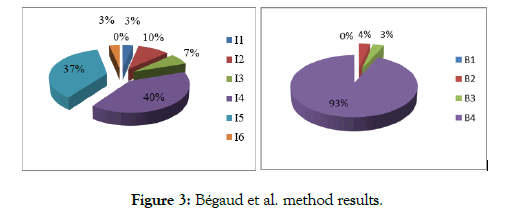
Figure 3: Bégaud et al. method results.
When using the three methods, we have noticed a difference in:
- The nature of involved drugs: the CIOMS scale could favor a drug "drug A" while the non-specific method favored another "drug B";
The number of involved drugs: the CIOMS method could favor a drug "drug A" while the non-specific method favored two drugs "drug A and drug B";
- The grading of causality: score and judgment.
- Comparison according to nature and number of involved drugs: we have considered:
• "match": the case where the two methods incriminated the same drugs in kind and in number.
• "mismatch": the case where the two methods did not incriminate the same drugs in kind or in number.
The results showed a better concordance between the CIOMS scale and the French method (93% of cases) than with the Naranjo and al. method (Percentage of match in the order of 86%) (Figures 4 and 5).
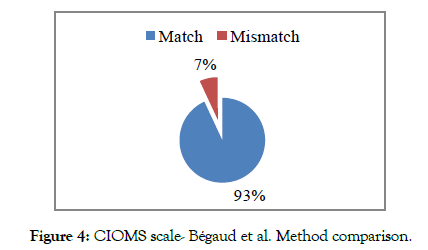
Figure 4: CIOMS scale- Bégaud et al. Method comparison.
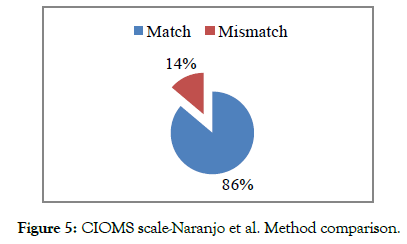
Figure 5: CIOMS scale-Naranjo et al. Method comparison.
- Comparison of causality grading:
The calculated kappa coefficient is 0.1186, so the agreement between the Naranjo et al method and the CIOMS scale is "bad".
Discussion
The assessment of causality must be carried out by qualified pharmacovigilant pharmacists who verify the quality of the provided information by physicians.
The CAM favored more than one drug in 15% of cases. The Paul H. Hayashi and al. study reported that in 31% of cases, more than one drug was involved [7]. These data showed that one drug cannot always be favored over others and that the evaluation of causality is far from being an exact science.
Comparison of the CIOMS scale to non-specific CAMs depending on the nature and number of drugs involved showed a better agreement between the CIOMS method and the French method than with the Naranjo and al method. and that both non-specific methods overestimated the causality assessment results compared to the CIOMS scale. Also, we found that the agreement between the CIOMS scale and the Naranjo et al. method was "bad" (k=0.12). A similar result was observed in the study of Garcia-Cortés et al. [8] with a kappa coefficient comparable to ours (k=0.15).
At last, our results join the criticisms described in literature that consider the non-specific CAMs, deficient and tending to overnotify and over-diagnose DILI [9].
The CIOMS method remains, therefore, the reference method. It has allowed us to shorten the time of bibliographic research (chronology of ADR, possible non-drug causes and risk factors). However, we have noticed some limitations of it:
- It does not consider the evolution of the impairment when treatment is continued at lower doses, since in some cases this measure is sufficient for the improvement of DILI.
- It does not list all possible non-drug causes and does not cite certain other important risk factors.
- It is unable to distinguish between lack of information and possible non-drug causes in unlikely cases.
- It tends to underestimate causality in most severe cases because of the lack of improvement following the drug discontinuation.
Conclusion
Our study showed that comparison between the different CAMs, has been useful to us to show their limits. A refinement of the specific method is, therefore, necessary for better use.
REFERENCES
- Haque T, Sasatomi E, Hayashi P. Drug-Induced Liver Injury: Pattern Recognition and Future Directions. Gut and liver. 2016;10(1):27.
- Khan LM, Al-Harthi SE, Osman AMM, Sattar MA, Ali AS. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm J. 2016;24(4): 485-493.
- Miremont-Salamé G, Théophile H, Haramburu F, Bégaud B. Accountability in pharmacovigilance: from the original French method to updated methods. Pharmacovigilance. 2016;17(2): 171-178.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-45.
- Teschke R, Eickhoff A, Schulze J. Drug- and Herb-Induced Liver Injury in Clinical and Translational Hepatology: Causality Assessment Methods, Quo Vadis?. J Clin Transl Hepatol. 2013;1(1):59-74.
- Frédéric Santos, Le kappa de Cohen : un outil de mesure de l’accord inter-juges sur des caractères qualitatifs, CNRS France,2017.
- Hayashi PH, Barnhart HX, Fontana RJ, Chalasani N, Davern TJ, Talwalkar JA, et al. Reliability of Causality Assessment for Drug, Herbal and Dietary Supplement Hepatoxicity in the Drug-Induced Liver Injury Network (DILIN). Liver Int. 2015;35(5):1623-1632.
- García-Cortés M, Lucena MI, Pachkoria K, Borraz Y, Hidalgo R, Andrade RJ, et al. Evaluation of Naranjo adverse drug reactions probability scale in causality assessment of drug-induced liver injury. Aliment Pharmacol Ther. 2008;27:780-789.
- Maria VJ, Victorino Andruim M. Development and Validation of a clinical scale for The Diagnosis Of Drug-Induced Hepatitis. Hepatology. 1997;26:3.
Citation: Berradia A, Fznmekaouche, Fetati H, Toumi H, et al. (2020) DILI Causality Assessment Methods Comparison: CIOMS Scale Versus Non- Specific Methods. Adv Pharmacoepidemiol Drug Saf. 9:232. doi: 10.35248/2167-1052.20.9.232
Copyright: © 2020 Berradia A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited

