Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 4
Development and Validation of a Rapid RP-HPLC Method for the Determination of Pemetrexed in Lyophilized Powder for Injection
Louis Kwame Dotse and Carlos Martin Infante Cordova*Received: 02-Aug-2023, Manuscript No. CPECR-23-22437; Editor assigned: 04-Aug-2023, Pre QC No. CPECR-23-22437; Reviewed: 18-Aug-2023, QC No. CPECR-23-22437; Revised: 25-Aug-2023, Manuscript No. CPECR-23-22437; Published: 01-Sep-2023, DOI: 10.35248/2161-1459.23.13.383
Abstract
An economic, rapid and versatile RP-HPLC method for the determination of pemetrexed, an effective antifolate in lyophilized powder for endovascular injection, was developed employing a Luna C18 Column (250 × 4.6 mm; 5 μm), and spectrophotometric detection (analytical wavelength at 254 nm). A column oven temperature of 25°C and a mobile phase composed of ACN: Sodium dihydrogen orthophosphate buffer of ratio 30 v/v:70 v/v with pH adjusted to 4. The flow rate was 0.8 ml/min in isocratic elution with a 10:1 of injection volume of sample. The method validation was performed in accord with the ICH guidelines, considering parameters as accuracy, precision, linearity, robustness, limits of detection and quantification. The linear dynamic range was between 80 μg/ml and 120 μg/ml with a correlation coefficient of 0.999 and a runtime of 10 minutes. This propose method stable after 10 hrs. At 25°C proving to be reliable a such it can be used in the determination of Pemetrexede in Pharmaceutical formulation for injectable.
Keywords
HPLC; Lyophilized powder; Pemetrexed; Antifolate; Pharmaceutical formulations
Introduction
Pemetrexede disodium (N-[4-[2-[2-Amino-4,7-dihy-dro-4-oxy-3Hpyrrolo[ 2,3-d]pyrimidine-5-yl ethyl]benzo-yl]-L-glutamic acid) is a multi-target novel antifolate of synthetic origin, extensively used as chemotherapeutic agent for the treatment of pleural mesothelioma, Non-Small Cell Lung Cancer (NSCLC) breast cancer, cervical cancer and colorectal cancer (Figure 1) [1-5]. This is formulated as a sterile lyophilized powder for intravenous application, a reconstituted with water is required prior to its administration. Pemetrexed limits cell proliferation through the interruption of folate dependent metabolic processes indispensable for cell growth. Despite the effectiveness of this drug it`s use is not without side effects some of which include chest pains, unusual weakness, chills, fever, nausea and pale skin [6]. The versatility of Liquid chromatography makes it the most used for routine analysis in biological, pharmaceutical and food industries [7]. Few analytical methods are available for the quantification of pemetrexed in pharmaceutical lyophilized powder for injectable. In general, this methods employing, isocratic and gradient HPLC coupled with reverse phase columns and spectrophotometric methods for the determination of pemetrexed in bulk pharmaceutical products [8-11]. On the other hand, HPLC and LC-MS methods are also currently accessible for the quantification of pemetrexed in biological fluids [12-15]. Analytical methods will be efficient for determining the purity and identity of drugs. It is paramount, efficient methods are developed not only to safeguard the optimization of resources, but rather their aptness towards their intended use [16]. The development and the validation of an analytical method is constant and evolves comparatively with the progress of the drug. Therefore, is methods purpose to reflect the phase of a giving drugs growth. Clearly, a method ought to meet all regulatory guidelines as well as be simple and robust [17-19]. Furthermore, method ought to fully evaluate to establish its technical soundness, a requirement that varies within the regulatory registration procedure. In general, it affords the analyst a clear understanding into the behavior of the designed method making it possible to institute performance limits [20]. The aim of this work is focused on acquiring results with high reproducibility, precision and in the least possible time, with little margin for error, RP-HPLC technique was chosen for the development of a simple, rapid and economic method for the quantification of pemetrexed in injectable powder.
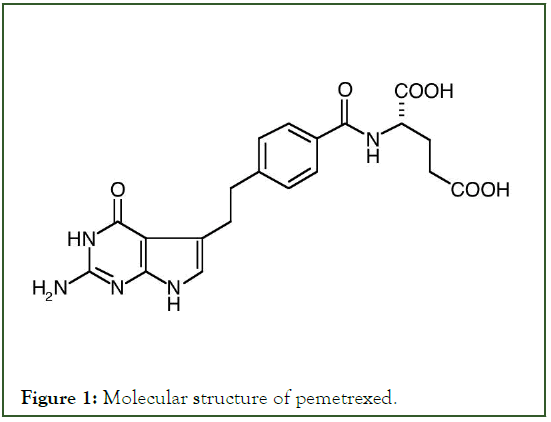
Figure 1: Molecular structure of pemetrexed.
Materials and Methods
Chemicals reagents and solutions
USP pemetrexed disodium heptahydrate of 78.3% purity, orthophosphoric acid, acetonitrile were obtained from LAS do Brasil Ltd and sodium dihydrogen orthophosphate was acquired form Merck. The medicament, lyophilized Pemetrexed disodium powder for injectable samples used for the study where provided by ChemicalTech Ltd.
Buffer preparation
2.76 mg of sodium dihydrogen orthophosphate was weighed, and further diluted with 800 ml of HPLC grade water after which the pH of the resulting solution was adjusted to 4 and then completed to the 1000 ml with the same diluent and passed through filter of 0.22 μm porosity.
Mobile phase and diluent solution
A mobile phase, consisting of acetonitrile and buffer was prepared in the ratio of 30 v/v:70 v/v and then filtered and degassed, this solution was as well a diluent for samples and standard.
Preparation of standard solution
About 35.1 mg of pemetrexed disodium heptahydrate was accurately weight, (equivalent to 27.7 mg of pemetrexed disodium) and transferred into a 25 ml volumetric flask dissolved and diluted to mark with water of HPLC grade. After which 2 ml of the resulting solution was then transferred to 20 ml volumetric flask and completed to the mark with the diluent.
Preparation of sample solution
A vial of the 500 mg presentation of pemetrexed was weighed, after which the contents of vial was reconstituted with 20 ml of HPLC grade water, transferred to 200 ml volumetric flask and the volume was completed to the mark with the same solvent. 2 ml of that previous solution was then transferred to a 100 ml volumetric flask and volume completed to the mark with the diluent solution.
Equipment
The HPLC systems (Rigol L3000 HPLC) consisting of a Rigol L3500 dual-wavelength absorbance detector with a Rigol L3240 quaternary pump. The system operating on the data acquisition, monitoring and processing software system empowered by Clarity. A pH meter model H2221 (Hanna), an ultrasound Bath of model S120 (Elmas) and an analytical balance of model aux 220 (Shimadzu) were used for the development of the method.
Chromatographic conditions
Samples separation was performed using Luna C18 Column (250 × 4.6 mm; 5 μm), at a flow rate of 0.8 ml/min in isocratic elution, column temperature maintained at 25°C ± 2°C, and a detection wavelength 254 nm. The programed run time was 10 mins with an injection volume 10 μl.
Results and Discussions
Multiple tests involving the use of diluents consisting of water, acetonitrile, and orthophosphate buffer solution of pH 3.5-6 and a mobile phase of the same solvents were performer and not significant variations were observed. The resulting optimized conditions selected were a C18 Column (250 × 4.6 mm; 5 μm), a mobile phase containing sodium dihydrogen orthophosphate buffer pH 4 and acetonitrile in the proportion of 70:30 v/v respectively, in isocratic elution mode. Furthermore, the validation is in accordance with ICH guidelines as well the ANVISA directives for analytical procedures [17-19]. Elected parameters adopted for the validation of this method were, specificity, Linearity, accuracy, precision, Limit of Quantification (LoQ), Limit of Detection (LoD) and robustness.
System suitability
Both sample and standard solutions were within the intervals of 0, 2, 4, and 10 hours for the verification of how storage of prepared samples could be relied upon. Both solutions showed stability, confirmed by the 99% recovery obtained, suggesting stability of pemetrexed ate room temperature.
Selectivity/ Specifity
In the Figure 2, showing chromatograms of diluent, pemetrexed standard and sample obtained by the proposed method. The superposition of sample and standard is practically total and the diluent (mobile phase) not present variation in the base line. No other signals are observed for the retention time of analyte and the method is satisfactory for the proposed application.
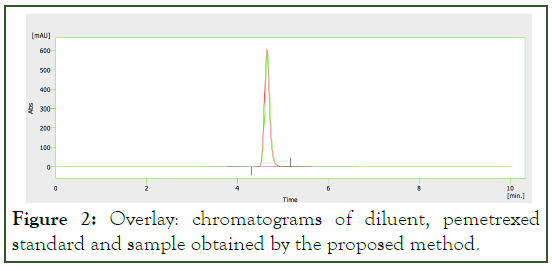
Figure 2: Overlay: chromatograms of diluent, pemetrexed standard and sample obtained by the proposed method.
Linearity
The method proved to be linear meeting the acceptance criteria within the range of 80% (80 μg/ml) to 120% (120 μg/ml) of the expected concentration of sample. The equation of regression was Y=(37.5+0.6)+(214.6+65.0) X, with a correlation coefficient (r2) of 0.99883 and %RSD less than 0.1% for all levels of concentration.
Precision
This test was performed through reproducibility were six replicate injections of both sample and standard solution of 100 μg/ml concentration where performed and their standard deviation, were checked for both analysts on different days, results are showing in Table 1. Intraday samples recorded %RSD=0.01 for each analyst. A inter day %RSD considering all the data of analyst was 1.8%. The acceptation criteria is that %RSD<2, then the precision of the method was considering satisfactory.
| Samples | Analyst 1 | Analyst 2 |
|---|---|---|
| Area | Area | |
| 1 | 4016145 | 4154747 |
| 2 | 4016914 | 4154214 |
| 3 | 4016018 | 4154341 |
| 4 | 4016228 | 4154928 |
| 5 | 4016483 | 4154590 |
| 6 | 4016995 | 4155315 |
| Mean | 4016464 | 4154689 |
| RSD%intra-days | 0.01 | 0.01 |
| Acceptance criteria | DPR ≤ 2,0% | |
| RSD%inter-days | 1.8 | |
| F cal | 1.0399 | |
| F critical | 5.0503 |
Table 1: Studies of precision: Reproducibility and intermediary precision.
Limit of detection and quantification
Were determinate considering: Limit of Detection (LoD)=3 S/N and Limit of Quantification (LoQ)= 10 S/N, where, S is the standard deviation of intercept and m the pendent of the analytical curve. The LoD by the method was 0.008 μg/ml and the LoQ determined by the method was 0.028 μg/ml, this values are acceptable for the application.
Robustness
The robustness was estimated by deliberately varying of four parameters of the method, the mobile phase pH, change in column, flow rate and proportion of mobile phase (Table 2). In accord with ICH (18) the method is robust if %RSD<2%. The method proved robust because all variables tested were between the determined limits, with the highest RSD being 0.7%.
| Variable | %RSD | |
|---|---|---|
| Standard | sample | |
| Change in flow rate to 0.7 mL/min. | 0.1 | 0.1 |
| Change in flow rate to 0.9 mL/min | 0.3 | 0.3 |
| Change in column temperature-10% | 0.3 | 0.1 |
| Change in column temperature+10% | 0.7 | 0.2 |
| Change in column Batch number | 0.1 | 0.2 |
| Change in proportion of the mobile phase-10% | 0.1 | 0.1 |
| Change in proportion of the mobile phase+10% | 0 | 0.3 |
Table 2: Results for robustness.
Accuracy
Method accuracy was determined by recovery for all the three levels of concentrations (80%, 100%, 120%) within the specified interval and in triplicates (Table 3). The mean recovery observed for all injections varies between 101.23% to 101.3%, with a % RSD 0.01, this recovery is accepted for the criteria because is greater to 98% and less than 102%.
| Injections | Recovery 80% |
Recovery 100% |
Recovery 120% |
|---|---|---|---|
| 1 | 101,3 | 101,3 | 101,4 |
| 2 | 101,3 | 101,4 | 101,1 |
| 3 | 101,3 | 101,2 | 101,2 |
| Mean% | 101.3 | 101.3 | 101.23 |
| %RSD | 0.01 | ||
| Acceptance criteria | 98 ≤ RC ≤ 102% | ||
Table 3: Results for accuracy.
Conclusion
The developed method is unique, rapid, precise, and highly robust as confirmed by this validation. Offering superior recovery and adequate selectivity with no interference at the acknowledged retention time. The method proves to be suitable for the determination of pemetrexed present in lyophilized powder for injection, encouraging its application in routine physicochemical quality control analysis directed towards safeguarding its use.
Acknowledgment
The authors express their gratitude to ChemicalTech for the assistance in the development of the experimental part of this work.
References
- Warner A, Piraner I, Weimer H, White K. Development of a purity control strategy for pemetrexed disodium and validation of associated analytical methodology. J Pharm Biomed Anal. 2015;105:46-54.
[Crossref] [Google Scholar] [PubMed]
- Reddy BP, Reddy KA, Reddy MS. Validation and stability indicating RP-HPLC method for the determination of tadalafil API in pharmaceutical formulations. Res Pharm Biotech. 2010;2(1):1-6.
- Agrawal SK, Rathore DS. Development and validation of pemetrexed by RP-HPLC method in bulk drug and pharmaceutical dosage forms. Indian J Res Pharm. Biotech. 2013;1(4):537.
- Cohen MH, Cortazar P, Justice R, Pazdur R. Approval Summary: Pemetrexed maintenance therapy of advanced/metastatic nonsquamous, Non-Small Cell Lung Cancer (NSCLC). Oncologist. 2010;15(12):1352-1358.
[Crossref] [Google Scholar] [PubMed]
- Tomasini P, Barlesi F, Mascaux C, Greillier L. Pemetrexed for advanced stage nonsquamous non-small cell lung cancer: latest evidence about its extended use and outcomes. Ther Adv Med Oncol. 2016;8(3):198-208.
[Crossref] [Google Scholar] [PubMed]
- Reddy LM, Reddy PR, Reddy LB, Reddy KJ. RP-HPLC method for the estimation of pemetrexed assay in formulations. 2011;1(1):1-10.
- Fekete S, Oláh E, Fekete J. Fast liquid chromatography: the domination of core–shell and very fine particles. J Chromatogr A. 2012;1228:57-71.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Trissel LA. Physical and chemical stability of pemetrexed solutions in plastic syringes. Ann Pharmacother. 2005;39(12):2026-2028.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Trissel LA. Physical and chemical stability of pemetrexed in infusion solutions. Ann Pharmacother. 2006;40(6):1082-1085.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Trissel LA. Physical instability of frozen pemetrexed solutions in PVC bags. Ann Pharmacother. 2006;40(7-8):1289-1292.
[Crossref] [Google Scholar] [PubMed]
- Patel AD, Parikh SK, Sen DJ, Patel CN. Development and validation of high performance liquid chromatographic and UV spectrophotometric method for estimation of Pemetrexed disodium in bulk drug and pharmaceutical formulation. Int J Drug Dev Res. 2011;3(2):301-307.
- Stapleton SL, Reid JM, Thompson PA, Ames MM, McGovern RM, McGuffey L, et al. Plasma and cerebrospinal fluid pharmacokinetics of pemetrexed after intravenous administration in non-human primates. Cancer Chemother Pharmacol. 2007;59:461-466.
[Crossref] [Google Scholar] [PubMed]
- Rivory LP, Clarke SJ, Boyer M, Bishop JF. Highly sensitive analysis of the antifolate pemetrexed sodium, a new cancer agent in human plasma and urine by high performance liquid chromatography. J Chromatogr B 2001; 765:135– 140.
[Crossref] [Google Scholar] [PubMed]
- Meesters RJW, Cornelissen R, van Klaveren RJ, de Jonge R, den Boer E. A new ultrafast and high-throughput mass spectrometric approach for the therapeutic drug monitoring of the multi-targeted antifolate pemetrexed in plasma from lung cancer patients. Anal Bioanal Chem. 2010;398(7-8):2943-2948.
[Crossref] [Google Scholar] [PubMed]
- Stoop MP, Visser S, van Dijk E, Aerts JG, Stricker BH, Luider TM. A new quantification method for assessing plasma concentrations of pemetrexed and its polyglutamate metabolites. J Pharm Biomed Anal. 2016 Sep 5;128:1-8.
[Crossref] [Google Scholar] [PubMed]
- FDA Guidance for Industry-Analytical Procedures and Methods Validation for Drugs and Biologics, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) Pharmaceutical Quality/CMC. 2015.
- BRASIL ANVISA. Agência Nacional de Vigilância Sanitária. Resolução RE n° 899 de 29 de maio de 2003a. Guia para a Validação de Métodos Analíticos e Bioanalíticos. 2003.
- BRASIL ANVISA. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada- RDC Nº 166, 24/07/2017. Guia para validação de métodos analíticos – julho. 2017.
- International Conference on Harmonization Quality Guidelines Q2 (R1).Validation of Analytical Procedures, Text and Methodology, Parent guideline dated 27 Oct 1994 Complementary guideline on methodology dated 6 Nov 1996, incorporated November 2005.
- USP 39 (2016): General Tests, Chapter 621 – Chromatography System Suitability, United States Pharmacopeial Convention (USP), Rockville, MD
Citation: Dotse KL, Cordova CMI (2023) Development and Validation of a Rapid RP-HPLC Method for the Determination of Pemetrexed in Lyophilized Powder for Injection. J Clin Exp Pharmacol. 13:383.
Copyright: © 2023 Dotse KL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

