Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 3
Development and Evaluation of Curcumin Floating Tablets
Kishor A. Bellad1, Basavaraj K. Nanjwade2*, Arindam Basu Sarkar3, Teerapol Srichana4 and Roopali M. Shetake12KJD Pharmaceuticals Pvt Ltd, Plot No. 14B, Naubad Industrial Area, Karnataka, India
3Department of Pharmaceutics, College of Pharmacy, University of Findlay, USA
4Drug Delivery System Excellence Centre, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand
Received: 06-Nov-2019 Published: 12-Jun-2020, DOI: 10.35248/2153-2435.20.11.622
Abstract
In the present study, curcumin an anti-inflammatory agent, was first complexed with the soluplus by hot melt extrusion machine to enhance the solubility of curcumin and was formulated into floating tablets by direct compression method. Tablets were formulated using various grades of Hydroxypropyl methylcellulose (HPMC) polymers and sodium bicarbonate as gas generating agent. Preformulation and micromeritic studies were performed. The floating tablets were evaluated for their physico-chemical properties, in-vitro release, in-vivo floating property, pharmacokinetic and stability studies. Formulations were found uniform with respect to thickness (5.11 to 5.27 mm) and hardness (4.4 to 4.8 kg/cm2). The friability (0.27 to 0.53 %), weight variation (1.28-1.89%) and Drug content (97.84 to 99.46 %). The order of swelling index of HPMC was found to be K100M > K15M > K4M. Sodium bicarbonate at the concentration of 16% w/w was found to be sufficient in attaining the buoyancy. The buoyancy lag time was found to be less than 1 minute for all the prepared tablets. The total floating time for different formulations was in the range of 12-24 hours. The optimized formulation F4, and F6 showed 98.85 and 98.10 % drug release at the end of 20 and 24 hrs respectively. The in-vivo floating study was carried out by X-ray imaging showed floating of the tablet in the stomach. The pharmacokinetic study showed, Cmax-260 ng/ml, Tmax-12 hrs, AUC -738.33 ng/hr/ml Kel as 0.061 and t1/2 was found to be 11.36 hrs. FT-IR, XRD and DSC studies revealed no chemical interaction between drug and polymers. During the stability period selected tablets were found to be stable with respect to floating behaviour and drug release characteristics.
Keywords
Curcumin; HPMC; Floating tablets; Direct-compression; Buoyancy; Swelling; In vitro release; In-vivo scintriography study; pharmacokinetic study
Introduction
Effective delivery of the curcumin floating tablets to the stomach, for local action and treatment of gastric disorders such as gastric cancer and gastric ulcers can be achieved by floating dosage forms like single and multiple unit gas generating systems, hollow microspheres, hydrodynamically balanced systems, swelling or expanding systems, mucoadhesive systems and other gastroretentive dosage forms [1].
Gastritis is an inflammation of the lining of the stomach, and has numerous possible causes [2]. It is also essential to be aware of the fact that an inflammation of the stomach lining is mainly considered by the medical fraternity to be a symptom of some medical condition or infection. The chief acute causes are excessive alcohol consumption or continued use of non-steroidal anti-inflammatory drugs (also known as NSAIDs) such as aspirin or ibuprofen. Occasionally gastritis develops after major surgery, traumatic injury, burns, or severe infections.
Curcumin is an orange–yellow powder. Curcumin 1,7-bis (4-hydroxy-3-methoxyphenyl)-1, 6- heptadiene-3, 5-dione, is a natural polyphenolic phytochemical extracted from the powdered rhizomes of turmeric (Curcuma longa) [3]. It has attracted considerable attention in recent years due to its countless variety ofbeneficial biological and pharmacological activities. Besides its effective antioxidant, Curcumin is also effective for preventing and ameliorating gastric lesions. It also possesses anti-inflammatory, antibacterial, antifungal and anti-yeast, anti-hypocholesterolemic, anticancer, anti-mutagen, anti-parasitic, anti-tumour promoting, anti-proliferative, MDR modulator effects [4].
Curcumin important activities in the human body is its ability to inhibit activation of the transcription factor, nuclear factor-kappa B (NF-kB), a potent inducer of chronic inflammation and also the anti-inflammatory effect of curcumin is mediated through its ability to inhibit COX-2, lipoxygenase and inducible nitric oxide synthase important enzyme that mediate inflammatory process .
Curcumin possess low systemic bioavailability attributed to a generally poor absorption and faster metabolic alterations [5]. Curcumin has a short half-life [6,7] and requires administration 3 times a day. Curcumin is poorly soluble in water [8]. Curcumin has a molecular weight of 368.37g/mol and a melting point of 183°C.
Thus to increase the solubility of curcumin various approaches has been made including Solid dispersions effectively improved curcumin’s solubility and dissolution in simulated gastric fluids. Cyclodextrin complexation also showed better solubility for curcumin and after oral administration this complex showed better anti-inflammatory activity in rat colitis model compared to pure curcumin [9]. Formerly biodegradable polymers were used to prepare curcumin nanoparticles and demonstrated enhancement in solubility and hence pharmacological activities [10,11].
Soluplus is a polymeric solubilizer with an amphiphilic chemical structure, which was chiefly developed for solid solutions. Due to its an amphiphilic character, it is able to act as a matrix polymer for solid solutions on the one hand, while being capable of solubilizing poorly soluble drugs in aqueous media on the other. Soluplus® is a polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (13% PEG 6000/57% vinyl caprolactam/30 % vinyl acetate). It has a PEG 6000 backbone with one or two side chains consisting of vinyl acetate randomly copolymerized with vinyl caprolactam.
The molecular weight of the polymer was determined by means of gel permeation chromatography and has an average value of 118,000 g/mol [12].
Curcumin floating tables, drug delivery system of gastro retentive is an approach to prolong gastric residence time, thus targeting sitespecific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects. Gastro retentive dosage forms can remain in the gastric region for longer period of time and hence considerably elongates the gastric retention time (GRT) of drugs [13].
Prepared curcumin floating tablets are hydrodynamically controlled drug delivery systems or floating drug delivery systems are lowdensity systems that have adequate buoyancy to float above the gastric contents and remain floating in the stomach without disturbing the gastric emptying rate for a prolonged period of time [14].
Complete knowledge about GI dynamics such as gastric emptying, small intestine transit, colonic transit, etc. is the key for the optimum design of oral controlled release dosage forms. The rate and extent of drug absorption from various sites of GI tract and aspects that govern the absorption further help’s the design of dosage form.
Thus due to the poor solubility of the curcumin the solid solution of the curcumin with the soluplus was being prepared by hot melt extrusion method to increase the solubility, and also by considering the short half-life, dosing frequency (3 times a day) the present study was aimed in the development and evaluation of curcumin floating drug delivery systems of anti-inflammatory.
Materials and Methods
Curcumin was obtained as a gift sample from Sami Labs Ltd, Bengaluru. Soluplus was procured from BASF India Ltd, Mumbai as gift sample. Various grades of HPMC were provided by the Colorcon Ltd. Goa.Sodium bicarbonate. All other ingredient used were of analytical grade.
Preparation of solid solution (Curcumin Complex)
Solid solutions of curucmin using soluplus were prepared by hot melt extrusion in a Thermo Fisher Polylab twin-screw extruder. The Drug: polymer ratio was maintained at 1:1. Extrusion parameters adjusted were as 80 RPM, 40 bar pressure, 60°C - 150°C temperature.
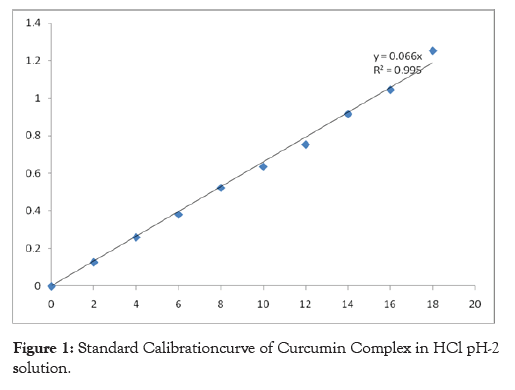
Standard calibration curve of Curcumin Complex in Hydrochloric Acid pH-2 solution
From the stock-That contains the concentration of 1mg/ml ie.1000 μg/ml, 10 ml solution was taken and then diluted up to 100 ml with Hydrochloric Acid pH-2 solution in a volumetric flask and then the concentration of this stock will be 100 μg/ml (II stock solution). From this II stock solution, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6 and 1.8 ml solutions were pipetted and volume was made to 10 ml using Hydrochloric Acid pH-2 solution to get concentrations of 2, 4, 6, 8, 10, 12, 14, 16 and 18 μg/ml respectively. The absorbance of these solutions was measured at 421 nm and standard calibration curve was plotted using the absorbance so obtained.
Solubility studies
An excess quantity of Curcumin and curcumin complex was separately added to 10 ml of water in a shaking water bath at room temperature for 24 hrs. The solutions were then filtered through Whatmann filter paper (No. 41) and the filtrate was suitably diluted with Hydrochloric Acid pH-2 and analysed using UV spectrophotometry at 421 nm [15].
Preparation of floating tablets
The floating tablets of Curcumin were prepared by employing different grades of HPMC polymers like K4M, K15M and K100M by direct compression method using 10 stations Rimek compression machine. For the preparation of floating tablets, the active ingredient was thoroughly mixed with polymer(s), diluents (MCC), sweetener (sodium saccharin) and gas forming agent (sodium bicarbonate) using a mortar and pestle for 10 min. Magnesium stearate, talc were added to the above blend as flow promoters. In all the formulations, the amount of Curcumin Complex was kept constant at 400 mg and different HPMC polymers like HPMC K4 M, K15M and K100M were used in different ratios i.e. 1:0.5, 1:1 with respect to drug. The formulae of different matrix tablets of Curcumin are given in the Table 1.
Table 1: Formulae of different floating tablet formulations of curcumin.
| SI. No. | Ingredients (mg) | Formulation code | |||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | ||
| 1. | Curcumin complex | 400 | 400 | 400 | 400 | 400 | 400 |
| 2. | HPMC K4M | 100 | 200 | - | - | - | - |
| 3. | HPMC K15M | - | - | 100 | 200 | - | - |
| 4. | HPMCK100M | - | - | - | - | 100 | 200 |
| 5. | NaHCO3 | 120 | 120 | 120 | 120 | 120 | 120 |
| 6. | Sodium saccharin | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| 7. | MCC | 107.5 | 7.5 | 107.5 | 7.5 | 107.5 | 7.5 |
| 8. | Mg.st | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| 9. | Talc | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| 10. | Total | 750 | 750 | 750 | 750 | 750 | 750 |
Angle of repose
Angle of repose was determined using fixed funnel method. The physical mixtures of drug with different excipients were prepared and the accurately weighed drug powder or its physical mixture was taken in a funnel. The height of the funnel was adjusted in such a way that the tip of the funnel just touches the apex of the heap of the drug powder. The powder was allowed to flow through the funnel freely onto surface [16]. The height (h) and diameter (r) of the powder cone was measured and angle of repose was calculated by following formula:
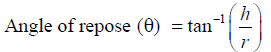
Apparent bulk density
Apparent bulk density was determined by placing pre-sieved drug excipient blend in to a graduated cylinder and measuring the volume and weight as it is [17]. Bulk density was determined by using following formula.
Tapped density
Weighed sample of powder mixture was transferred to a graduated cylinder and was tapped for a fixed time or for a fixed number of taps (100) [17]. The tapped density was determined by using the following formula.
Compressibility index
Based on the apparent bulk density and the tapped density, the percentage compressibility of the powder mixture was determined by the following formula [18].
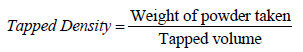
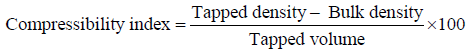
Hausner’s ratio
Hausner’s ratio is an indirect index of ease of measuring the powder flow. It was calculated by the following formula [18].
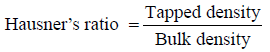
Lower Hausner ratio (<1.25) indicates better flow properties than higher ones (>1.25).
Hardness
The hardness of the tablet was determined using hardness tester [19]. It was expressed in Kg/cm2.
Weight variation
Twenty tablets were selected randomly from the lot and weighed individually to check for weight variation [17]. IP limit for weight variation in case of tablets weighing 130-324 mg ± 7.5% and more than 324 mg ± 5%.
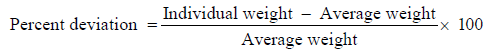
Thickness
The thickness of the tablets was measured by varnier calliper scale [19]. It is expressed in mm.
Friability
Friability of the tablets was determined using Rolex friability test apparatus. This device subjects the tablets to the combined effect of abrasions and shock in a plastic chamber revolving at 25 rpm and dropping the tablets at a height of 6 inches in each revolution. A preweighed prepared tablet was placed in the friabilator and was subjected to 100 revolutions. Tablets were dedusted using a soft muslin cloth and reweighed. The friability (F) it was calculated by the following formula [20].
F = (1- W0 / W) × 100
Where, W0 is the weight of the tablets before the test and W is the weight of the tablet after the test. (Lieberman HA et al)
Drug content
The drug content was carried out by weighing five tablets from each batch and calculated the average weight. Then the tablets were triturated to get a fine powder. From the resulting triturate, powder was weighed accurately which is equivalent to 100 mg of curcumin and dissolved in a 100 ml volumetric flask containing 50 ml of Hydrochloric Acid pH-2 and volume was made up to 100 ml with same solvent. The volumetric flask was shaken using sonicator for 1 hrs and after suitable dilution with Hydrochloric Acid pH-2 the drug content was determined using UV- spectrophotometry at 421 nm [17].
Swelling studies
The extent of swelling was measured in terms of % of weight gained by the tablet. One prepared tablet from each formulation was weighed and kept in Petri dish containing 50 ml of 0.1 N HCl. At the end of studies specified time intervals tablets were withdrawn from Petri dish and excess buffer blotted with tissue paper and weighed [21]. The % of weight gained by the tablet was calculated by using following formula:
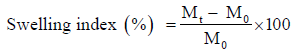
Where, Mt weight of tablets at time‘t’; M0 weight of tablets at time ‘0’ Buoyancy lag time determination and total floating time The in vitro buoyancy was determined by the floating lag time [22].
The prepared tablets were placed in a 100 ml beaker containing 0.1N HCl. The time required for the tablet to rise to the surface for floating was determined as the floating lag time and further floating duration of all tablets was determined by visual observation.
In vitro dissolution study
In vitro drug release studies for the prepared floating tablets were conducted for a period of 24 hours using USP XXIV type- II (Paddle) dissolution apparatus (Electro lab, Mumbai.) at 37 ± 0.5°C and 75 rpm speed using 900 ml ofHydrochloric Acid pH-2 as dissolution medium. At predetermined interval of time, 10 ml of sample was withdrawn from the dissolution medium and replaced with fresh medium to maintain the sink condition. After filtration and appropriate dilution, the samples were analyzed for Curcumin by UV spectrophotometer at 421 nm. The amount of drug present in the samples was calculated.
Fourier transform infrared spectroscopy (FTIR) study The compatibility between drug and polymer was detected by IR spectra obtained by Shimadzu 8400, Japan. The pellets were prepared on KBr press (spectra lab,). The spectra were recorded over the number range of 4000 to 400 cm-1.
X-ray powder diffractometry (X-RD)
X-ray powder diffractometry was carried out to investigate the effect of polymer on drug. Powder XRD patterns were recorded on PW 3710 based diffractometer using a voltage of 40kV and a current of 30mA. The scanning rate employed was 50 min-1, over the 50 to 400 diffraction angle (2θ) range. The X-RD patterns of drug and its physical mixture were recorded.
Differential scanning calorimetry (DSC) study
Thermograms were obtained by using a differential scanning calorimeter (DSC Q20 V24.4 Build 116, Japan) at a heating rate 10 oC/min over a temperature range of 0-300 oC. The sample was hermetically sealed in an aluminium crucible. Nitrogen gas was purged at the rate of 10 ml/min for maintaining inert atmospheres.
Evaluation of gastric retention using X-Ray imaging
The selected Curcumin tablet formula (F6) for in vivo investigation was reformulated with 10% BaS04 as opaquing agent and prepared by direct compression method [23]. The X-ray studies were was performed in 3 healthy meal New Zealand white strain rabbits weighing from 2.0-2.5 kg. After fasting for 24 hours, rabbits were allowed to asses water for 12 hours just before start of study. The permission was taken from animal ethical committee prior to experiment. The X-ray was taken just before the administration of the reformulated curcumintablet to ensure the absence of radio-opaque material in the stomach. The formulation was administered to the rabbit through gastric tube with add of 3-4 ml distilled water followed by 50 ml of water. The X-ray imaging was taken from each animal, and the distance between the source of X-rays and the animal was kept constant for all imaging, thus the observation of the reformulated curcumintablet movements could be easily noticed. Gastric imaging was done at 6 and 12 hrs using an X-ray machine.
In-vivo bioavailability studies
Blood samples of 5 ml were collected from the marginal ear vein of the rabbits into heparinized [24].
Pharmacokinetic analysis
Centrifuge tubes at 1, 3, 6, 12, 18 and 24 h after the drug administration. The blood samples were centrifuged at 3000 × g for 10 min to obtain the plasma and stored at -20 °C until analysis. The extraction of drug from plasma was carried out as reported previously and then injected into the HPLC system. The mobile phase used was methanol: phosphate-citrate buffer pH 3.0 and analyzed at 262 nm wavelength using HPLC.
Pharmacokinetic parameters were derived from the plasma concentration vs. time plot. The area under the curve (AUC), the peak plasma concentration (Cmax) and the time to attain peak concentration (tmax) were obtained from these plots. The elimination rate constant (Kel) was determined from the semi-logarithmic plot of plasma concentration vs. time. Elimination half-life (t1/2) was calculated using the formula; t1/2 = 0.693/Kel. AUC was statistically analyzed applying one-way ANOVA at 0.05 levels in the GraphPad Prism version 5.01 software.
Stability studies
From the six batches of floating curcumin tablet, formulation F6 was tested for stability studies [25]. Formulation F6 was divided into 3 sample sets and stored at:
» 4o ± 1 oC
» 25o± 2 oC and 60% RH ± 5% RH.
» 37o± 2oC and 65% RH ± 5% RH.
After one-month, the drug release of selected formulation (F6) was determined by the method discussed previously in vitro drug release studies, floating lag timeand total floating time was also carried out for the same formulation.
Results and Discussion
Description
The pure drug curcumin used was yellow in colour with no odour and bitter taste.
Melting point
The melting point of the curcumin was found to be 184°C. Mean of three readings was noted. The results of melting point of Curcumin are shown in Table 2.
Table 2: Observations for calibration curve of curcumin complex in HCl pH-2 solution.
| Sr.No | Concentration ( µg/ml ) | Absorbance ( 421nm ) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 2 | 0.127 |
| 3 | 4 | 0.260 |
| 4 | 6 | 0.381 |
| 5 | 8 | 0.523 |
| 6 | 10 | 0.636 |
| 7 | 12 | 0.754 |
| 8 | 14 | 0.916 |
| 9 | 16 | 1.045 |
| 10 | 18 | 1.253 |
R2- 0.990 Y- 0.066
Solubility
The available literature on solubility profile of indicated that, Curcumin is poorly soluble in water, but dissolves in methanol followed by acetone, normal amyl methyl ketone, ethanol, tetrahydrofuran, acetylacetone, chloroform, acetic acid, dimethyl sulfoxide, benzene, toluene and CCl4. Hence to increase solubility in water, curcumin-soluplus complex was prepared by using hot melt extrusion method through extruder. Thus the solid solution formed using soluplus showed that the solubility of the curcumin was increased. The results of solubility of Curcumin are shown in Table 3.
Table 3: Pre-formulation studies of curcumin.
| Studies | colour | odour | taste | Melting point | Solubility in water | Solubility in water after formation of complex |
|---|---|---|---|---|---|---|
| Reported Values | Yellow | Odourless | Bitter | 183°C | Poorly soluble | - |
| Observed Values | Yellow | Odourless | Bitter | 184°C | Practically insoluble | 6.5µg/ml |
Micromeritic properties
In the present work, direct compression technique was used for tableting. Hence the mixture of drug and polymers should have good flow and compaction properties. Table 4 represents the micromeritic property of pre-compressional mixture. All formulations showed the angle of repose value between the 23.65- 26.27% that was further supported by good compressibility index value between 13.45-15.48 % and Hausner’s ratio between 1.12- 1.29, thus demonstrating the suitability of pre-compressional powder blend for direct compression. The results are shown in Table 4.
Table 4: Micromeritic properties of pre-compressional powder blend of different curcumin floating tablet.
| Code | Angle of repose (θ) | Bulk density (g/cm3) | Tapped density (g/cm3) | Carr’s compr. Index (%) | Hausner's ratio |
|---|---|---|---|---|---|
| F1 | 23.65 ± 0.54 | 0.46 ± 0.45 | 0.46 ± 0.38 | 13.45 ± 0.32 | 1.18 ± 0.05 |
| F2 | 24.21 ± 0.52 | 0.53 ± 0.07 | 0.61 ± 0.16 | 13.56 ± 0.35 | 1.24 ± 0.03 |
| F3 | 26.27 ± 0.03 | 0.52 ± 0.59 | 0.62 ± 0.15 | 14.25 ± 0.45 | 1.25 ± 0.08 |
| F4 | 24.63 ± 0.83 | 0.47 ± 0.76 | 0.59 ± 0.34 | 14.37 ± 0.38 | 1.29 ± 0.06 |
| F5 | 25.83 ± 0.59 | 0.52 ± 0.23 | 0.64 ± 0.16 | 14.92 ± 0.14 | 1.12 ± 0.04 |
| F6 | 23.95 ± 0.52 | 0.45 ± 0.13 | 0.59 ± 0.12 | 15.48 ± 0.46 | 1.28 ± 0.02 |
*All values are expressed as mean ± SD. n=3.
Physico-chemical evaluation of tablets
The tablets formulated using various grades of HPMC (K4M, K15M and K100M) were found uniform with respect to thickness (5.11- 5.27mm) and hardness (4.4 to 4.8 kg/cm2). The friability (0.27 to 0.53 %) and weight variation (1.28 to 1.89 %) of different batch of tablets were found within prescribed limits. Drug content (97.84 to 99.46%) was also uniform within the batches of different tablets. Hence tablets containing drug, polymer, diluent and lubricants could be formulated satisfactorily by using direct compression technique. The results of physico-chemical evaluation of tablets are given in Table 5.
Table 5: Physico-chemical evaluation of curcuminfloating tablets.
| Code | Hardness test+ (kg/cm2) | Friability† (%) | Weight variation* (%) | Thickness† (mm) | Drug content+ (%) |
|---|---|---|---|---|---|
| F1 | 4.8 ± 0.42 | 0.29 ± 0.16 | 1.38 ± 0.57 | 5.24 ± 0.12 | 99.26 ± 0.68 |
| F2 | 4.4 ± 0.24 | 0.32 ± 0.12 | 1.28 ± 0.36 | 5.27 ± 0.08 | 98.38 ± 0.41 |
| F3 | 4.5 ± 0.46 | 0.27 ± 0.13 | 1.46 ± 0.57 | 5.17 ± 0.06 | 97.84 ± 0.26 |
| F4 | 4.7 ± 0.51 | 0.48 ± 0.08 | 1.55 ± 0.39 | 5.11 ± 0.17 | 99.46 ± 0.24 |
| F5 | 4.5 ± 0.26 | 0.53 ± 0.09 | 1.43 ± 0.17 | 5.22 ± 0.23 | 98.59 ± 0.41 |
| F6 | 4.6± 0.39 | 0.44 ± 0.06 | 1.89 ± 0.16 | 5.15 ± 0.06 | 99.32 ± 0.68 |
*All values are expressed as mean ± SD. n=3.
Swelling study
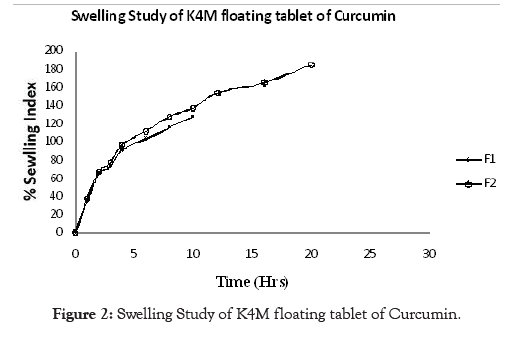
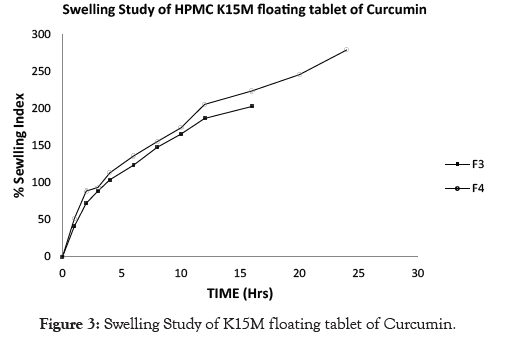
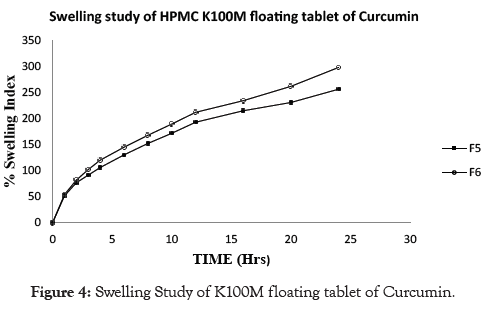
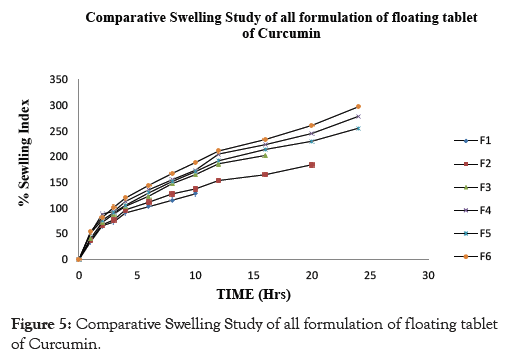
Examination of polymer swelling and erosion is important application to better understand the mechanism of release and the relative significance of participating parameters. Swelling ratio defines the extent of water that is confined within the hydrogel at equilibrium and is a function of network structure, hydrophilicity and ionization of functional group. Swelling study was done on all the batches of floating tablet for 24 hrs. The obtained results of swelling index are given in Table 6. The plot of % swelling index against time (Hrs.) is depicted in Figures 1-5. All the floating tablets were found intact throughout the period of swelling (10 to 24 hrs) in 0.1 N HCl. The % of swelling index was proportional to the polymer level regardless of grade of the polymer. The swelling index was higher for tablets prepared using high viscosity HPMC polymer like HPMC K100M (F6, 297.53%) than the lower viscosity grades such as HPMC K15 M (F4, 278.93%) and HPMC K4M (F2, 184.63%). The order of swelling index witnessed with altered grades of HPMC polymers is HPMC K100M > HPMC K15M > HPMC K4M.
Table 6: Swelling study of different floating tablets of curcumin.
| Time (hrs) | Swelling Index (%) | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 34.46 | 37.87 | 41.18 | 51.42 | 51.59 | 53.88 |
| 2 | 65.59 | 67.72 | 72.29 | 88.63 | 76.74 | 81.73 |
| 3 | 73.38 | 77.43 | 88.76 | 93.61 | 91.25 | 101.81 |
| 4 | 90.89 | 96.89 | 103.88 | 113.28 | 105.69 | 119.88 |
| 6 | 102.94 | 111.78 | 123.57 | 135.96 | 129.91 | 144.64 |
| 8 | 115.43 | 127.85 | 147.74 | 155.35 | 151.87 | 167.54 |
| 10 | 127.98 | 137.55 | 165.40 | 173.92 | 171.27 | 188.98 |
| 12 | - | 153.95 | 186.59 | 205.49 | 192.49 | 211.45 |
| 16 | - | 165.28 | 202.72 | 223.69 | 214.42 | 233.79 |
| 20 | - | 184.63 | - | 245.53 | 230.33 | 261.23 |
| 24 | - | - | - | 278.93 | 255.76 | 297.53 |
Average of 3 determinations.
Figure 1: Standard Calibrationcurve of Curcumin Complex in HCl pH-2 solution.
Figure 2: Swelling Study of K4M floating tablet of Curcumin.
Figure 3: Swelling Study of K15M floating tablet of Curcumin.
Figure 4: Swelling Study of K100M floating tablet of Curcumin.
Figure 5: Comparative Swelling Study of all formulation of floating tablet of Curcumin.
Thus comparing of the swelling indices of different floating formulations, it was found that, the viscosity of the polymer has important influence on swelling process and the matrix integrity of the prepared tablets. Hence from the results obtained it can be concluded that there exists a linear relationship between viscosities of polymer and the swelling process.
Buoyancy lag time and total floating time determination
Gastro retentive floating tablets need to retain certain characteristics. Therefore experiments were performed for floating lag time as well as total flotation time. Floating lag time shows time taken by a tablet, under in vitro simulated conditions to float over the gastric fluid.
It was observed that, at optimum concentration of sodium bicarbonate (16%) all the floating tablets (F1-F6) batches had floating lag time below 40 seconds irrespective to the viscosity and the concentration of HPMC used, because the generation of CO2 resulted from interaction between sodium bicarbonate and dissolution medium, entrapment of gas inside the hydrated polymeric matrices permits the dosage form to float by lowering the density of the matrices. No variation in floating lag time was observed with tablets prepared by using different viscosity grades of HPMC. The total floating time was found to be in the range of 12 - 24 hours for all the tablets formulated. The results of the floating lag time (FLT) and total floating time (TFT) for the different floating tablet formulations are given in Table 7 and Figure 6.
Table 7: Floating behaviour of different floating tablets of curcumin in 0.1 N HCl.
| Sr. No | Formulation Code. | Floating lag time (sec) | Total floating time (hrs) |
|---|---|---|---|
| 1 | F1 | 23 | 12 |
| 2 | F2 | 35 | 15 |
| 3 | F3 | 25 | 20 |
| 4 | F4 | 29 | 24 |
| 5 | F5 | 17 | 24 |
| 6 | F6 | 18 | More than 24 |
Average of 3 determinations.
In vitro release study
Figure 6: Photographs indicating the floating lag time of optimized formulation (F6).
In vitro release study
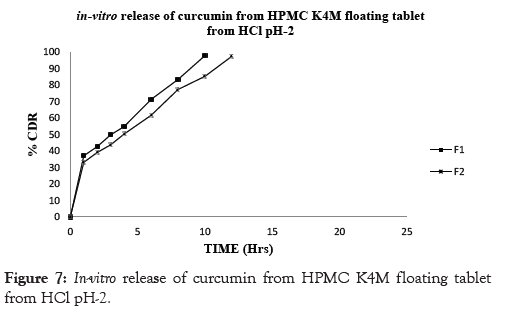
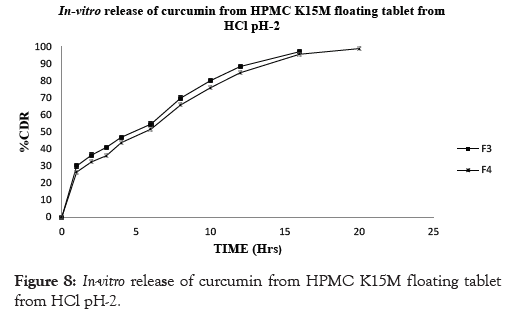
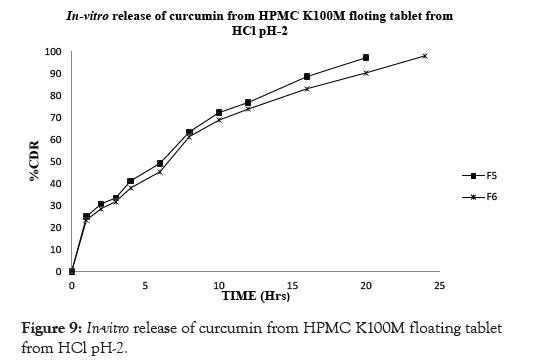
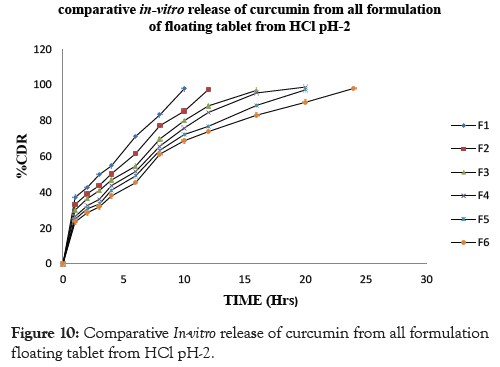
In conclusion, the results of the in vitro drug release studies showed that, floating tablets prepared using drug: polymer ratio (1:1) of viscosity grade HPMC polymer HPMC K100M showed drug release profiles for F6 98.10% at the end of 24 hrs however the formulation prepared using lower grades of polymer like HPMC K4M and HPMC K15M showed that the drug release profile for F2 97.41% and F4 98.85% showed release at 12 and 20 hrs respectively. The similar effect was seen with drug: polymer ratio (1:0.5) the F1 showed 97.82 % drug release at end of 10 hrs. F3 showed 97.06 % drug release at end of 16 hrs whereas F5 showed 97.27 % drug release at end of 20 hrs. Thus it can be concluded that the F6 formulation showed drug release for complete 24 hrs and there by showed good sustained effect as compared to the other formulation. This could be due higher concentration of the polymer in the matrices that effectively retarded the drug release. The results are shown in Table 8 and Figures 7-10.
Table 8: In-vitro drug release of curcumin floating tablets in HCl pH-2.
| SR.NO | TIME(Hrs) | % CDR | |||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | ||
| 1 | 1 | 37.15 | 33.15 | 30.05 | 26.47 | 24.88 | 23.37 |
| 2 | 2 | 42.67 | 39.15 | 36.40 | 32.47 | 30.74 | 28.54 |
| 3 | 3 | 49.84 | 43.77 | 40.95 | 36.05 | 33.43 | 31.78 |
| 4 | 4 | 54.80 | 50.32 | 46.87 | 43.70 | 41.15 | 37.91 |
| 5 | 6 | 71.28 | 61.63 | 54.60 | 51.63 | 49.15 | 45.36 |
| 6 | 8 | 83.34 | 77.28 | 69.83 | 65.83 | 63.49 | 61.35 |
| 7 | 10 | 97.82 | 85.27 | 80.03 | 75.90 | 72.24 | 68.80 |
| 8 | 12 | - | 97.41 | 88.31 | 84.65 | 76.86 | 73.90 |
| 9 | 16 | - | - | 97.06 | 95.55 | 88.65 | 83.14 |
| 10 | 20 | - | - | - | 98.85 | 97.27 | 90.31 |
| 11 | 24 | - | - | - | - | - | 98.10 |
Figure 7: In-vitro release of curcumin from HPMC K4M floating tablet from HCl pH-2.
Figure 8: In-vitro release of curcumin from HPMC K15M floating tablet from HCl pH-2.
Figure 9: In-vitro release of curcumin from HPMC K100M floating tablet from HCl pH-2.
Figure 10: Comparative In-vitro release of curcumin from all formulation floating tablet from HCl pH-2.
Compatibility studies
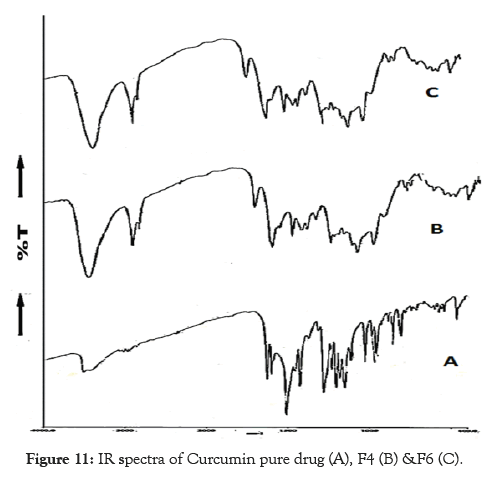
The IR spectra of pure curcumin and its optimized formulations F4 and F6 showed their characteristic absorption bands in the IR region as follows. Functional groups bands/peaks observed in following IR region for pure Curcumin and its optimized formulations. The IR spectrum of pure drug and optimized formulations F4, F6 are used to establish the characteristic behaviour of the drug and in its formulations. It is found from the spectral study, that thereis no difference worth mentioning in the portions of characteristic absorption bands of various functional groups and bonds present in the drug molecule and the formulations prepared from it. The drug has not lost its characteristic properties when it is converted into its different formulations. These observations support the fact that the drug has maintained its integrity in its formulations as it retained its physical characteristics without undergoing any change in its properties. This suggests that there is no interaction of the drug with polymer and excipients used in preparation of the formulations. Hence, it can be concluded that the drug has not under gone any type of interaction with the excipients used in the formulation development. The results of the FT-IR are shown in Figure 11.
Figure 11: IR spectra of Curcumin pure drug (A), F4 (B) &F6 (C).
X-ray powder diffractometry (X-RD)
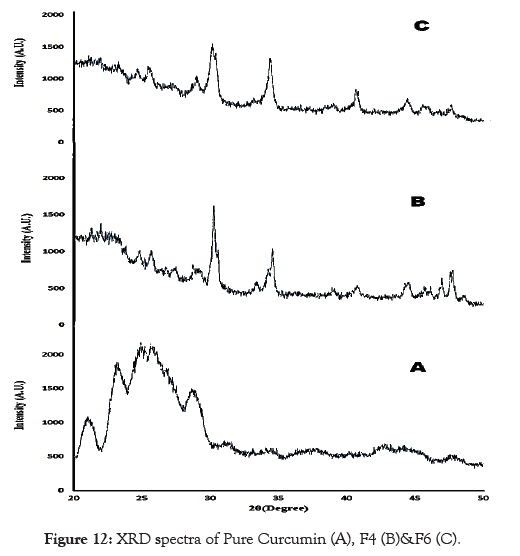
XRD directly detects the crystallinity properties of a material. The diffraction pattern for pure curcumin showed five peaks at 21.12º, 23.40º , 24.50°, 25.70° and 28.60º, 2θ the peaks showed are broadthus indicating that the pure drug was amorphous. Whereas F4 and F6 showed two main peak at 30.94°, 34.82°, 30.10°, 34.66° respectively. The peak obtained was sharp thus indicating that formulation were crystalline in nature. The powder X-ray diffraction patterns obtained for pure drug, F4 and F6 are shown in Figure 12.
Figure 12: XRD spectra of Pure Curcumin (A), F4 (B)&F6 (C).
Differential scanning calorimetry (DSC) study
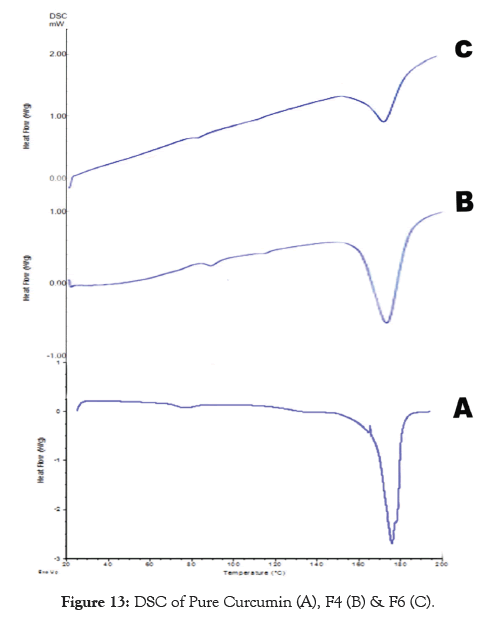
In addition to IR spectra and XRD, DSC studies were also carried out to establish the compatibility of the drug with its formulation ingredients. The DSC thermograms of the pure drug and its formulation F4 and F6 exhibited the endothermic peak at the temperature 175.46°c, 174.58 °C, and 173.20°C respectively. These values clearly suggest that there is no significantalteration in the physical characteristic of the drug in its normal form and formulations. This specifies that there is no interaction of the drug with the different polymers and other excipients used in the study. The results are shown in Figure 13.
Figure 13: DSC of Pure Curcumin (A), F4 (B) & F6 (C).
Gastric retention by X-Ray imaging
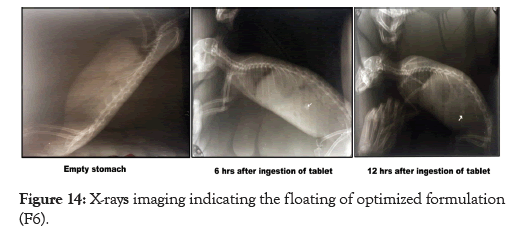
The in-vivo Floating characteristic of the optimized formulation (F6) was carried out on the male albino rabbits. The tablets were formulated using BaSO4 an opaqueing agent. The X-ray was taken just before the administration of the reformulated curcumintablet to ensure the absence of radio-opaque material in the stomach. Also X-ray was taken at 6 hrs and 12 hrs respectively after ingestion of tablet. The imaging confirmed the absence of radio-opaque material in the stomach prior to ingestion of tablet whereas it also confirmed that the tablet remain floating in the stomach for more than 12 hrs which is confirmed by the imaging. The results are shown in Figure 14.
Figure 14: X-rays imaging indicating the floating of optimized formulation (F6).
Pharmacokinetic analysis
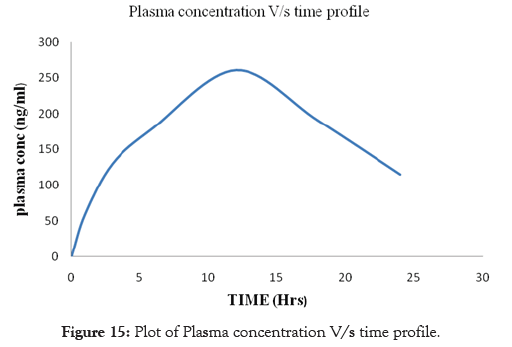
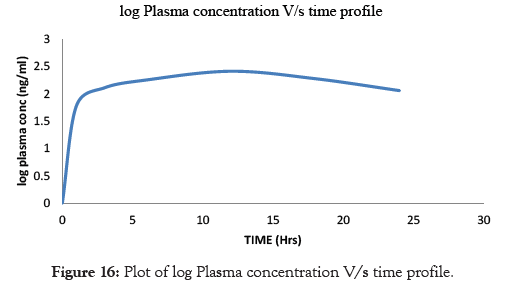
Pharmacokinetic study was conducted on male albino rabbits with the Formulation F6. From the Plasma concentration versus time profile of the Formulation F6, Cmax after oral dosing was found to be 260 ng/ml, Tmax as 12 hrs, AUC as 738.33nng/hr/ml, Kel as 0.061. The t1/2 of Formulation F6 was found to be 11.36 hrs. The results for the Plasma concentration versus time profile are shown in Table 9, log plasma concentration versus time profile is shown in Table 10. The obtained pharmacokinetics parameters are shown in Table 11. Plot of Plasma concentration versus time profile are shown in Figure 15, Plot of log plasma concentration versus time profile is shown in Figure 16.
Table 9: Plasma concentration V/s time profile.
| Time in hours | 1 | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|---|
| Formulation F6(ng/ml) | 60 | 130 | 180 | 260 | 190 | 115 |
Table 10: Log plasma concentration V/s time profile.
| Time in hours | 1 | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|---|
| Formulation F6(ng/ml) | 1.778 | 2.111 | 2.255 | 2.414 | 2.278 | 2.060 |
Table 11: Pharmacokinetic parameters of formulation F6.
| Formulation Code | Pharmacokinetic parameters | ||||
|---|---|---|---|---|---|
| F6 | Cmax (ng/ml) | Tmax (hrs) | AUC (ng/hr/ml) | Kel | t1/2(hrs) |
| 260 | 12 | 738.33 | 0.061 | 11.36 | |
Stability studies
Figure 15: Plot of Plasma concentration V/s time profile.
Figure 16: Plot of log Plasma concentration V/s time profile.
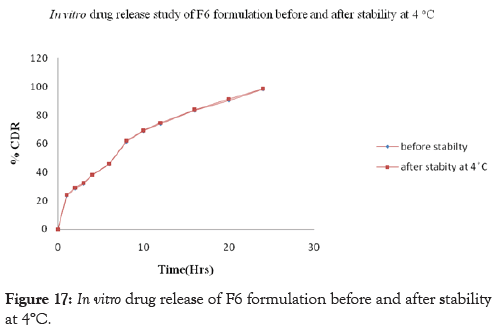
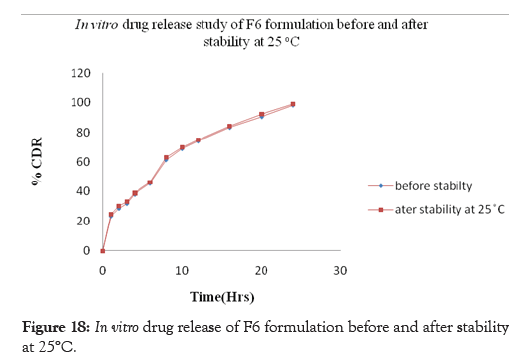
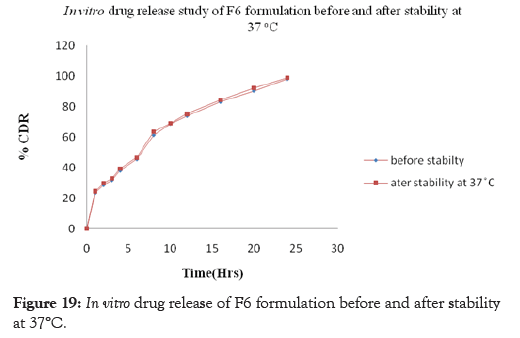
Stability studies of the prepared curcumin floating tablet were carried out by storing the formulation F6 at 4° ± 1°C, 25° ± 2°C 60% ± 5% RH and 37° ± 2°C 65% ± 5% RH for one month. The parameters namely floating lag time, Total Floating time and in-vitro release studies were carried out. These studies revealed that, there is a slight increase in Floating lag time along with the increase in drug release whereas no effect is seen on total floating time after storage for one month at 4°± 1°C, 25°± 2°C; 60% ± 5% RH and 37°± 2°C; 65% ± 5% RH. The drug content was found above 97% and no significant variation was observed at the end of one month stability study. This indicates that, the prepared tablets remained fairly stable during storage conditions. The results of Floating lag time, Total Floating time and in-vitro releaseafter one month of storage are shown in Tables 12 and 13 and Figures17-19 respectively.
Table 12: Stability studies-floating lag time and total floating time after 30 days storage of selected formulation F6.
| Formulation | Before stability studies | FLT and TFT at 4° ± 1°C |
FLT and TFT at 25° ± 2°C and 60% Rh ± 5% RH |
FLT and TFT at 37° ± 2°C and 65% Rh ± 5% RH |
||||
|---|---|---|---|---|---|---|---|---|
| FLT (sec) |
TFT (Hrs) |
FLT (sec) |
TFT (Hrs) |
FLT (sec) |
TFT (Hrs) |
FLT (sec) |
TFT (Hrs) |
|
| F6 | 18 | More than 24 | 18.42 | More than 24 | 18.48 | More than 24 | 18.39 | More than 24 |
Table 13: Stability studies-In Vitro release studies of selected formulation F6.
| Time | Before stability studies | %CDR at 4° ± 1°C | %CDR at 25° ± 2°C and 60% Rh ± 5% RH | %CDR at 37° ± 2°C and 65% Rh ± 5% RH |
|---|---|---|---|---|
| 1 | 23.37 | 24.17 | 24.67 | 24.44 |
| 2 | 28.54 | 29.04 | 30.14 | 29.64 |
| 3 | 31.78 | 32.48 | 33.17 | 32.88 |
| 4 | 37.91 | 38.11 | 38.91 | 39.12 |
| 6 | 45.36 | 45.89 | 46.26 | 46.43 |
| 8 | 61.35 | 62.25 | 63.05 | 63.35 |
| 10 | 68.80 | 69.28 | 69.80 | 68.80 |
| 12 | 73.90 | 74.60 | 74.90 | 75.00 |
| 16 | 83.14 | 83.94 | 84.14 | 84.37 |
| 20 | 90.31 | 91.51 | 92.31 | 91.97 |
| 24 | 98.10 | 98.54 | 98.92 | 98.69 |
Figure 17: In vitro drug release of F6 formulation before and after stability at 4°C.
Figure 18: In vitro drug release of F6 formulation before and after stability at 25°C.
Figure 19: In vitro drug release of F6 formulation before and after stability at 37°C.
Conclusion
The prepared curcumin-soluplus complexed showed increase in solubility. Preformulation studies on curcumin fairly validate with the described limits. The implemented method generated uniform and reproducible floating tablets with all the polymers used. The hardness, friability, weight variation, drug content, floating lag time, total floating time, swelling index and in vitro release were uniform and reproducible. Swelling index was found to be higher with HPMC K100M than HPMC KM 4 and HPMC K15M. FLT was found to be less than 40 sec at 16% NaHCO3 concentration for all formulations. All floating tablets gave complete drug release but F4 & F6 showed controlled drug release over a period of 20- 24 hrs as compared to all other formulations. FT-IR, DSC and XRD studies revealed the absence of any chemical interaction between drug and polymers used. In-Vivo floating study by x-ray study showed that the selected F6 formulation showed that tablet remained floated in the stomach of animal. Prepared matrix tablets were found to be stable with respect to Floating lag time, Total Floating time and in-vitro release. Hence, floating tablets of curcumin showed promising results.
REFERENCES
- Gupta N, Aggarwal N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin-“a technical note”. AAPS pharmscitech. 2008;9:810-813.
- https://simple.wikipedia.org/wiki/Gastritis
- Kilambi P, Hiral ND. Simultaneous determination of curcumin and berberine in their pure form and from the combined extracts of curcuma longa and berberis aristata. Int J Appl Sci Eng. 2010;8:19-26.
- Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J Nanotech. 2012.
- Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131:682-691.
- Ridhima D, Shweta P, Upendra K. Formulation and evaluation of floating microspheres of curcumin. Int J Pharm Pharm Sci. 2012;4:334-336.
- Preetha A, Ajaikumar BK, Robert AN, Bharat BA. Bioavailability of Curcumin: Problems and Promises. Mol Pharmaceutics. 2007;4:807-818.
- Zaveri M, Gajjar H, Kanaki N, Patel S. Preparation and evaluation of drug phospholipid complex for increasing transdermal penetration of phytoconstituents. Int J Inst Pharm Life sci. 2011;1:80-93.
- Jithan AV, Madhavi K, Madhavi M, Prabhakar K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Invest. 2011;1:119.
- Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597-6611.
- Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, et al. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403:285-291.
- Thomas R. Solubility Enhancement with BASF Pharma Polymers. 2011.
- Nayak A, Maji R, Das B. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res. 2010;3:1-10.
- Vedha H, Chaudhary J. The recent developments on gastric floating drug delivery system: An overview. Journal of Pharmaceutical Technology and Research. 2010;2:524-534.
- Mutalik S, Naha A, Usha AN, Ranjith AK, Musmade P, et al. Preparation, in vitro, preclinical and clinical evaluation of once daily sustained release tablet of aceclofenac. Arch Pharm Res. 2007;30:222-234.
- Lieberman HA, Lachman L, Schwartz JB. Pharmaceutical dosage forms: Tablets. 1990;2:201-243.
- Lakade SH, Bhalekar MR. Formulation and evaluation of sustained release matrix tablets of anti-anginal drug, influence of combination of hydrophobic and hydrophilic matrix former. Res J Pharm Tech. 2008;1:410-413.
- Aulton ME. Pharmaceutics: The science of dosage form design. Churchill livingstone. 2002.
- Gupta AK. Introduction to pharmaceutics. CBS Publications. 1993;1:270.
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Lea & Febiger. 1986; 286-300.
- Nerurkar J, Jun HW, Prince JC, Park MO. Controlled release matrix tablets of ibuprofen using cellulose ethers and carrageenans: effect of formulation factors on dissolution rate. Eur J Pharm Biopharm. 2005;61:56-68.
- Puneeth KP, Kavitha K, Tamizh MT. Development and evaluation of rosiglitazone maleate floating tablets. Int J Appl Pharm. 2010;2:6-10.
- Patel A, Modasiya M, Shah D, Patel V. Development and in vivo floating behavior of verapamil HCl intragastric floating tablets. Aaps Pharmscitech. 2009;10:310-315.
- Madhavi M, Madhavi K, Jithan AV. Preparation and in vivo/in vitro characterization of curcumin microsphere intended treat colon cancer. J Pharm Bioallied Sci. 2012;4:164-171.
- http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1150.html
Citation: Kishor AB, Basavaraj KN, Arindam BS, Teerapol S, Roopali MS (2020) Development and Evaluation of Curcumin Floating Tablets. Pharm Anal Acta 12:622. doi: 10.35248/2153-2435.20.11.622.
Copyright: © 2020 Kishor AB, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.