Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 4
Development and Evaluation of Aerodynamic Particle Size Distribution of Inhalable Spray Dried Rifampicin Loaded Steric Acid-Chitosan Nanomicellar Micro-Composites Dry Powder Inhaler by Qbd Approach with Dual Analysis by TSI/ACI Deposition Study
Ashwin J Mali*, Nirav J Javiya and Atmaram P PawarReceived: 18-Jul-2023, Manuscript No. PAA-23-22225; Editor assigned: 20-Jul-2023, Pre QC No. PAA-23-22225; Reviewed: 03-Aug-2023, QC No. PAA-23-22225; Revised: 10-Aug-2023, Manuscript No. PAA-23-22225; Published: 18-Aug-2023, DOI: 10.35248/2153-2435.23.14.741
Abstract
The present work deals with the development of spray-dried Rifampicin-Loaded Nanomicellar Micro Composites (RFP-NMC) by Quality by Design (QbD) approach. The RFP-NMC was prepared by oil in water emulsion solvent evaporation technique and optimized by the 3 full factorial designs. The stearic acid (X1) and chitosan (X2) were selected as Critical Quality Attributes (CQA’s) while particle size (Y1) and % drug content (Y2) as dependent factors. The data was represented by QbD Minitab software. The in vitro lung deposition of RFP-NMC was evaluated by Twin Stage Impinger (TSI) and by Andersen Cascade Impactor (ACI). Further, in vitro release was carried out by dialysis method in Simulated Lung Fluid (SLF) and cytotoxicity was assessed by MTT assay. The optimized formulation depicted a particle size of 5.85 µm ± 0.38 µm and drug content of 77.20% ± 1.04%. The infrared spectra, X-ray diffraction spectra and scanning electron microscopy revealed no significant structural alternations with the amorphous and spherical nature of the microcomposites. The in vitro dialysis study reflected 90% of drug release in 24 hours. The optimized formulation exhibited Fine Particle Fraction (FPF) and Mean Median Aerodynamic Diameter (MMAD) of 52.66% ± 1.23% and 2.96 µm ± 0.04 µm with optimum flow properties. The in vitro cytotoxicity study on A 549 cell line established a lack of toxicity of formulation. The present investigation has proved the excellent aerodynamic properties and deposition efficiency of RFP-NMC which further can be explored for enhanced lung delivery.
Keywords
Rifampicin; Quality by design; Fine particle fraction; Mass median aerodynamic diameter; Twin stage liquid impinger; Andersen cascade impactor
Introduction
Tuberculosis (TB) is deadly and infectious disease which has been reemerged as a major health concern in India. There has been an alarming increase in the drug resistance resulting in Multi- Drug Resistance (MDR), Extensively Drug Resistance (EDR) and Totally Drug Resistance (TDR). The prolonged treatment of these conditions results in a severe systemic toxicity. Globally World Health Organization (WHO) reported 4.1% of new cases of TB with estimated death of 2.5 lakhs annually. India is one of the high burden countries resulting about 47% of MDR/EDR/TDR-TB cases. The TB is more difficult to treat and requires drugs therapies which are costly and accountable for more side effects [1,2].
At present Fixed Dose Combination (FDC’s) of Isoniazid (75 mg) (BCS class I, III), Rifampicin (150 mg) BCS Class II, Pyrazinamide (400 mg) (BCS III) and Ethambutol (275 mg) (BCS III) are administered to the TB patients up to six months by oral route as a first line therapy. The second line drugs such as streptomycin, ofloxacin, levofloxacin etc. are preferred for the better therapeutic outcome in case of MDR-TB. The number of cases of MDR-TB was reported due to noncompliance and adverse drug effects of respective drugs. Moreover, the failure of oral chemotherapy of anti TB drugs mainly attributed with number of drawbacks such as first pass metabolism, high doses and sub therapeutic concentration of drugs at macrophages, prolonged and severe toxic effects [3-5].
Lung is the primary site of infection, so when anti TB agents are administered orally, very less fraction of drug reaches the lung. In this regard multi-particulate systems have shown a great promise to be novel means of delivering actives to the respiratory tract. A number of attempts have been made till now to prepare microspheres, micro particles, liposomes etc. for effective and enhanced delivery to the lungs [6,7]. But these systems are supported by various drawbacks such as high polymer consumption, lower drug entrapment, use of toxic substances and higher cost. Moreover, these particulate systems are rapidly taken up from the blood by mononuclear phagocytes such as kuffer cells in the liver. So there is a need for the development of novel drug delivery system. Pulmonary drug delivery comprising of polymeric micelles can be emerged as one of the finest modalities among multiparticulate systems in case of tuberculosis due to their characteristics such as high drug loading, selective accumulation at the target site, enhanced permeability and retention effect, controlled release of drug and ability to invade the alveolar macrophages [8-10].
Rifampicin (RFP) is one of the most recognized drugs used in TB and it is an inseparable part of fixed drug combination therapy in TB patients. Despite being such an effective and efficacious molecule it has certain drawbacks like high dose of 300 mg to 600 mg which results in systemic adverse effects and toxicity [3,11,12]. Moreover, the failure of antitubercular chemotherapy is mainly attributed to prolonged duration of 6 to 9 months and complex nature of therapy resulting in non-compliance of patients thus MDR-TB becomes an imminent threat to the patient.
Previous work with RFP for lung targeting has been reported on PLGA nanoparticles, mannitol microspheres, lactose microparticles, chitosan microparticles, liposomes etc. [3,13-15]. Notably, there was not much research has been carried out on polymeric micelles for the purpose of effective delivery to lungs. The novel RFP loaded Nanomicellar Composites (RFP-NMC) carrier system with better; safer, less toxic, shorter and cheaper regimen is therefore useful to reduce patient suffering and mortality [16-18]. Moreover, to overcome the issues of MDR/EDR/TDR-TB, the RFP-NMC having a site specific release will be helpful to resolve the toxicity issues of RFP by maintaining high payloads of RFP too deep in the lung for a prolonged period.
To overcome these constraints the attempt has taken to produce RFP-NMC for dry powder inhaler. In the current investigation, RFP-NMC were prepared by solvent evaporation method and were spray dried using mannitol as carrier to obtain dry powder for inhalation. The obtained spray dried powder formulations containing RFP-NMC was characterized in terms of particle size, entrapment efficiency, drug content, flow property, Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Powder Diffraction (XRPD) and in vitro drug release. The spray dried powder was characterized to confirm the possessed desirable physical and aerodynamic properties suitable for deposition in the lungs. The in vitro lung deposition was checked by using Twin Stage Impinger (TSI) and Andersen Cascade Impactor (ACI) to analyze the MMAD, GSD and FPF. Further, in vitro cytotoxicity study was assessed on A 549 lung carcinoma cell line [19-21].
Materials and Methods
Materials
Rifampicin was obtained as a gift sample from Lupin Limited, Pune, and Maharashtra. Stearic acid was purchased from Sigma Aldrich Chemicals Inc, Bangalore, India. Chitosan was obtained from Marine Chemicals, Cochin, and India. Mannitol was procured from Loba Chemie Pvt. Ltd, Mumbai, Maharashtra. The dialysis bag (Molecular weight cut off 12,000) was purchased from Sigma Aldrich Chemicals Inc, Bangalore, India. The Methanol, Potassium dihydrogen phosphate, Sodium Dihydrogen Phosphate (GR grade) obtained from Merck Chemicals Ltd, Mumbai, India. Other chemicals were of analytical grade. Distilled water was used throughout the study.
Preparation of rifampicin loaded nanomicellar composites
The oil in water emulsion solvent evaporation method was used to prepare RFP-NMC. The stearic acid and low molecular weight chitosan were chosen as hydrophobic and hydrophilic polymers respectively. The RFP and stearic acid were dissolved in methanol while chitosan was dissolved in distilled water containing 1% acetic acid. Both solutions were allowed to stir for 30 minutes to solubilizing polymer and drug. Then organic solution was added drop wise to the aqueous portion by syringe. The later micellar solution was allowed to stir overnight to evaporate methanol. Further, formulation was sonicated on high intensity probe sonicator for 1 minute to reduce the size of polymeric micelles.
Critical micellar concentration determination
For the determination of Critical Micellar Concentration (CMC) conductivity measurements were carried out at 25 ± 0.1°C by adding 0.4 g/100 cm3 chitosan and stearic acid which were added to the 50 mL of chitosan solution of the same concentration. The solution or blend was stirred using magnetic stirrer after addition of every portion of stearic acid until the steady value of conductivity was achieved. The conductometer (Make-E1, model 601) was used to measure a specific conductance of solutions and blends.
Experimental design
A 32 full factorial design was applied for optimization of RFP-NMC. The Critical Quality Attributes (CQA’s) such as concentration of stearic acid (X1) and chitosan (X2) were evaluated at 3 levels. The particle size (Y1) and drug content (Y2) were selected as dependent variables. The experimental trials were performed at all possible combinations. The statistical model incorporating interactive and polynomial terms was used to evaluate the responses. The following polynomial equation was obtained
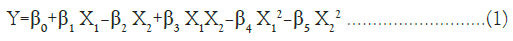
Where, Y is dependent variable, β0 is arithmetic mean response and β1-β5 is estimated coefficients for the factors X1 and X2.
Characterization of polymeric micelles
Particle size analysis by laser diffraction: The particle size analysis was performed by using laser diffraction technique (Malvern 2000 SM, Instruments, UK). The particle size measurements were carried out at 90°scattering angle. The sample of polymeric micelles dispersed in distilled water and the average particle size was determined.
Drug content and entrapment efficiency: The Drug Content (DC) of RFP-NMC was determined by UV-visible spectrophotometer at λmax of 473 nm by using Jasco UV spectrophotometer. The DC was measured by dissolving 1 mL of micellar solution in 10 mL of methanol and then the content was analyzed spectrophotometrically by using the following formula:
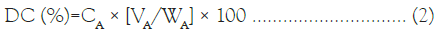
Where, CA is the total concentration of RFP loaded polymeric micelles WA is the theoretical amount of RFP, VA is the volume of polymeric micelles
For determining the Entrapment Efficiency (EE), polymeric micelles were allowed to centrifuge at 25000 rpm for 20 minutes at 4°C. Then supernatant was separated and measured for unentrapped RFP. The EE was calculated from the following formula:
EE(%)=[(Total drug content-Free dissolved drug)/Drug amount used]×100 ………… (3)
Preparation of inhalable dry powder of RFP-NMC by spray drying: The mannitol (2% w/v) was added into micellar suspension as a cryprotectant. The resultant dispersion was spray dried using laboratory spray dryer (Model LU 20., Labultima, Mumbai, India) at following set of conditions: Inlet temperature 120°C, Outlet temperature 140°C, Nozzle air flow (1-3 bar), Feed rate 2.5 mL/ min, Atomization air pressure 1 kg/cm -3 kg/cm and passed through 0.7 mm nozzle at aspiration pressure of 70%-80%. The product was collected from cyclone and collecting vessel. The powders were stored in a desiccator at ambient temperature until used for further characterization. The conventional formulation of RFP was prepared by blending the inhalation grade lactose (Respitose SV003) with ball milled AGP (Retsch Ltd, United Kingdom, Model No CM 2000 PM) at 70% of critical velocity. The ratio of RFP to lactose was maintained 1:67.5% w/w. The prepared RFP-NMC and conventional form of RFP (C-RFP) dry powder was stored in a desiccator at ambient temperature until used for further characterization. The compatibility of the polymer and drug was tested prior to development of formulation.
Characterization of dry powder
Particle size analysis and drug content: The particle size distribution was measured with a laser diffractometer (Mastersizer, Malvern instruments, UK) in dry powder form after dispersing with compressed air. The approximately 100 mg of powder was used to achieve the required obscuration. The size was expressed as volume weighted mean particle size.
Measurement of flow properties: The bulk density of the spray dried RFP-NMC and C-RFP of dry powder was determined by pouring known mass of powder (approximately 0.5 g) under gravity into a calibrated 10 mL measuring cylinder and volume occupied by the powder was recorded. The tapped density was measured using a tap density tester (ELECTROLAB, tap density tester, USP). The measurement was performed in triplicate (n=3). The Carr’s index and Hausner’s ratio were calculated accordingly. For measuring the angle of repose, powders were poured through a funnel to form a cone shaped pile which had an angle of θ to the horizontal. The radius (r) and height (h) of pile was measured and angle of repose was calculated based on formula:
θ=tan-1 (h/r) …………….. (4)
In vitro aerosol performance by twin stage liquid impinger: The deposition of RFP-NMC and C-NMC formulation was determined using a Twin-Stage Liquid Impinger (TSLI; Apparatus A, European Pharmacopoeia, 2000, Westech instruments, UK). The obtained 25 mg of powder equivalent to 2 mg of RFP was encapsulated in Hydroxyl Propyl Methyl Cellulose (HPMC) stick free capsule ≠ 3. The aerosolization was carried out at flow rate of 60 ± 5 L/min having 7 mL and 30 mL of methanol in stage 1 and 2 of TSLI respectively. Each stage was rinsed with methanol and DC was determined by the UV spectrophotometry method after appropriate dilution. The Rotahaler with filled capsule to be tested was placed into a rubber mouthpiece attached to the throat of the TSLI and the pump was switched on. The capsule was released by operating the inhalation device and the pump was allowed to run for another 5 second which allowed the aspiration of 5 L of air in the apparatus as recommended by the European Pharmacopoeia (2000). Each section (inhaler, capsule shell, stages 1 and 2) was rinsed with methanol. The rinsed solvent was collected and diluted to an appropriate volume. The RFP content was determined by UV spectrophotometer at 473 nm (Jasco-v-530). The formulation having the highest RF was chosen for the further deposition studies using an ACI.
Fourier transforms infrared spectroscopy: The Fourier Transforms Infrared Spectroscopy (FTIR) spectra of RFP, RFP-NMC, stearic acid, chitosan and mannitol were checked. The spectras were recorded after appropriate background subtraction using FTIR spectrometer equipped with a diffuse reflectance accessory (DRS- 8000, Shimadzu Corporation, Japan) and a data station. About 2 mg-3 mg of the sample was mixed with 100 mg of dry potassium bromide and the samples were scanned from 4000 cm-1-400 cm-1 wave numbers at a resolution of 2 cm-1. The characteristic peaks were recorded using the software FTIR 8000 SCS.
X-ray diffraction study: The RFP-NMC, pure RFP, physical mixture and mannitol were studied by X-Ray Diffraction (XRD) using X-ray diffractometer (PW 1729; Philips, The Netherlands). The samples were irradiated with monochromatized Cu Kα radiation (1.542Å).
Scanning electron microscopy: The surface morphology of RFP-NMC and C-RFP was determined by Scanning Electron Microscopy (SEM) (JEOL JSM-6360A). The samples were mounted on a double faced adhesive tape and sputtered with thin palladium layer by sputter coated unit (VG-Microtech, Uckfield, East Sussex, and UK) and surface topography was analyzed.
In vitro drug release study: The in vitro release of RFP-NMC and pure RFP was carried out in SLF using dialysis bag diffusion technique. The calibration curve of RFP in SLF was carried out by dissolving RFP in methanolic stock solution. The formulation equivalent to 2 mg of RFP added into the dialysis bag (cellulose membrane, mw cut off 12,000 Da) which was hermetically sealed and immersed into 100 mL of dissolution medium. The entire system was kept at 37 ± 0.5°C with continuous magnetic stirring at 100 rpm/min. At selected time interval, sample was removed and replaced with fresh medium in order to maintain sink condition. The sample was analyzed by UV spectrophotometer at 473 nm. The RES release mechanism from RFP-NMC and C-RFP formulation was analyzed by different kinetics models such as zero-order release Mt/M∞=k0t, first-order release Mt/M∞=1–exp (–k1t) and Higuchi Mt/M∞= kHt1/2 equation. The data analysis was performed using PCP Disso software (V3; Poona College of Pharmacy, Pune, India). The results of triplicate measurements and their means were reported.
In vitro deposition study using Anderson cascade impactor: An aerodynamic characteristic of RFP–NMC formulation was studied with an eight stage, nonviable Anderson Cascade Impactor (ACI) (Westech Private Instruments, UK, and Model Number: WP- ACISS-0289). The formulation equivalent to 2 mg of RFP was filled into Hydroxyl Propyl Methyl Cellulose (HPMC) stick free capsule ≠ 3. The filled capsule was placed in the Rotahaler as delivery device. The device was fitted into moulded rubber mouthpiece attached to the induction port of cascade impactor. The collecting plates were coated with 1% of HPMC solution and dried to avoid bouncing effect of formulation. The runs were conducted at a flow rate of 60 L/min for 4 seconds after stabilizing the assembly. The capsule shell was removed from inhaler device. The extra four capsules were actuated in the same manner. The cut-off particle aerodynamic diameters at 60 L/min for each stage were as follow: pre-separator (8.6 μm), stage 0 (6.5 μm), stage 1 (4.4 μm) stage 2 (3.3 μm), stage 3 (2.0 μm), stage 4 (1.1 μm), stage 5 (0.54 μm) and stage 6 (0.25 μm).
After dosing, collected plates were washed with 10 mL of methanol. The collected solution was sonicated in a bath-type sonicator for 15 min. The obtained solution was centrifuged at 25,000 rpm for 30 min and RFP content in the supernatant was determined spectrophotometrically at 473 nm in order to find out RFP deposition on each stage of the impactor. Each stage of the impactor was rinsed individually for adequate time and the solution was made up to fixed volume. The Recovered Dose (RD), Emitted Dose (ED) and Fine Particle Dose (FPD) were calculated. The MMAD was calculated as the 50th percentile of aerodynamic particle size distribution by mass. The GSD was calculated as square root of ratio of particle size at 84.13th percentile to the 15.87th percentile. Both MMAD and GSD were determined from the linear region of plot of cumulative mass distribution as a function of logarithm of aerodynamic diameter [6,7].
In vitro cell viability study: The in vitro cell viability was evaluated for RFP loaded powder of nanomicellar composites against alveolar epithelial cancer cell line A549 (obtained from NCCS, Pune, and Maharashtra) using MTT assay. The results were compared with free RFP and formulation excipients. The cells were cultured in DMEM/F12 medium, supplemented with 10% v/v fetal bovine serum and 2 mM L-glutamine. The medium was maintained at humidity atmosphere less than 5% carbon dioxide at 37°C. The trypsin-EDTA solution was used for sub culturing and cell isolation. The cells were harvested on the 4th day of subculture. The cells were seeded at the density of 5 × 103 cells per well and grown in 96- well tissue culture plates in a final volume of 150 μL in humidified atmosphere for 48 hours. Each formulation was dispersed in water and tested in varying RFP concentration over the range of 15 μM to 1000 μM. After 24 hours incubation, 10 μL of MTT labeling agent (5 mg/mL in PBS) was added, incubated for further 4 hours in humidified condition. After incubation, 100 μL of solubilizing solution (10% SDS in 0.01 M HCl) was added to each well. The plate was incubated overnight. The optical density was measured at 570 nm with a reference wavelength at 630 nm using an ELISA reader. The cell viability was calculated using following equation:
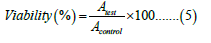
Where Atest is the absorbance of the test solutions and Acontrol is the absorbance of control (PBS).
Statistical methods: All the experiments were performed in triplicates. Data were recorded as mean ± standard deviation. Statistical analysis was carried out using online Quick Calcs Graph Pad software. The data analyzed in following manner; *p ≤ 0.1(not statistical significant), **p ≤ 0.01 (statistical significant), ***p ≤ 0.001 (very statistical significant) and **** p ≤ 0.0001 (extremely statistical significant).
Results and Discussion
TB, a deadly and infectious disease which has been reemerged as a major health concern is associated with MDR, EDR and TDR TB. Further, the prolonged treatment in these conditions results in a severe systemic toxicity. RFP is one of the most recognized first line drugs used in TB and it is an inseparable part of fixed drug combination therapy. The high dose of 300 mg to 600 mg of RFP results in systemic adverse effects and toxicity [3,21]. Moreover, oral antitubercular chemotherapy is mainly attributed to prolonged duration of 6 to 9 months and complex nature of therapy resulting in non-compliance of patients resulting MDR-TB. By considering these facts, the study was designed to develop spray dried RFP- NMC and C-RFP formulation for DPI in order to achieve higher concentration of RFP in the lung resulting enhanced antitubercular activity. The oil in water solvent evaporation was preferred due to simplicity and ability to produce nano size range carriers. Polymeric micelles were composed of stearic acid, chitosan wherein RFP was encapsulated in the core of micelles and shell was composed by chitosan. Further, the micron size composites of nanomicelles were prepared by the spray drying of the micellar formulation.
Critical micellar concentration determination
Conductometric measurements are widely used for CMC determination [22–24]. The specific conductivity of chitosan was depicted in Figure 1, at constant concentration of stearic acid. The curve of specific conductance demonstrated in three regions. The first break point was at the end of the first region which was below CMC. This is the indication of beginning of polymer-polymer association depicting critical aggregation concentration (CAC). The second break point which was appeared after second region over CMC represents the Polymer Saturation Point (PSP) [25,26]. The results obtained by determination of specific conductance of stearic acid-chitosan blends indicated existence of interactions of respective polymers. The CMC for chitosan was found to be 0.16 g/cm3 at constant concentration of stearic acid. The change in slope is the result of interaction between two components. The more significant changes occurred for 0.12 g/100 cm3 of stearic acid after CAC. The significant changes in behavior of specific conductance of chitosan at CMC might be because stearic acid molecules entrained into chitosan network was slower which was reflected in specific conductance.
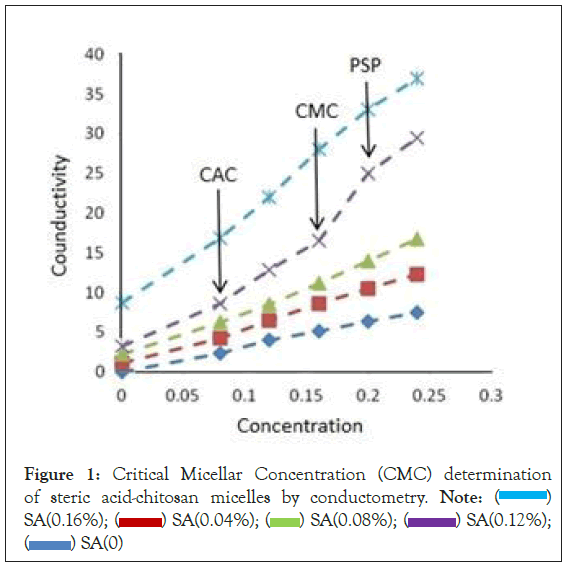
Figure 1: Critical Micellar Concentration (CMC) determination of steric acid-chitosan micelles by conductometry. Note:  SA(0.16%);
SA(0.16%);  SA(0.12%);
SA(0.12%);  SA(0)
SA(0)
Optimization of design
The effect of formulation variables such as X1 and X2 were studied using statistical optimization by applying 32 full factorial design [27,28]. Total 9 batches were proposed by the design which varied at 3 different levels (High, Medium and Low). The Y1 was obtained in between 0.73 ± 0.17 nm-1.01 ± 0.04 nm and Y2 was between 54.88% ± 1.15%-77.20% ± 2.47%. The data indicated significant effect of X1 on Y1 and Y2 . The polynomial equations can be used to draw conclusions after considering the magnitude of coefficient and mathematical sign. The multiple regression analysis for mean particle size of factorial batches revealed the good fit depicting R2=0.89. The positive coefficient of X1 influencing the Y1 was given by the following equation:
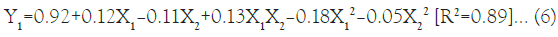
As per the model fitting equation, X1 has positive effect as compared to X2 while interaction term X1X2 also indicated positive effect which might be due to dominant effect of X1 on particle size over X2. A significant increase in the Y1 was observed with increase in the amount of X1 as depicted in the Figure 2, which might be the cause of ionic and lipophilic interactions of anionic stearic acid with the positively charged groups of RFP.
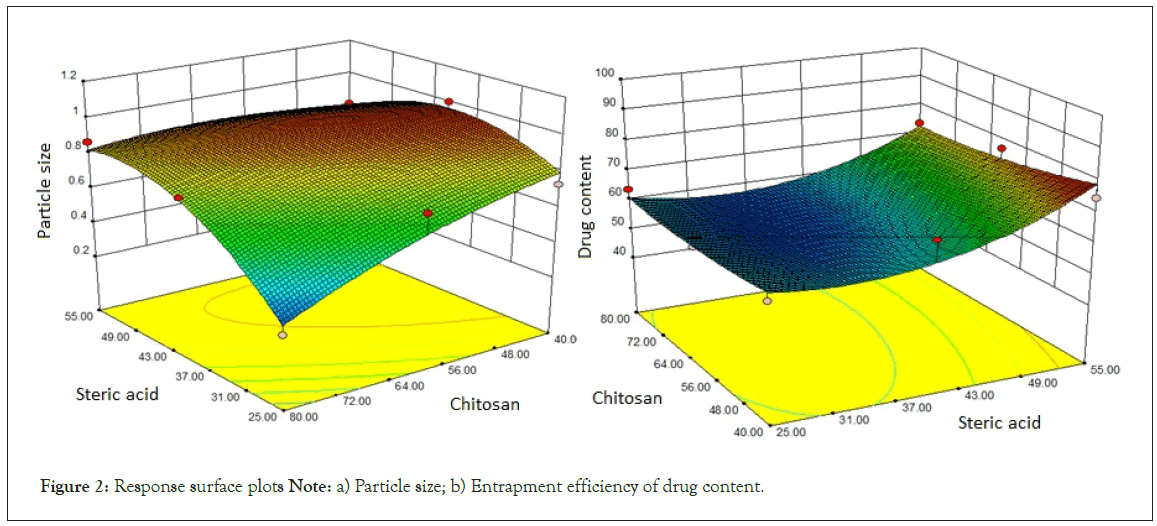
Figure 2: Response surface plots Note: a) Particle size; b) Entrapment efficiency of drug content.
The slight reduction in Y1 with rise in the amount of Y2 was observed due to cationic nature of X2 which may interfere with the interaction of RFP with Y1 by repulsion effect. The multiple regression analysis for the Y2 revealed good fit depicting R2=0.80. The positive coefficient of X1 variable influencing the Y2 was given by the following equation:
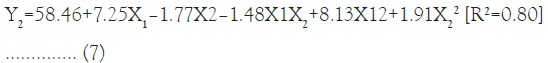
The response surface quadratic model was utilized to produce three dimensional response surface plots to analyze the influence of independent variables. The design-Expert 8.0.6.1 software (Stat-Ease Inc., USA) was used for generation and evaluation of the statistical experimental design. The matrix of coded design including investigated factors and responses is shown in Table 1. As per R2 value, multiple regression analysis revealed good fit for Y2. In the model fitting equation 7, X1 showed positive effect as compared to X2 and interaction term X1X2. The effect of X1 and X2 on the Y2 was depicted in Figure 2. The higher Y2 was observed with increased amount of X1 which may be due to ionic and lipophilic interactions. The %EE of the developed RFP-NMC was observed in the range of 21.03 ± 2.12 to 69.26 ± 5.10 which was parallel to the DC (Table 1).
| Formulation Number | Steric acid Chitosan X1, X2 | Micelles size Y1 (µm) | Drug content Y2 (%) | Dry NMC size (µm) | Entrapment efficiency (%) | Recovered Dose (%) | FPF (%) | Dispersibility (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0,-1 | 1.01 ± 0.04 | 69.15 ± 2.22 | 12.70 ± 0.87 | 21.03 ± 1.22 | 32.34 ± 2.09 | 44.16 ± 4.24 | 44.91 ± 2.96 |
| 2 | -1, 1 | 0.27 ± 0.03 | 63.68 ± 1.12 | 7.56 ± 0.76 | 21.17 ± 2.12 | 34.03 ± 2.09 | 49.25 ± 3.17 | 61.748 ± 2.3 |
| 3 | 1,-1 | 0.87 ± 0.02 | 74.66 ± 2.42 | 7.62 ± 1.21 | 58.24 ± 3.17 | 33.12 ± 3.39 | 38.04 ± 3.41 | 57.56 ± 4.13 |
| 4 | 0, 0 | 0.87 ± 0.01 | 55.21 ± 5.01 | 9.42 ± 0.81 | 37.41 ± 1.02 | 29.48 ± 1.28 | 34.87 ± 2.63 | 94.32 ± 4.34 |
| 5 | 0, 1 | 0.76 ± 0.03 | 54.88 ± 3.17 | 9.36 ± 1.85 | 54.88 ± 4.11 | 41.94 ± 1.67 | 50.55 ± 2.85 | 61.74 ± 3.27 |
| 6 | 1, 1 | 0.86 ± 0.05 | 73.51 ± 1.17 | 10.61 ± 2.65 | 36.95 ± 1.12 | 37.69 ± 4.03 | 43.38 ± 2.72 | 53.62 ± 5.62 |
| 7 | -1, 0 | 0.739 ± 0.03 | 59.24 ± 2.72 | 8.23 ± 1.33 | 47.04 ± 2.02 | 33.23 ± 2.30 | 28.47 ± 3.41 | 36.44 ± 4.5 |
| 8 | 1, 0 | 0.78 ± 0.07 | 77.20 ± 1.04 | 5.85 ± 0.38 | 35.08 ± 1.17 | 44.056 ± 3.47 | 52.66 ± 6.79 | 61.88 ± 2.44 |
| 9 | -1,-1 | 0.73 ± 0.17 | 58.92 ± 2.02 | 7.18 ± 0.90 | 69.26 ± 5.10 | 44.51 ± 4.65 | 34.4 ± 1.87 | 38.01 ± 1.88 |
Table 1: Matrix of 32 full factorial designs (1=Higher level, 0=Middle level, -1=Lower level) and evaluation of dry powder formulations on Twin stage liquid Impinger (Values=mean ± S.D, n=3)
Spray drying of polymeric micelles
Spray drying was utilized for preparation of microcomposites of nanomicelles for pulmonary administration. The technique has better control over size and shape of the composites. The method was preferred to get the spherical particles. This might be due to the higher shearing forces produced at the nozzle due to spray drying conditions depicting the significant effect of different concentrations of polymers on the particle size and drug content.
The response surface quadratic model was utilized to produce three dimensional response surface plots to analyze the influence of independent variables. The design-Expert 8.0.6.1 software (Stat-Ease Inc., USA) was used for generation and evaluation of the statistical experimental design. The matrix of coded design including investigated factors and responses is shown in Table 1. As per R2 value, multiple regression analysis revealed good fit for Y2. In the model fitting equation 7, X1 showed positive effect as compared to X2 and interaction term X1X2. The effect of X1 and X2 on the Y2 was depicted in Figure 2. The higher Y2 was observed with increased amount of X1 which may be due to ionic and lipophilic interactions. The %EE of the developed RFP-NMC was observed in the range of 21.03 ± 2.12 to 69.26 ± 5.10 which was parallel to the DC (Table 1).
Characteristics of dry powder
Particle size analysis of dry powder: Particle size analysis of each batch of dry powder was carried out and has been mentioned in Table 1. Particles volume weighted mean diameter d (0.9) was found to be in the range of 5 μm-13 μm. The F8 formulation has shown the lowest particle size of 5.853 μm ± 0.38 μm as shown in the Figure 3. The Particle size of 1 μm-5 μm is an ideal to achieve maximum lung deposition. The particles less than 1 μm are mostly exhaled resulting less deposition efficiency whereas the particles more than 5 μm are preferably deposited in the throat region. The developed RFP-NMC formulation was found to be within the limits of inhalable range. Further, the less span value of developed formulation was the indication of uniform and narrow size distribution of particles with no evidence of aggregation. The C-RFP dry powder formulation has shown enhanced particle size of 5.47 μm ± 1.89 μm. This was observed due to the drawback of micronization and blending process.
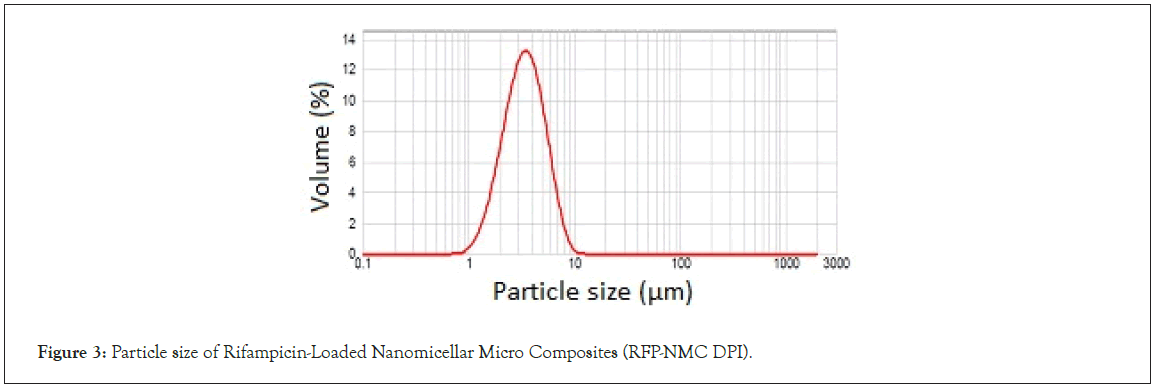
Figure 3: Particle size of Rifampicin-Loaded Nanomicellar Micro Composites (RFP-NMC DPI).
Flowability and packability: Spray drying produced fine and porous nature of composites of polymeric micelles which imparted lower density to the powder obtained from all the formulations. The flowability of all formulations was shown in Table 2. The bulk density was in the range of 0.25 ± 0.01 to 0.28 ± 0.04 g/cm3 while tapped density was found to be in the range of 0.31 ± 0.05 to 0.41 ± 0.03 g/cm3. The Carr’s index of all batches was in the range of 20.17 ± 1.17-40 ± 4.01. The angle of repose was observed in the range of 18.63 ± 0.61- 40.91 ± 3.36 and Hausner’s ratio has range between 1.25 ± 0.11 to 1.66 ± 0.24. The lowest tapped density was observed for F8 formulation [29,30].The aerolization of the formulation is mainly governed by the flow properties. The better angle of repose, Carr’s index and Hausner’s ratio of RFP-NMC would be one of the contributing factors for better aerosolization and lung deposition as compared to the C-RFP formulation.
| Formulation Number | Bulk Density (gm/cm3) | Tapped Density (gm/cm3) | Hausner’s Ratio | Carr’s Index | Angle of Repose [θ] |
|---|---|---|---|---|---|
| F1 | 0.25 ± 0.05 | 0.36 ± 0.09 | 1.43 ± 0.17 | 29.99 ± 0.97 | 33.15 ± 1.67 |
| F2 | 0.28 ± 0.04 | 0.35 ± 0.02 | 1.29 ± 0.12 | 22.21 ± 1.45 | 32.54 ± 1.27 |
| F3 | 0.27 ± 0.01 | 0.33 ± 0.04 | 1.33 ± 0.16 | 24.99 ± 2.34 | 18.63 ± 0.61 |
| F4 | 0.25 ± 0.02 | 0.35 ± 0.03 | 1.43 ± 0.14 | 29.99 ± 2.99 | 39.09 ± 2.89 |
| F5 | 0.25 ± 0.01 | 0.39 ± 0.06 | 1.53 ± 0.15 | 34.99 ± 3.22 | 36.57 ± 2.75 |
| F6 | 0.27 ± 0.05 | 0.38 ± 0.01 | 1.53 ± 0.22 | 34.99 ± 3.38 | 33.39 ± 3.14 |
| F7 | 0.26 ± 0.02 | 0.41 ± 0.03 | 1.66 ± 0.24 | 40.00 ± 4.01 | 40.91 ± 3.66 |
| F8 | 0.25 ± 0.06 | 0.31 ± 0.05 | 1.25 ± 0.11 | 20.17 ± 1.17 | 38.96 ± 2.44 |
| F9 | 0.26 ± 0.05 | 0.35 ± 0.02 | 1.32 ± 0.21 | 26.32 ± 2.40 | 22.99 ± 2.02 |
Table 2: Characterization of flow properties of all formulations
In vitro deposition study using twin stage impinger: The amount of RFP deposited on the second stage of impinger (effective cut-off diameter <6.4 μm) was considered as Fine Particle Dose (FPD). The Recovered Dose (RD) is the amount of RFP present on the stage 1 and stage 2 of the impinger, inhaler device and capsule shell. The Fine Particle Fraction (FPF) was the ratio of FPD to RD and was expressed in the percentage. The FPF for all the formulations ranges from 28.47% ± 3.41% to 52.66% ± 6.79%. As per the obtained results depicted in Table 1, RD and dispersibility for all the formulations ranges from 29.48% ± 1.28% to 44.56% ± 3.47% and 36.44% ± 4.50% to 61.748% ± 2.30%. The FPF for F8 was 52.66% ± 6.79%. The highest FPF of F8 can be attributed to the cumulative effect of spherical nature, less bulk density and good flow property of formulated DPI [31,32]. The F8 batch showed optimum EE, FPF, angle of repose, bulk density, tapped density, Carr’s index, Hausner’s ratio and percentage dispersibility which were subjected to further evaluation for physicochemical properties and lung deposition by ACI.
Zeta potential determination: The zeta potential of optimized batch F8 was measured for stability and charge distribution. The zeta potential was found to be +7.56 mV as shown in Figure 4. The positive charge was observed probably due to outer shell layer formed by cationic nature of X2 [29].
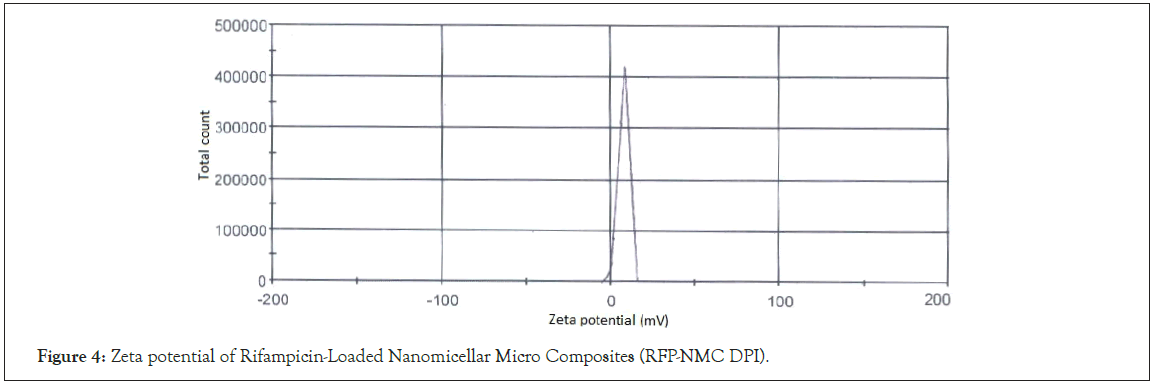
Figure 4: Zeta potential of Rifampicin-Loaded Nanomicellar Micro Composites (RFP-NMC DPI).
Fourier transforms infrared spectroscopy: The lower intensity characteristic peaks indicating the functional groups at 2930 cm-1 for –CH3 stretching and 1720 cm-1 for –C=O acetyl stretching were observed due to entrapment of RFP in the formulation. The prominent peak at around 950 cm-1-1000 cm-1 for mannitol was observed in the formulation spectra. The X1 and X2 has not depicted any characteristic peaks in spectra of formulation which indicated complete absence of chemical interaction between drug and polymers (Figure 5) [33].
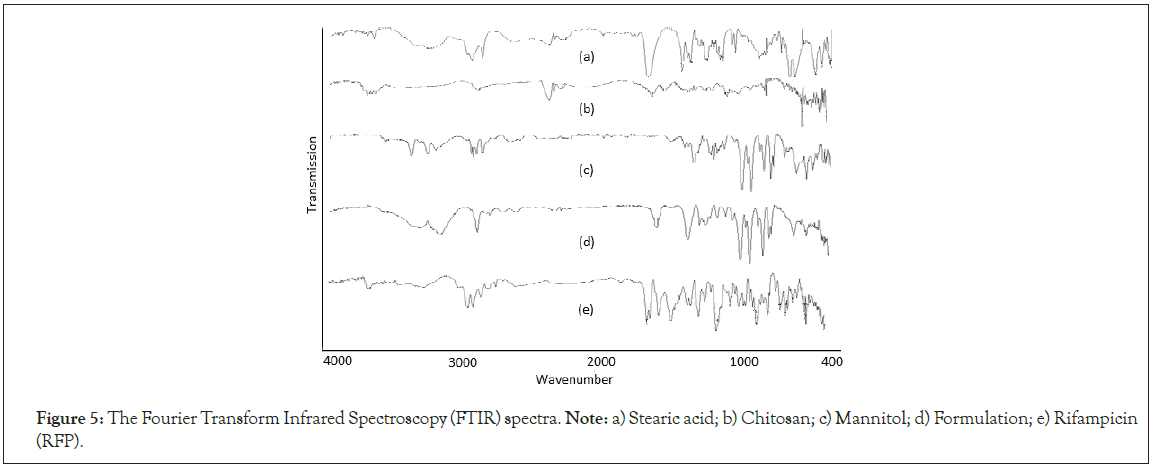
Figure 5: The Fourier Transform Infrared Spectroscopy (FTIR) spectra. Note: a) Stearic acid; b) Chitosan; c) Mannitol; d) Formulation; e) Rifampicin (RFP).
X-ray powder diffraction: The X-Ray Powder Diffraction (XRPD) patterns of mannitol, physical mixture, formulation and RFP are shown in Figure 6.The diffraction spectra of RFP exhibited sharp crystalline peaks in the range of 10-22° (θ values) which indicated the crystallinity of pure RFP. The formulation pattern showed less intense, broad and diffused peaks compared to pure drug reflecting amorphous nature of RFP. Moreover, the change in maximum peak intensity of RFP, physical mixture and formulation were (6866, 5206 and 3815 respectively) reflected an amorphous nature of formulation which may ultimately results in increased solubility (Figure 6) [9,34].
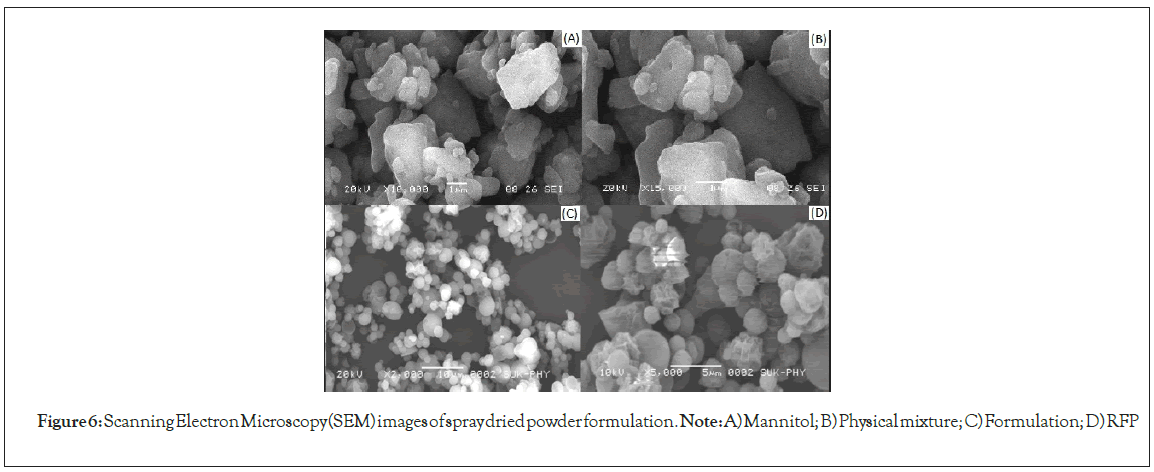
Figure 6: Scanning Electron Microscopy (SEM) images of spray dried powder formulation. Note: A) Mannitol; B) Physical mixture; C) Formulation; D) RFP
Scanning Electron Microscopy (SEM): The Scanning Electron Microscopy (SEM) revealed the spherical nature of the spray dried particles as depicted in Figure 7. The Nano-aggregates of polymeric micelles were prepared from spray drying and size was appeared to be within the range of 5.85 μm ± 0.384 μm to 12.70 μm ± 0.874 μm. The microcomposites were supposed to be deagglomerated in the airways of lung where micron size composites were converted in to RFP nanoparticles which would be further travel into deep lung regions (Figure 7).
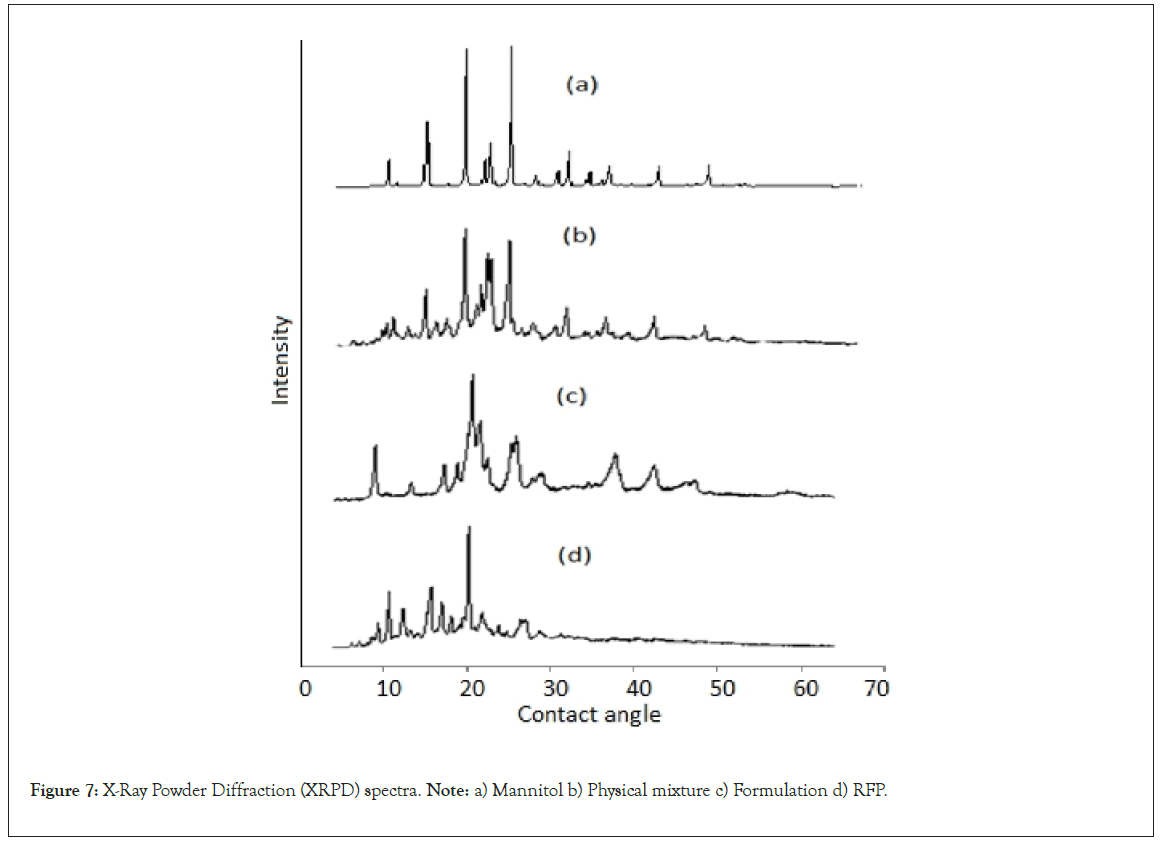
Figure 7: X-Ray Powder Diffraction (XRPD) spectra. Note: a) Mannitol b) Physical mixture c) Formulation d) RFP.
In vitro drug release study: The in-vitro drug release of RFP-NMC and pure RFP was carried out by dialysis technique using diffusion membrane. The Artificial Lysosomal Fluid (ALF) is one of the widely used simulated lung fluids and was used as dissolution medium. The ALF is analogous to the fluid with which inhaled particles would come into contact after phagocytosis by alveolar and interstitial macrophages in the lung. The in vitro release profile of the formulations F5, F8 and pure RPF illustrated 86.67%, 94.5% and 74.83% of RFP release at the end of 24 hours as depicted in Figure 7. The F8 has exhibited faster RFP release as compared to F5 batch which might be due to less amount of X2 which was resulted in less retardation of RFP. The kinetic model was applied to further evaluation of release pattern and higuchi model was found to be the best fitted for formulations F5, F8 and pure RFP based on the values of regression coefficient (R2) as depicted in Table 3. The values indicated a matrix type release by diffusion mechanism based on the principle of Fick’s law [35].
| Formulation Number | Zero order | First order | Higuchi model | Peppas model |
|---|---|---|---|---|
| Pure Drug | 0.90 ± 0.01 | 0.48 ± 0.07 | 0.97 ± 0.02 | 0.80 ± 0.03 |
| F5 | 0.92 ± 0.03 | 0.60 ± 0.02 | 0.94 ± 0.06 | 0.89 ± 0.02 |
| F8 | 0.73 ± 0.05 | 0.38 ± 0.05 | 0.95 ± 0.08 | 0.67 ± 0.05 |
Table 3: Kinetic modeling of in vitro drug release study
In vitro aerosol performance: The in vitro aerosol performance study of dry powder was assessed on TSLI and ACI. Deposition of all 9 batches was evaluated on TSLI as shown in Table 1 with excellent FPF of 52.66% ± 6.79%. The highest FPF was obtained for batch F8 due to lowest tapped density signifying the porous nature among all batches. Therefore, F8 batch was assessed for deposition efficiency on ACI. The in vitro aerodynamic drug deposition behavior of RFPNMC and RFP-DPI were tested using ACI assembly equipped with commercially available inhaler device Rotahaler (low resistance device). The MMAD and GSD were found to be 2.96 μm ± 0.04 μm and 2.04 μm ± 0.24 μm. The MMAD of RFP-NMS-DPI reflected the better deep lung deposition. It was indicated that the RFP deposition on Induction Port (IP) and Pre-Separator (PS) was less displaying a good deposition on the deep sizing part of the DPI [36]. In brief, the RFP-NMS formulation was able to give better aerolization and lung deposition as compared to the conventional form of DPI. The spray drying produced nanoparticle aggregates of polymeric micelles having spherical shape suitable for flow through airways. The uniform spherical shaped particles with an uneven surface may be the major contributing factor which results in the enhanced lung deposition. This characteristic shape of the NMC’s was useful to increase the trajectory and the in vitro aerodynamic efficacy to reach the deeper region of the lungs. Moreover, the SEM images indicated desired range of 1 μm-5 μm particle size [31]. Further, mannitol must have probably improved deposition and aerosolization efficiency as a carrier while porosity of dry powder was clearly reflected in flowability properties (Figure 8).

Figure 8: In vitro drug release profile of formulation F5, F8 and RFP. Note:  F8
F8
In vitro cytotoxicity assessment: In vitro cytotoxicity study was carried out on A 549 lung carcinoma cell line [37,38]. The equivalent 100 μg/mL was the concentration used for comparative study of RFP, optimized formulation and excipients [39]. The graph of optical density vs. concentration is presented in Figure 8. The optical density was the parameter used to evaluate the extent of cytotoxicity and further percentage viability was calculated for each concentration of sample. The optical density of zero conc was considered as 100% viability. The excipients and pure RFP didn’t express any toxicity while formulation demonstrated lesser percentage viability than pure drug probably because of the increase in the solubility of the RFP which may become effective to reduce the dose (Figure 9).

Figure 9: Percentage cell viability against alveolar epithelial cancer cell line A549 of formulation and excipients. Note:  ) Rifampicin pure;
) Rifampicin pure;  Citric acid
Citric acid
Conclusion
Based on experimental evidences, RFP-NMC ascertained a controlled and improved delivery towards the respiratory tract. The spray drying produced fine and porous microcomposites which can aid in the better delivery of drug. The zeta potential demonstrated a positive charge distribution around the micelles which may be advantageous in penetrating the alveolar barrier, increasing the deposition efficiency and uptake by macrophages. In the present work, effect of stearic acid and chitosan on the polymeric micelles was established by 32 full factorial designs. The XRD data reflected an increase in amorphous nature, while IR spectra concluded that group’s essential for the activity of drug remained unchanged in the formulation. In vitro drug release study showed 90% release up to 24 hours while no toxicity signs were observed for excipients on A549 cell line. The TSLI data revealed high respirable fractions while MMAD has displayed good aerosolization properties. These results concluded that optimized RFP-NMC dry powder formulation is suitable for inhalation in the therapy of tuberculosis.
Acknowledgements
We declare that this study was conducted by the authors named in this article. AJM: Data drafting and interpretation of the work. Designing and presentation of data. NJ: The execution of work and collection of data. APP: The idea generation, analysis and execution.
All authors read and approved the manuscript.
Sources of Funding
There is no any funding received for this performed work.
Conflict of Interest
The author declared no conflict of interest in the manuscript.
References
- Liu Y, Sung J, Prud’homme RK, Edwards DA. Nanoparticle of conjugated rifampicin for aerosol drug delivery and sustained release. PrincetonEdu. 2010.
- Farooq U, Ahmad T, Khan A, Sarwar R, Shafiq J, Raza Y, et al. Rifampicin conjugated silver nanoparticles: a new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int J Nanomed. 2019;29:3983-3993.
- Manca ML, Sinico C, Maccioni AM, Diez O, Fadda AM, Manconi M. Composition influence on pulmonary delivery of rifampicin liposomes. Pharmaceutics. 2012;4(4):590-606.
- Moreno-de-Viguri E. Design, synthesis and study of quinoxaline-2-carboxamide 1, 4-DI-N-Oxide derivatives as anti-tuberculosis agents .
- Pandey R, Khuller GK. Antitubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother. 2005;55(4):430-435.
- AKDAĞ ÇAYLI YA. Development of dry powder inhaler formulations for drug delivery systems. J Res Pharm. 2019;23(6).
- Chishti N, Dehghan MH. Nano-embedded microparticles based dry powder inhaler for lung cancer treatment. J. Res. Pharm. 2020;24:425-435.
- Naikwade S, Bajaj A. Preparation and in vitro evaluation of budesonide spray dried microparticles for pulmonary delivery. Sci Pharm. 2009;77(2):419-42.
- Mali AJ, Pawar AP, Purohit RN. Development of budesonide loaded biopolymer based dry powder inhaler: optimization, in vitro deposition, and cytotoxicity study. J Pharm. 2014.
- Telli GÖ, Tel BA, Büyükafsar K, Gümüşel BÜ. Effects of GPER-1 receptor activation on the reactivity of pulmonary vascular bed and its possible protective role on ischemia/reperfusion. Marmara Pharm J. 2018;22(3).
- Tukulula M, Hayeshi R, Fonteh P, Meyer D, Ndamase A, Madziva MT, et al. Curdlan-conjugated PLGA nanoparticles possess macrophage stimulant activity and drug delivery capabilities. Pharm Res. 2015;32:2713-2726.
[Crossref] [Google Scholar] [PubMed]
- Stoimenovski J, MacFarlane DR, Bica K, Rogers RD. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm Res. 2010;27:521-526.
- Ober CA, Kalombo L, Swai H, Gupta RB. Preparation of rifampicin/lactose microparticle composites by a supercritical antisolvent-drug excipient mixing technique for inhalation delivery. Powder Technol. 2013;236:132-138.
- Ohashi K, Kabasawa T, Ozeki T, Okada H. One-step preparation of rifampicin/poly (lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosis. J Control Release. 2009;135(1):19-24.
- Singh DJ, Jain RR, Soni PS, Abdul S, Darshana H, Gaikwad RV, et al. Preparation and evaluation of surface modified lactose particles for improved performance of fluticasone propionate dry powder inhaler. Indian J Nov Drug Deliv. 2015;28(4):254-267.
- Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009 ;376(1-2):176-185.
- Gill KK, Nazzal S, Kaddoumi A. Paclitaxel loaded PEG5000–DSPE micelles as pulmonary delivery platform: Formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur J Pharm Biopharm. 2011 ;79(2):276-84.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Liu D, Wang L, Zhang J, Zhang N. Docetaxel-loaded pluronic p123 polymeric micelles. Int J Mol Sci. 2011;2:1684–1696.
[Crossref] [Google Scholar] [PubMed]
- Mehta P, Bothiraja C, Kadam S, Pawar A. Potential of dry powder inhalers for tuberculosis therapy: Facts, fidelity and future. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S791-S806.
[Crossref] [Google Scholar] [PubMed]
- Viswanathan V, Pharande R, Bannalikar A, Gupta P, Gupta U, Mukne A. Inhalable liposomes of Glycyrrhiza glabra extract for use in tuberculosis: formulation, in vitro characterization, in vivo lung deposition, and in vivo pharmacodynamic studies. Drug Dev Ind Pharm. 2019;45(1):11-20.
[Crossref] [Google Scholar] [PubMed]
- Mehta P, Bothiraja C, Kadam S, Pawar A. Potential of dry powder inhalers for tuberculosis therapy: Facts, fidelity and future. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S791-S806.
[Crossref] [Google Scholar] [PubMed]
- Jadhav P, Bothiraja C, Pawar A. Methotrexate-loaded nanomixed micelles: Formulation, characterization, bioavailability, safety, and in vitro anticancer study. J Pharm Innov. 2018;13:213-225.
- Bothiraja C, Kapare HS, Pawar AP, Shaikh KS. Development of plumbagin-loaded phospholipid–Tween® 80 mixed micelles: Formulation, optimization, effect on breast cancer cells and human blood/serum compatibility testing. Ther Deliv. 2013;4(10):1247-1259.
[Crossref] [Google Scholar] [PubMed]
- Jeong YI, Kim DH, Chung CW, Yoo JJ, Choi KH, Kim CH, et al. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly (DL-lactide-co-glycolide) copolymer. Int J Nanomedicine. 2011:1415-1427.
[Crossref] [Google Scholar] [PubMed]
- Krstonošić V, Dokić L, Milanović J. Micellar properties of OSA starch and interaction with xanthan gum in aqueous solution. Food Hydrocoll. 2011;25(3):361-367.
- Kharia AA, Hiremath SN, Singhai AK, Omray LK, Jain SK. Design and optimization of floating drug delivery system of acyclovir. Der Pharm Lett. 2010;72(5):599.
- Shah V, Sharma M, Gandhi K, Suthar V, Parikh RK. Quality by Design (QbD) approach for optimization of microemulsion based topical gel. Marmara Pharmaceutical Journal. 2016;20(3):415-424.
- Makarand G, Gambhire V, Mangesh B. Development of rifampicin nanoparticles by 32 factorial design. Int J Pharm Sci Nanotechnol. 2010;3(3):1085-1091.
- Thomas D, Nair VV, Latha MS, Thomas KK. Theoretical and experimental studies on theophylline release from hydrophilic alginate nanoparticles. Futur J Pharm Sci. 2019;5:1-7.
- Wang L, Zhang Y, Tang X. Characterization of a new inhalable thymopentin formulation. Int J Pharm. 2009;375(1-2):1-7.
[Crossref] [Google Scholar] [PubMed]
- Draper B, Yee WL, Pedrana A, Kyi KP, Qureshi H, Htay H, et al. Reducing liver disease-related deaths in the Asia-Pacific: The important role of decentralised and non-specialist led hepatitis C treatment for cirrhotic patients. Eur J Pharm Sci. 2022;20.
[Crossref] [Google Scholar] [PubMed]
- Bothiraja C, Pawar AP, Mali AJ, Shaikh KS. Improved pharmaceutical properties of surface modified bioactive plumbagin crystals. Int J Surf Sci Eng. 2013;7(2):181-195.
- Sudhakar TD, Rajan MR, Srinivas KN, Prabu RR, Narmadha TV, Krishnan MM. Modeling and simulation of distribution network with the integration of distribution generator using Matlab. Int J Pharm Sci Nanotechnol. 2016 ;9(12):1-7.
- Mali AJ, Bothiraja C, Purohit RN, Pawar AP. In vitro and in vivo performance of novel spray dried andrographolide loaded scleroglucan based formulation for dry powder inhaler. Curr Drug Deliv. 2017;14(7):968-980.
[Crossref] [Google Scholar] [PubMed]
- Mali AJ, Pawar AP, Bothiraja C. Improved lung delivery of budesonide from biopolymer based dry powder inhaler through natural inhalation of rat. J Nanosci Nanotechnol. 2014 ;29(6):350-357.
- Naikwade SR, Bajaj AN, Gurav P, Gatne MM, Singh Soni P. Development of budesonide microparticles using spray-drying technology for pulmonary administration: Design, characterization, in vitro evaluation, and in vivo efficacy study. Pharm Sci Tech. 2009 ;10:993-1012.
[Crossref] [Google Scholar] [PubMed]
- Jayan H, Leena MM, Sundari SS, Moses JA, Anandharamakrishnan C. Improvement of bioavailability for resveratrol through encapsulation in zein using electrospraying technique. J Funct Foods. 2019 ;57:417-424.
- Vicente E, Pérez-Silanes S, Lima LM, Ancizu S, Burguete A, Solano B, et al. Selective activity against Mycobacterium tuberculosis of new quinoxaline 1, 4-di-N-oxides. Bioorganic Med Chem. 2009;17(1):385-389.
[Crossref] [Google Scholar] [PubMed]
- Mali AJ, Pawar AP, Purohit RN. Development of budesonide loaded biopolymer based dry powder inhaler: optimization, in vitro deposition, and cytotoxicity study. J Pharm. 2014;1–12.
[Crossref] [Google Scholar] [PubMed]
Citation: Mali AJ, Javiya NJ, Pawar AP (2023) Development and Evaluation of Aerodynamic Particle Size Distribution of Inhalable Spray Dried Rifampicin Loaded Steric Acid-Chitosan Nanomicellar Micro-Composites Dry Powder Inhaler by Qbd Approach with Dual Analysis by TSI/ACI Deposition Study. Pharm Anal Acta. 14:741.
Copyright: © 2023 Mali AJ, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


