PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 10, Issue 2
Delta-Globin Gene Mutations Complicate the Diagnosis of ò-Thalassemia
Hassan S1*, Ahmad R1, Esa E1, Yusoff YM1, Sahid ENM1, Aziz NA1, Hamid FSA1, Omar SL1, Bidin MB1, Hamid AH1, Zakaria Z1 and Mokhri NM22Department of Pathology, Queen Elizabeth Hospital, 13A, Jalan Penampang, Kota Kinabalu, Sabah, Malaysia
Received: 17-Dec-2018 Published: 08-Mar-2019
Abstract
Increased Hemoglobin A2 (HbA2) levels are used as invaluable markers for the detection of beta-thalassemia (β-thalassemia) carriers. However, a concomitant delta-globin gene (HBD) mutation reduces the HbA2 level resulting in the confusion of the β-thalassemia status.
Objectives: We sought to identify HBD mutations leading to low HbA2 level β-thalassemia carriers receiving the molecular diagnosis at the Institute for Medical Research (IMR), Malaysia.
Methods: Thirty-seven β-thalassemia carriers were ruled out of alpha-thalassemia (α-thalassemia) and underwent HBD genotyping by Sanger sequencing.
Results: Twenty-two β-thalassemia carriers with HBD mutations were identified. The most common mutations were HbA2-Indonesia and HbA2-Deventer. The HbA2-Deventer was found in ethnics from Sabah. Two new δ-globin mutations, Cap +48 (A>T) and HbA2-Shah Alam were identified among the Malays. Altogether, we identified seven δ-globin gene mutations. Relying upon HbA2 levels for β-thalassemia carrier status prediction is risky. A misdiagnosis could occur when β-thalassemia carrier interacts with delta-thalassemia (δ-thalassemia) lacking additional HbA2 fraction, leading to normal or borderline HbA2. Family screening and ethnicity are important to facilitate the accurate diagnosis of complicated cases.
Conclusions: Since δ-thalassemia modifies HbA2 levels, diagnosing borderline HbA2 β-thalassemia among Malaysians is vital. HBD mutations are heterogeneous and its deoxyribonucleic acid (DNA) analysis is important in our multi-ethnic populations.
Keywords
Reduced HbA2; δ-Globin gene mutation; Novel HbA2 variant; δ-thalassemia; HBD mutations; β-thalassemia; Haemoglobinopathies
Introduction
In normal adult life, hemoglobin (Hb) is predominated by hemoglobin A (HbA) comprising over 95% of total Hb. About 2.5%- 3.5% of the Hb content will comprise hemoglobin A2 (HbA2) with traces of hemoglobin F (HbF) of less than 1% [1]. In β-thalassemia, HbA2 levels are often increased (>3.5%). This characteristic and microcytosis are invaluable markers for the detection of β-thalassemia carriers.
HBD (MIM# 142000) is expressed at a low level of adult human hemoglobin. Like other globin genes, mutations that occur in the δ-globin gene can affect the structure or the expression level of the δ-globin chain, leading to reduced HbA2 level. Structural defects may produce a second stable and visible HbA2 fraction, whereas the unstable defects are undetectable by basic methods, and a DNA analysis is required. Although δ-globin mutations have no clinical effects, it reduces the HbA2 level of β-thalassemia carriers resulting in the confusion of the β-thalassemia status [2,3].
Molecular diagnosis in thalassemia prevention program and its utilization in the clinical decision has become a standard practice in patient management particularly in the region where the mutation frequency and heterogeneity are high. Multiple genes have contributed to the complex genotypic and phenotypic expression of thalassemia syndromes, thus molecular study is important for the diagnostic confirmation, treatment and counseling purposes.
Mutations within the δ-globin gene have never been investigated in Malaysia. However, we noted increasing cases of β-thalassemia carriers with reduced HbA2 levels. Here, we described the molecular characterization, frequency, and geographic distribution of δ-globin gene mutation and HbA2 variants. In addition, we report its necessity in our molecular diagnosis program for thalassemia syndrome in Malaysia.
Materials and Methods
Study subjects
Institute for Medical Research (IMR) is the national referral centre for molecular diagnostic of genetic diseases in Malaysia. Under the National Thalassemia Prevention and Control Program in Malaysia, Molecular Genetic Laboratory (MGM) in IMR provides DNA analysis for thalassemia syndromes and hemoglobinopathies. Samples were submitted for DNA analysis of thalassemia and variants when abnormal indices of Mean Cell Volume (MCV) <78 g/dL and/or Mean Corpuscular Hemoglobin (MCH) <27 pg is observed.
We retrospectively reviewed 10,485 subjects referred to our laboratory from 2011 to the second quarter of 2018 and selected 37 subjects for δ-globin gene sequencing. The selection criteria were β-thalassemia carriers that had low HbA2 level, with and without visible HbA2 fraction variant. The exclusion criteria included: (i) Mild β++-thalassemia with consistent borderline HbA2 comprising HBB:c.-50A>C [Cap+1 (A>C)], HBB:c.112A>G [Poly A (A>G)], HBB:c.110T>C [Poly A (T>C)], and Hb Malay [HBB:c.59A>G (Codon 19 A>G)], and (ii) concomitant α- and β-thalassemia that may influence the HbA2 levels.
Complete Blood Count (CBC) and Hb analysis were performed at the referring hospital laboratory using automated hematology analyzers. For Hb analysis, Bio-Rad Variant or Variant II highperformance liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA) or capillary electrophoresis (CE) instruments (Sebia SA, France) were used. Blood specimens were cold-shipped (2°C-8°C) in K3 etylenediaminetetraacetic acid (EDTA) tubes to MGM from the referring hospitals along with the request form, CBC, and Hb analysis results.
Molecular analysis
Genomic deoxyribonucleic acid (DNA) was extracted from whole blood using QIAamp® DNA Blood Midi Kit (Qiagen® GmbH, Hilden, Germany) or Maxwell® 16 LEV Blood DNA Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocols. Molecular diagnoses of α-, β-, and δ-globin genes were performed to determine the thalassemia status leading to such HbA2 levels. α-thalassemia screening panel comprised of seven deletions [4], and five-point mutations using multiplex gap-Polymerase Chain Reaction (PCR) and amplification refractory mutation system (ARMS) were performed for α-thalassemia genotyping [5].
For β-thalassemia genotyping, first-time screening was carried out using multiplex ARMS to detect 20 common point mutations [6] and multiplex gap-PCR for the detection of eight common β-cluster deletions [7]. Unknown β- and δ-globin genes were genotyped by sequencing. The β-globin gene was amplified using primers generating 2156 bp amplicon. The δ-globin gene was amplified using δ1F (5’-CTGAGTCAAGACACACATGACAG-3’) and δ2R (5’-TAATTTCTGCTCTTTGGAGGTAG-3’) primers generating 2005 bp amplicon [8]. Complementation test was performed to β°-FIL deletion/HbA2-Deventer carriers by direct sequencing. A forward primer (δ1F: 5’-CTGAGTCAAGACACACATGACAG-3’) [8] complementing reverse primers P5 (5’-CATTTAGCTCCCACACTCCT-3’) [9] and Filipino Fw (5’- CCTTGAAGCTGGGTAGTGTGA-3’) 4 were used to amplify the normal (3623 bp) and β°-FIL deletion (3890 bp) alleles, respectively.
All the amplification reactions were performed on an Eppendorf Mastercycler® (Eppendorf Scientific, Hamburg, Germany). Direct sequencing was performed on an Applied Biosystems® 3730 DNA Analyzer (Life Technologies™, Carlsbad, CA, USA). Direct sequencing data was analyzed using CLC Main Workbench (Qiagen® GmbH, Hilden, Germany). The pathogenicity of the new δ-globin variants was evaluated by using MutationTaster2 web-based software [10].
The samples were classified into three groups; Group 1 consists of β- with δ-thalassemia carriers, while Group 2 and 3 consists of β- with δ-variant carriers, and β-thalassemia carriers without HBD mutation, respectively. Distributions of hematological parameters of Hb, Red Blood Cell (RBC) Count, MCV, MCH, Red cell Distribution Width (RDW), and the Hb molecules of HbA2 and HbF from the High- Performance Liquid Chromatography (HPLC) were checked using the Anderson‐Darling test. A one-way between groups Analysis of Variance (ANOVA) was conducted to compare these parameters. All measurements were expressed as the mean (standard deviation, SD). A p‐value <0.05 was considered significant. For significantly different parameters, a two‐tailed unpaired t‐test was conducted to compare each groups. Analyses were performed using MaxStat Lite v3.6 (Jever, Germany).
Results
Twenty-two (59%) out of the 37 β-thalassemia carriers studied were positive for HBD mutations. We detected five known mutations, HbA2-Deventer [HBD:c.202G>A (p.Val68Met)(δcd 67 GTG>ATG)], HbA2-Indonesia [HBD:c.208G>C (p.Gly70Arg)(δcd 69 GGT>CGT)], HbA2-Walsgrave [HBD:c.157G>C (p.Asp53His)(δcd 52 GAT>CAT)], HbA2’ (Hb B2) [HBD:c.49G>C (p.Gly17Arg)(δcd 16 GGC>CGC)], and -30 (T>C) [HBD:c.-80T>C]; and two new mutations, 5’ UTR +48 (A>T) [HBD:c.-6 A>T] and [HBD:c.170G>A (p.Gly597Asp) (δcd56 GGC>GAC)].
They were classified into three groups. In Group 1, three HBD mutations were found. They were β-thalassemia carriers with a borderline HbA2 percentage lacking additional fraction typical of HBD variant carrier. The β°-FIL deletion with HbA2-Deventer was found in multiple ethnics from Sabah totaling to 12 cases involving six Dusun, two Kadazan, one Murut and Sungai, and two subjects that their ethnicity was not mentioned. The other two subjects were Chinese and Malay that had β°cd 41/42 (-TTCT) (HBB:c.126_129delCTTT) and δ -30 (T>C) mutations, and IVS 1-5 (G>C) (HBB:c.92+5G>C) with a new mutation, +48 (A>T), respectively (Supplementary Table, and Figure 1). Complementation test revealed the HbA2-Deventer carriers were presented in trans to the β°-FIL deletion. This ruled out any HbA2- Deventer among β°-FIL deletion homozygotes.
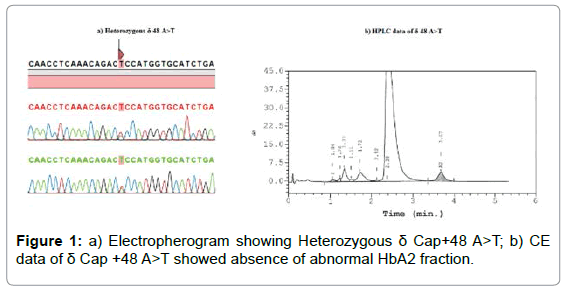
Figure 1: a) Electropherogram showing Heterozygous δ Cap+48 A>T; b) CE data of δ Cap +48 A>T showed absence of abnormal HbA2 fraction.
All concomitant β-thalassemia and δ-variant carriers (Group 2) and β-thalassemia carriers without HBD mutation (Group 3) were the Malays. The eight subjects in Group 2 were concomitant β-thalassemia and δ-variant carriers (Table 1). The most common δ-globin variant was HbA2-Indonesia in four β-thalassemia carriers of IVS 1-5 (G>C), Cd 41(TTC>TT-) (HBB:c.126 delC), and Cd 71/72 (+A) (HBB:c.216_217insA), respectively. Two IVS 1-5 (G>C) carriers had HbA2-Walsgrave and an IVS 2-1 (G>A) (HBB:c.315+1G>A) carrier had HbA’ (Hb B2) mutations. They were presented with a small fraction on the CE Zone 1 or/and the HPLC S-window. A new variant, Cd 56 (GGC>GAC) HbA2-Shah Alam were identified in a boy with a small fraction on the CE Zone 6 instead (Supplementary Table and Figure 2). Fifteen samples (Index 23-37, Supplementary data) were without HBD mutation. They were mostly carriers of a common β-thalassemia mutation, IVS 1-5 (G>C). One and two Cd 41/42 (-TTCT) and Cd 126 (GTG>GGG) Hb Dhonburi (HBB:c.380T>G), respectively.
| Group | Total | Ethnicity | Frequency | HBD Genotype | HBB Genotype | % variant fraction on HPLC | % CE Z1 (HbA') |
|---|---|---|---|---|---|---|---|
| Group 1: Concomitant β- and δ-thalassemia carriers | 14 | Dusun | 6 | δcd 67 (GTG>ATG)/δ | βFIL/β | Absent | Absent |
| Kadazan | 2 | ||||||
| Not given | 2 | ||||||
| Murut | 1 | ||||||
| Sungai | 1 | ||||||
| Malay | 1 | δ+48 (A>T)/δ | βIVS 1-5 (G>C)/β | ||||
| Chinese | 1 | -δ30 (T>C)/δ | βCd 41/42 (-TTCT)/β | ||||
| Group 2: Concomitant β-thalassemia and δ-variant carriers | 8 | Malay | 2 | δcd 69 (GGT>CGT)/δ | βIVS 1-5 (G>C)/β | Present | Present |
| 1 | βCodon 41 (TTC>TT-)/β | ||||||
| 1 | βCodon 71/72 (+A)/β | ||||||
| 2 | δcd 52 (GAT>CAT)/δ | βIVS 1-5 (G>C)/β | |||||
| 1 | δcd 16 (GGC>CGC)/δ | βIVS 2-1 (G>A)/β | |||||
| 1 | δcd56 GGC>GAC)/δ | βIVS 1-5 (G>C)/β | |||||
| Group 3: β-thalassemia carriers | 15 | Malay | 7 | δ/δ | βIVS 1-5 (G>C)/β | Absent | Absent |
| 5 | βIVS 1-1 (G>T)/β | ||||||
| 2 | βCd 41/42 (-TTCT)/β | ||||||
| 1 | βCd 126 (T>G)/β |
Table 1: Distribution of β-thalassemia carriers with and without δ-globin gene mutations among Malaysian ethnics.
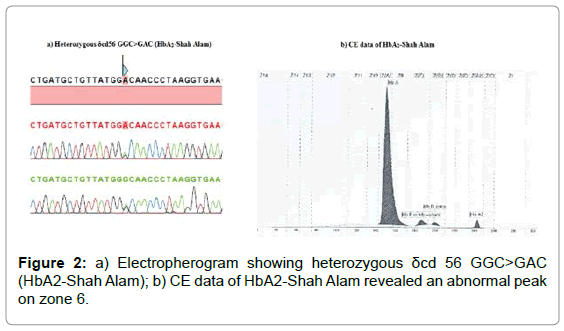
Figure 2: a) Electropherogram showing heterozygous δcd 56 GGC>GAC (HbA2-Shah Alam); b) CE data of HbA2-Shah Alam revealed an abnormal peak on zone 6.
A one-way between groups ANOVA was conducted to compare the hematological parameters of Hb, RBC, MCV, MCH, RDW; and the Hb molecules of HbA2 and HbF among the groups. All the hematological parameters between groups were not statistically significant (Table 2). Meanwhile, a significant difference was observed for HbA2 (p<0.0001) and HbF (p=0.02). Group 3 had the highest HbA2 (mean=3.689), while Group 1 had the highest HbF percentage (mean=3.675). Mean HbA2 for Group 2 was significantly different to that of Group 1 (p=0.0002) and Group 3 (p<0.0001) (Table 3). A two‐tailed unpaired t‐test was conducted to each group’s HbA2 and HbF. There was also a significant difference between HbF mean for Group 1 and 3 (p=0.0039) (Table 4).
| Parameter | Group 1 | Group 2 | Group 3 | ANOVA |
|---|---|---|---|---|
| n, mean (SD) | n, mean (SD) | n, mean (SD) | p-value | |
| RBC, X1012/L | n=14, 5.420 (1.268) | n=8, 5.138 (0.953) | n=15, 5.453 (1.178) | 0.8136 |
| Hb, g/dL | n=14, 10.793 (2.176) | n=8, 9.912 (1.245) | n=15, 11.053 (1.953) | 0.4007 |
| MCV, fL | n=14, 67.150 (7.845) | n=8, 60.938 (5.114) | n=15, 68.347 (8.351) | 0.0868 |
| MCH, pg | n=14, 20.564 (2.693) | n=8, 19.350 (1.257) | n=15, 20.627 (3.022) | 0.4977 |
| RDW, % | n=8, 16.462 (3.027) | n=6, 17.7 (1.197) | n=11, 17.082 (3.488) | 0.7423 |
| HbA2, % | n=12, 3.567 (0.311) | n=7, 2.914 (0.261) | n=9, 3.689 (0.176) | <0.0001 |
| HbF, % | n=12, 3.675 (2.606) | n=7, 1.286 (0.564) | n=9, 0.789 (0.289) | 0.002 |
Table 2: Comparison of hematological parameters and Hb molecules between group 1, 2, and 3. The n refers to number of samples used in the statistical analysis. The HbA2 mean (SD) were taken from HPLC only
The new δ-thalassemia and variant were found in Malay subjects. The 5’ UTR +48 is located at the Cap-site of the HBD and lacking additional fraction on the Hb electrophoresis (Figure 1). Index 13 was a carrier for β+ IVS 1-5 (G>C) with borderline HbA2 (3.7%). The HbA2-Shah Alam changes the second nucleotide of the 56th amino acid (Figure 2a), substituting glycine with an aspartic acid. Index 22 was a Malay toddler having a normal HbA2 (2.5%), an elevated HbF (3%), microcytosis and hypochromia (Supplementary data). A small fraction on the CE Zone 6 (1.2%) was recorded (Figure 2b). He was diagnosed with β+ IVS 1-5 (G>C) and HbA2-Shah Alam, respectively.
Discussion
Malaysia is a multiracial tropical country and its geography is divided into West and East Malaysia. West Malaysia is in Mainland Southeast Asia where the majority of ethnics are Malays, Chinese, Indians, and Orang Asli (indigenous). Whereas, East Malaysia comprises of Sarawak and Sabah states in the north part of Borneo Island inhabited by many indigenous sub-groups. The Malays formed 63.1% of the total Malaysians population while the Kadazan-Dusun and Iban formed the largest indigenous groups made up 24.5% and 30.3% of Sabah and Sarawak populations, respectively [11].
Geographically, we found HbA2-Deventer among ethnicities from Sabah. The HbA2-Deventer was encountered for the first time in a Rungus ethnic family whom several children were lacking HbA2 (Supplementary Figure). Both parents were not consanguineous, suggesting that the mutation is common in individuals of Sabah ethnicity. Therefore, we suspect previous β°-FIL deletion carriers with borderline HbA2 were due to HbA2-Deventer, and that was the case. However, HBD was not considered because there was no additional HbA2 fraction on the HPLC and CE. In total, 12 β°-FIL deletion with HbA2-Deventer cases were identified: six cases among Dusun, two among Kadazan, one Murut, and Sungai, and two unrecorded ethnicities (Supplementary data). Index 6, 7, and 12 showed examples of possible misdiagnosis in β°-FIL deletion with HbA2-Deventer. Index 6 and 7 had normal HbA2 and elevated HbF were tested with β-MGAP to rule out High Persistent Fetal Hemoglobin (HPFH) and δβ-thalassemia, but β°-FIL deletion was found. Index 12 was submitted by a West Malaysia’s hospital without mentioning her ethnicity. Direct sequencing was performed to rule out borderline HbA2 β-thalassemia and no mutation was found. However, her -globin gene was homozygous leading to the suspicion of a deletional δ -thalassemia and β°-FIL deletion with HbA2-Deventer were identified.
Ethnicity and β-globin gene zygosity can be used to predict a possible β°-FIL deletion with HbA2-Deventer. When ethnicity is not mentioned, we refer to the identity card number containing the patient’s birthplace. Sequencing data showing homozygous β-globin gene can be used to suspect a deletional δ -thalassemia.
We compared each group’s HbA2 to determine if it was possible to anticipate HbA2 interference by δ-globin defects among the β-thalassemia carriers. The HbA2 of Group 2 and 3 were significantly different, and detectable by the additional fractions. However, mean of Group 1 was not significantly different to that of Group 3 (Table 3). Since Group 1 lacks additional fraction and resembled borderline HbA2 β-thalassemia, we compared the HbF and found β- and δ-thalassemia carriers had a significantly higher HbF than β-thalassemia alone (Table 4). However, this may not be a true representation of β- with δ-thalassemia since majorities were β°-FIL deletion that is known to have elevated HbF.
| Group | Difference of means | C.I (95%) of mean difference | p-value |
|---|---|---|---|
| Group 1 vs Group 3 | -0.122 | ± 0.243 | 0.3054 |
| Group 1 vs Group 2 | 0.652 | ± 0.296 | 0.0002 |
| Group 2 vs Group 3 | -0.775 | ± 0.234 | <0.0001 |
Table 3: Comparison of HbA2 percentage between groups
| Group | Difference of means | C.I (95%) of mean difference | p-value |
|---|---|---|---|
| Group 1 vs Group 3 | 2.886 | ± 1.838 | 0.0039 |
| Group 1 vs Group 2 | 2.389 | ± 2.130 | 0.0301 |
| Group 2 vs Group 3 | 0.497 | ± 0.464 | 0.0375 |
Table 4: Comparison of HbF percentage between groups.
The rest of the mutations were detected only among the Malays, the predominant ethnicity of West Malaysia. Five types of HBD mutations were recorded; the most frequent was HbA2-Indonesia. It was found co-exists with common and rare δ-thalassemia. Two new mutations of δ Cap +48 (A>T) and δ cd 56 (GGC>GAC) HbA2-Shah Alam were discovered. The HbA2-Shah Alam was found in a 1-year-old patient heterozygous for β+ IVS 1-5 (G>C). Unlike other variants, the CE showed an additional fraction at Zone 6.
The δ Cap +48 (A>T) changes conserved A/G (purine) of Kozak consensus into a T (pyrimidine). The Kozak consensus sequence was originally defined as ACCAUGG following an analysis of the effects of single mutations surrounding the initiation codon (AUG) on the translation of the preproinsulin gene [12]. Subsequent mutagenesis studies and vertebrate mRNAs survey extended the consensus sequence for translation initiation to GCCGCC(A/G)CC(AUG)G, where the highest conserved position in that motif is the purine in position -3, with purine A found in 97% of vertebrate mRNAs [13,14]. Later, functional studies on preproinsulin and α-globin translation in cells showed that a purine (usually A) in position -3 (to the start codon) is crucial for efficient initiation of translation [15].
The HBD mutations co-exist with β-thalassemia of heterozygous IVS 1-5 (G>C), Cd 41 (TTC>TT-), Cd 41-42 (-TTCT), IVS 2-1 (G>A), Cd 71/72 (+A), and β°-FIL deletion. Some of them could be distinguished by a visible on the CE (Zone 1 and 6) and HPLC (S-window) while others were otherwise; highlighting the needs for thorough analysis of Hb parameters to suspect co-existence of multiple globin gene defects. For any δ-globin variant presented with HbA2 fraction other than the common CE Zone 1 or HPLC S-window, α- and β-globin variants must be ruled out. Since the β°-FIL deletion with HbA2-Deventer carriers may appear as borderline or normal HbA2 β-thalassemia and elevated HbF, the δ-globin status of any case involving such Hb molecules among ethnics from Sabah must be investigated. One of our major concerns is that interracial marriage between Sabahan ethnics with West Malaysians may pose a misdiagnosis when children of mix ethnicities are not mentioned. β°-FIL deletion carriers tend to have higher HbA2 than those of the point mutational β-thalassemia carriers. Although the HbA2-Deventer among the point mutational β-thalassemia carriers was not found, it may lead to a normal HbA2 β-thalassemia without additional fraction that is undiagnosed altogether.
The complexity of molecular diagnosis of the multi-globin gene tells us that relying upon HbA2 levels for excluding and confirming β-thalassemia carrier status is risky. Although the δ-globin defects are not clinically important, a misinterpretation could occur especially when the additional HbA2 fraction is absent as observed in our patients. Since δ-thalassemia modifies HbA2 levels, diagnosing borderline HbA2 β-thalassemia is vital. Ideally, HbA2 and HbF ranges of each β-globin mutation must be known to distinguish mild and severe mutation types so that any HbA2 interference by δ-globin defects can be predicted.
Conclusion
In conclusion, the study of δ-globin gene status is compulsory in our populations where thalassemia and hemoglobinopathies are heterogeneous. To improve the existing molecular diagnostic under National Prevention and Control Program for Thalassemia in Malaysia, DNA analysis of the δ-globin gene has been included in the program.
Acknowledgements
The authors would like to acknowledge the support of the Director General of Health and the Director of the Institute for Medical Research for their permission to publish this paper. We would also thank the National Prevention and Control Program for Thalassemia Fund in supporting our efforts.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
REFERENCES
- Mosca A, Paleari R, Ivaldi G, Giordano PC. The role of haemoglobin A2 testing in the diagnosis of thalassaemias and related haemoglobinopathies. J Clin Pathol. 2009;62:13-17.
- Pirastu M, Ristaldi MS, Loudianos G, Murru S, Sciarratta GV, Parodi MI, et al. Molecular analysis of atypical beta-thalassemia heterozygotes. Ann NY Acad Sci 1990;612:90-97.
- Trifillis P, Ioannou P, Schwartz E, Surrey S. Identification of four novel d-globin gene mutations in Greek Cypriots using polymerase chain reaction and automated fluorescence-based DNA sequence analysis. Blood. 1991;78:3298-305.
- Chong SS, Boehm CD, Higgs DR, Cutting GR. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassaemia. Blood. 2000;95:360-362.
- Eng B, Patterson M, Walker L, Chui DH, Waye JS. Detection of severe nondeletional a-thalassemia mutations using a single-tube multiplex ARMS assay. Genet Test. 2001;5:327-329.
- Hassan S, Ahmad R, Zakaria Z, Zulkafli Z, Zakaria Z. Detection of ß-globin Gene Mutations Among ß-thalassaemia Carriers and Patients in Malaysia: Application of Multiplex Amplification Refractory Mutation System-Polymerase Chain Reaction. Malays J Med Sci. 2013;20:13-20.
- Tritipsombut J, Phylipsen M, Viprakasit V, Chalaow N, Sanchaisuriya K, Giordano PC, et al. A single-tube multiplex gap-polymerase chain reaction for the detection of eight ß-globin gene cluster deletions common in Southeast Asia. Hemoglobin. 2012;36:571-580.
- Amirian A, Karimipoor M, Jafarinejad M, Taghavi M, Kordafshari A, Fathi Azar S, et al. First report on the co-inheritance of beta-globin IVS-I-5 (G>C) thalassemia with delta globin Cd12 {Asn>Lys (AAT>AAA)} HbA2-NYU in Iran. Arch Iran Med. 2011;14:8-11.
- Waye JS, Eng B, Hunt JA, Chui DHK. Filipino ß-thalassemia due to a large deletion: identification of the deletion end-points and polymerase chain reaction (PCR)-based diagnosis. Hum Genet. 1994;94:530-532.
- Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nature Methods. 2014;11:361-362.
- Population Distribution and Basic Demographic Characteristic Report 2010 Malaysia.2017;
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283-292.
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947-950.
- Kozak M. An analysis of 5´-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res 1987;15:8125-8148.
- Kozak M. The scanning model for translation: An update. J Cell Biol. 1989;108:229-241.
Citation: Hassan S, Ahmad R, Esa E, Yusoff YM, Yasin NM, et al. (2019) Delta-Globin Gene Mutations Complicate the Diagnosis of β-Thalassemia. J Blood Disord Transfus 10:420.
Copyright: © 2019 Hassan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

