Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 12, Issue 1
De-Constructing the TState/RState Structure Change of Human Hemoglobin
Francis Knowles* and Douglas MagdeReceived: 29-Dec-2022, Manuscript No. BABCR-23-19474; Editor assigned: 02-Jan-2023, Pre QC No. BABCR-23-19474 (PQ); Reviewed: 17-Jan-2023, QC No. BABCR-23-19474; Revised: 25-Jan-2023, Manuscript No. BABCR-23-19474 (R); Published: 01-Feb-2023, DOI: 10.35248/2161-1009.23.12.479
Abstract
The dimensionless equilibrium constant for the allosteric structure change is shown to be comprised of: (i) An endothermic change in structure, from Tstate to Rstate, of 24.3 kJ/mol; (ii) Exothermic conversion of Tstate TαO2- chains to Rstate RαO2-chains of-13.8 kJ/mol; (iii) Exothermic binding of BPG by R-states. Equation (1) defines the component steps whereby the Tstate structure is converted to the Rstate structure. ΔG°(R(Hb4), BPG) describes the endothermic decomposition of the binary complex, THb4/BPG into RHb4 and BPG, equal to +33.7 kJ/mol (DeBruin et al. (1973). J. Biol. Chem. 248, 2774-2777). ΔG° of the equilibrium constant for ΔG° (KΔ) and Ʃ ΔG° for binding of O2 by the pair of equivalent Tstate α-chains, ΔG°(Tα*O2), +9.41 kJ/mol and-49.6 kJ/mol, respectively, are determined by fitting of O2 equilibrium binding data to the Perutz-Adair equation.
Keywords
Hemoglobin; Allosteric structure; E-molecules; Standard free energy change; Structure (protein) changes; Allosteric mechanisms
Introduction
ΔGo for reaction of a pair of equivalent Rstate α-chains with O2, ΔG°(RαO2), was estimated from the known affinity of myoglobin for O2 at 37°C. (Biochem. Z., 268, 73-81),-63.4 kJ/mol. The unknown quantity, ΔG°(R(HbO2)4/BPG), was obtained by solving Equation (1), being-10.5 kJ/mol, K (HbO2)4/BPG)=58.4 L/mol. The value of the equilibrium constant for binding BPG to the R-state structure represents 0.0073% of the value of the binding constant of BPG to the Tstate structure: 800,000 L/mol. The value of KΔ; (i) Accounts for the ability of O2 to escape, virtually unhindered from red blood cells and (ii) Provides a biophysical basis for manifestation of high resting rates of metabolism in warm blooded species. The Perutz/ Adair equation of state, Equation (1), imposes the elements of the Perutz stereochemical model on the Adair equation. The Perutz/ Adair equation, comprised of only three unknown quantities, accurately predicts equilibrium binding curves of whole blood with both O2 and CO.
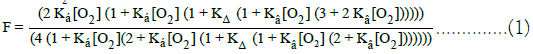
These observations are particularly relevant insofar as they define the properties of haemoglobin in human RBCs in vivo, in the presence of the naturally occurring E-molecule, BPG. The result obtained with the Perutz/Adair equation permits unambiguous assignment of structural states, R and T, to the subunits in each intermediate species in the reaction sequence, Equation (a). Species I and II, as encountered in rbcs of whole blood, are Tstate. Species III and IV, as encountered in red blood cells of whole blood, are Rstate. Species l is in a special category insofar as it does not exist in vivo. Species I, however, is readily prepared in the laboratory [1,2].
The value of the equilibrium constant for the structural change, KΔ=0.02602, is of particular interest. It accounts for the ability of molecules of O2 to escape, virtually unhindered, from RBCs. This single insight, by itself, provides strong justification for the model underlying the Perutz-Adair equation. Upon release of O2 atoms from β-chains, species III and IV, 98% of species III revert to species II. Species II is unable to bind O2 molecules to Tstate β-chains, precisely defining the boundary condition of the molecular mechanism releasing O2 from arterial blood into the systemic circulation. The value of KΔ provides a biophysical basis for manifestation of high resting rates of metabolism in warm blooded species.
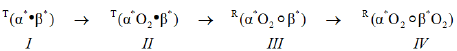
The endothermic Tstate → Rstate structure change is coupled with: (i) exothermic conversion of TαO2-chains (species II, Equation (a)) to RαO2-chains (species III, Equation (a)) and (ii) marked decrease in affinity of the Rstate for BPG. ΔG° for formation of THb4/BPG is- 33.7 kJ/mol at 25°C. The Rstate structure forms a less stable binary complex with BPG. The Perutz/Adair equation is based on the assumption that: (i) Tstate protein structures, species I and II Equation (a), bind BPG with the exactly the same high affinity and (ii) Rstate protein conformations, species III and IV Equation (a), bind BPG with exactly the same relativity low affinity, in comparison with Tstate structure. Nevertheless, the diminished affinity of Rstate structures for BPG is large enough to be exothermic, estimated to be-10.5 kJ/ mol. These reactions, endothermic and exothermic, taken together, account for the observed value of KC, 0.02602. Equation (2) and (3) define the reactions predicted to account for the observed value of the equilibrium constant for the Tstate to Rstate structure change [3,4].
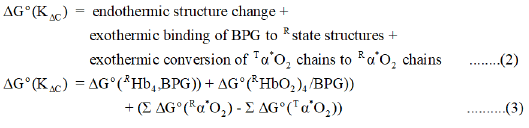
Literature Review
Determination of Unknown Quantities in Eq. (3): Calculation of the Values of ΔG° (KΔC) and ΔG° (Tα*O2) at 37°C
These values are returned by curve fitting of O2-binding date of whole blood, under standard conditions, to the Perutz/Adair equation:
ΔG°(KΔC)=9.41 kJ/mol; ΔG°(Tα*O2)=-49.6 kJ/mol. Results are summarized in Table 1.
| Reactions | K L/mol | ΔGo kJ/mol |
|---|---|---|
| RHb4+DPG → THb/DPGa | KDPG=8 x 105 | -33.69 |
| In Whole Blood | ||
| O2+T(α)2 → T(αO2) T(α) | 2Kα=30,180 | -26.6 |
| O2+T(αO2) T(α) → T(αO2)2 | Kα/2 = 7,545 | -23.02 |
| 2 O2+2 T(α) → T(αO2)2 | -49.62 | |
| T-State → R-State | KΔC=0.02602b | 9.41 |
| O2+R((α*O2)●( β*)) →R((α*O2)●((βO2)β) | 2 Kβ=787,800 | -35.01 |
| O2+R((α*O2)○((βO2)β)→ R(α*O2○β*O2) | Kβ/2=196,950 | -31.43 |
| Horse heart myoglobin | ||
| Mb+O2 → MbO2 For two molecules of Mb | K=2.20 x 105 | -63.4 |
| Whole Blood Standard Conditions | ||
| Kα=15,090 | ||
| 4 O2+T(α*●β*)→ | KC=9.41 | -106.7 |
| R((α*O2)○(β*O2)) | Kβ=393,900 | |
| E-Free Electrolyte | Kα=789,000 | |
| 0.05 M BisTris, pH 7 with HCl, 20°C | Kβ=272,000 | -134.6 |
Table 1: Equilibrium Constants and ΔG° for the Sequence of Reactions Comprising the Perutz-Adair Equation, 37°C.
Estimation of the Value of ΔG°(Rα*O2) at 37°C. The model described in Equation (a) does not permit determination of ΔG° for binding of a molecule of O2 to equivalent Rstate (Rα*)-chains. Molecules of O2 have only equivalent Tstate deoxy-(Tα*) chains to form equivalent (Tα*O2) chains. (Rα*O2) chains are formed directly from T(α* O2) when the Tstate → Rstate structure change relaxes proximal strain. One can estimate ΔG° for formation of RαO2 in two ways: (i) assume that ΔG° (MbO2) is similar to ΔG° (RαO2); (ii) assume that ΔG° (RαO2) for E-free hemoglobin is an upper limit [5-7]. Theorell elaborated O2-binding data for horse heart myoglobin at 37O, with half-saturation of myoglobin occurring at a partial pressure of O2 in the gas phase of approximately 3.5 Torr, corresponding to a concentration of O2 of 4.60 µmol/L. This allows an assignment of 2.2 x 105 L/mol as the O2-binding equilibrium constant of Rstate (Rα*O2) chains: ΔG°=-63.4 kJ/mol. Using equilibrium constants obtained for E-free hemoglobin at 20°C Results are summarized in Table 1.
Assignment of the value of ΔG°(THbO2)4/BPG) for the endothermic change, Tstate to Rstate, in protein structure
BPG, a potent E-molecule, binds avidly to RHb4. In the process of forming the binary complex, the structure of RHb4 changes to that of T(Hb4/BPG). In contrast to the great stability of THb4/ BPG, the equilibrium constant for the binary complex of BPG, yielding the Rstate binary complex, R(HbO2)4/BPG, is low. ΔG° for reversing the binding of BPG to RHb4, ΔG° (R(Hb)4, BPG), is +33.7 kJ/mol at 25°C. A correction due to the increase in temperature was not applied. ΔG° for binding of BPG by Rstate conformations, ΔG°(R(HbO2)4/BPG), can be calculated directly from Equation (3) since all other values are known, estimated, or assigned. This procedure computes a value of ΔG° for binding of BPG to an Rstate structure, ΔG°(Rstate/BPG)=-10.5 kJ/mol, corresponding to an equilibrium constant for binding of BPG to R-state conformations, K(R(HbO2)4/BPG)=58.9 L/mol. O2-Equilibrium binding curves at pH 9.1 demonstrate binding of IHP to R-state conformations.
Discussion
RHb4, free of E-molecules, such as BPG or 0.10 M chloride ions demonstrates a high affinity for O2, being half saturated with O2 in 2 μM O2/L. Addition of stoichiometric amounts of BPG to solutions of RHb4 results in formation of a binary complex with markedly diminished affinity for O2. The product is a Tstate binary complex. The reaction can be written as follows, where BPG is indicated by a bullet: and superscript * indicates equivalent binding by a pair of subunits.
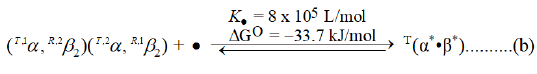
The reaction of BPG with RHb4 is exothermic: ΔG°=-33.7 kJ/ mol. The history of observations of the properties of human hemoglobin, until approximately 1967, were conducted without knowledge of the effect of BPG on the properties of hemoglobin. Analysis of the O2 equilibrium binding curve of E-free preparations of human Hb4 reveals a pair of equivalent cooperative dimers: (α1β2) and (α2β1). It may be incorrect to describe these cooperative dimers as being Rstate. An Rstate β-chain regulates an α-chain of diminished affinity. α-Chains in cooperative dimers of RHb4, then, are not Rstate until an Rβ-chain binds O2. The cooperative dimer, nevertheless, demonstrates a much higher affinity for O2 than does T(α*●β*), requiring 35 μmol O2/L for half saturation. Significant allosteric structure is present in RHb4. Addition of BPG to RHb4 results in: (i) formation of T(Hb4)/BPG; (ii) increased proximal strain in Tα-chains; (iii) imposition of steric hindrance to O2-binding by β-chain heme moieties. β-chain heme moieties in T(Hb4/BPG) may be Rstate in reactivity and simply unable to gain access to an O2 molecule due to distal side steric hindrance. If it was not possible to reverse the effects of binding BPG on Tβ-chain reactivity, the O2 equilibrium binding curve would be described by a simple equation of state accounting only for species I and II of Equation (a).
Imposition of the stereochemical model on the Adair (1925) sequence of four O2-binding reactions results in an equation of state acknowledging the existence of a Tstate → Rstate structure change. The discussion concerning binding of BPG to RHb4 suggests that an exothermic input of as much as-33.7 kJ/mol would be required. It is unlikely that BPG actually separates from the globin moiety in the Tstate → Rstate structure change. Our procedure is viewed from the perspective of thermodynamics: assuming release of BPG from a Tstate intermediate and rebinding to form an Rstate intermediate. The molecule of BPG bound to Tstate conformation remains, in all likelihood, attached to the globin moiety throughout the Tstate → Rstate transition. The physical realty of an equilibrium constant for a Tstate → Rstate change is supported by the virtual identity of the values for KΔ returned by equilibrium binding curves for both CO and O2, in whole blood, as well as purified human hemoglobin for O2 in the presence of 0.1 M NaCl: 0.0574; 0.02602; 0.03252; respectively Following the Tstate → Rstate change, step II to step III, there are only equivalent O2-binding reactions by Rstate β-chains, these reactions further stabilizing the Rstate conformation. Binding of O2 to Rβ-chains and release of O2 from RβO2-chains is free of changes in elements of allosteric structure. A Tstate structure is entirely different than an Rstate. Tstate and Rstate structures are, in fact, different molecules [8-14].
Since the curve fitting procedure is based on a model in which a pair of α-chains are equivalent and a pair of β-chains are equivalent, there can only be only be one Tstate → Rstate change and, therefore, only two structures: Rstate and Tstate. That single Tstate → Rstate transition occurs between species II and species III, Equation (a). Although there are two structural states, the model which underlies the Perutz/Adair equation is incompatible with the two state models in which the reactant, product, and all intermediate species are capable of being in either of the two states. These conclusions do extend to hemoglobin in whole blood from other species. These conclusions, however, do not address the validity of the two state models in other systems.
The value of KΔ, 0.02602, establishes the ability of the binary complex of hemoglobin and DPG in red blood cell to release O2 from arterial blood. The release of O2 from the equivalent Rstate βO2-chains is, kinetically, first order. Recapture of O2 by species III is extinguished by the thermodynamically favoured conversion of species III to species II, which is not capable of binding O2 to β-chains. If O2 depleted β-chains remained as Rstate haemoglobin molecules, the ability of the red blood cell to release O2 would be dramatically impaired, O2 molecules being subject to recapture before diffusing through the red blood cell membrane, thereby escaping from the RBC. The efficiency of O2 transport to mitochondria of the components of the systemic circulation from arterial blood is dependent upon the conversion of species III to species II. Using the Perutz/Adair equation to fit equilibrium binding date, the value of ΔG°(KΔ) is revealed to be remarkably well fitted to the purpose of O2 transport from arterial blood to mitochondria.
Conclusion
Variation in values of KΔ across the spectrum of mammals, birds and lizards offers new insights into comparative respiratory physiology. A significant increase in the value of KΔ would lower the rate at which rbcs could deliver O2 to mitochondria. The Perutz/Adair equation of state is the first probe of respiratory function to reveal insight into the mechanism of enhanced transport of O2 from the lungs to the respiratory tissues. The picture, however, is incomplete without consideration of the consequences of the cyclic variation in the pH of the interior of red blood cells. Doing so is the subject of another communication.
Acknowledgement
Francis Knowles would like to acknowledge the help of Professor Glen Lo at Nicholls State University in providing assistance with curve fitting procedures and providing copies of XYCALC. Francis Knowles would to thank Professor Quentin Gibson and the Department of Biochemistry at Cornell University for their hospitality and assistance in elaborating instrumentation for determination of equilibrium binding curves of hemoglobin for O2 (1972-1977). Francis Knowles would like to thank the Department of Chemistry and Biochemistry at UCSD for continuing appointments as a Lecturer and Professors Douglas Magde and Michael Tauber for hosting the author as a Visiting Scholar.
References
- Knowles F, Doyle SJ, Magde D. Architecture of allosteric structure. rate equations, rate constants, and equilibrium constants for reaction of: Hb4 with O2 and (HbO2)4 with dithionate, in the presence of 2, 3-Bisphosphoglycerate.
- Perutz MF, Fermi G, Luisi B, Shaanan B, Liddington RC. Stereochemistry of cooperative mechanisms in hemoglobin. Cold Spring Harb Symp Quant Biol. 1987 ;20(9): 309-321.
[Crossref] [Google Scholar] [PubMed]
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970; 228(5273): 726-739.
- Adair GS, Bock AV, Field Jr H. The hemoglobin system: VI. The oxygen dissociation curve of hemoglobin.J Biol Chem. 1925;63(2): 529-545.
- Kernohan JC, Roughton FJ. The precise determination of the bottom of the oxyhemoglobin dissociation curve of human blood. 1972: 54-64.
- 6 Roughton FJ, Deland EC, Kernohan JC, Severinghaus JW. Some recent studies of the oxyhemoglobin dissociation curve of human blood under physiological conditions and the fitting of the Adair equation to the standard curve. 1972.
- Severinghaus JW, Roughton FJ, Bradley AF. Oxygen dissociation curve analysis at 98.7–99.6% saturation. 1972:63.
- Roughton FJ. The equilibrium of carbon monoxide with human hemoglobin in whole blood. Ann N Y Acad Sci. 1970;174(1): 177-188.
[Crossref] [Google Scholar] [PubMed]
- de Bruin SH, Janssen LH. The interaction of 2, 3-diphosphoglycerate with human hemoglobin: effects on the alkaline and acid bohr effect. J Biol Chem. 1973;248(8): 2774-2777.
[Google Scholar] [PubMed]
- Garby L, DE Verdier CH, Gerber G. Binding of 2, 3‐diphosphoglycerate and adenosine triphosphate to human haemoglobin A. Eur J Biochem. 1969;10(1): 110-115.
[Crossref] [Google Scholar] [PubMed]
- Knowles FC. Binding of organic phosphates by human hemoglobin at alkaline pH-values. Biochem Biophys Res Commun. 1980;92(3): 1060-1065.
[Crossref] [Google Scholar] [PubMed]
- Knowles F, Magde D. Resolution of the equilibrium constant for the t state→ rstate conformational change of human hemoglobin into endothermic and exothermic component reactions.1934.
- Chanutin A, Curnish RR. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967;121(1): 96-102.
[Crossref] [Google Scholar] [PubMed]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12(1): 88-118.
[Crossref] [Google Scholar] [PubMed]
Citation: Knowles F (2023) De-Constructing the Tstate/Rstate Structure Change of Human Hemoglobin. Biochem Anal Biochem. 12:479
Copyright: © 2023 Knowles F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


