Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2021) Volume 12, Issue 1
Cultural, Morphological and Pathogenic Variability of Colletorichuma kahawae Isolate of Gurage Zone
Dereje Amare1*, Beira Hailu2 and Gerba Daba22Jimma University College of Agriculture and Veterinary Medicine, Ethiopia
Received: 07-Jan-2021 Published: 27-Jan-2021, DOI: 10.35248/2157-7471.21.12.532
Abstract
Arabica Coffee is an important crop in the national economy of Ethiopia. Coffee berry disease caused by the fungus Colletotrichum kahawae Waller and Bridge is the most devastating threat to Coffea arabica L. production in Africa at high altitude. Hence, this study was carried out for Variation of a representative Colletotrichum isolates of Gurage zone of major coffee producing areas using cultural, morphological and pathological criteria. Out of 33 sample 13 representative C. kahawae isolates from the study area and one Gera isolate were isolated from infected green coffee berry which showed significant variations in their cultural, morphological characteristics and pathogenicity. Mean radial colony growth rate of isolate showed significant variation (p<0.001) with the range of 2.67 to 4.08 mm/24hrs on PDA in EZA and CA1 isolates, respectively. Conidial size also showed significant difference (p<0.001) in the range of 5 to 6.04 and 9.24 to 10.0 µm in width and length, respectively. Similarly, conidia production varied from 182.25 to 432.92 × 104 conidia/ml of isolate EK1 and EZD, respectively. All isolates were found to be pathogenic to Arabica coffee with highly significant variation (P < 0.01) and infection percentage in the ranges of 45.83 to 68.06%. Aggressive isolate EZD should be used for screening of coffee variety for CBD resistance evaluations.
Keywords
Coffee berry disease; Colletotrichum kahawae; Pathogenecity
Introduction
Coffee is the most important cash crop worldwide; more than 125 million people in the coffee growing areas derive their income directly or indirectly from Coffee products [1]. Ethiopia is the center of origin and genetic diversity of Arabica coffee. In Ethiopia coffee farming provides a livelihood income for around 15-16 million peoples, based on four million small-holder farms,10 percent of agricultural production, and about 34 percent of total export earnings over the past decade [2-4]. The occurrence of major severe diseases is one of the main limiting factors of coffee production. Coffee berry disease (CBD) caused by the fungus Colletotrichum kahawaeWaller and Bridge, is the most devastating threat to Coffea arabicaL. production in Africa at high altitude.
C. kahawae attacks all stages of the crop from flower to ripe fruits and occasionally leaves, but the maximum crop loss occurs following the infection of green berries [5]. Yield loss due to CBD is estimated to range from 24% to 30%, but it may reach up to 100% in high rainfall, high humidity and high altitude areas [6,7]. Different research has been indicated that variation among coffee berry disease pathogen through morphological, cultural and pathogenic characteristics [6-9]. According to Tefestewold study indicated variation/similarities within C. kahawae isolates collected from Hararge, Illubabor, Kaffa and Sidamo areas and recorded a presence of variations in aggressiveness and absence of races within C. kahawae populations based on pathogenicity tests [9]. Eshetu and Waller also reported presence of physiologic races within C. kahawae isolates in Ethiopia, it would be useful to look at a profile of several isolates from widely differing coffee types existing in the country, in a locality over time [6]. Apart from the host, the variation in CBD severity could be associated with differences in virulence between C. kahawae isolates occurring in different coffee growing regions.
The variability within C. kahawae populations from different Gurage zone coffee producing areas of Ethiopia so far was not known. Therefore, the present study was carried out on variations within representative C. kahawae isolates from different Gurage zone coffee producing areas were studied using cultural, morphological and pathological criteria.
Materials and Methods
Description of the study area
The study was conducted in laboratory and growth room of Jimma Agricultural Research Center from July 2017 to June 2018.
Collection of samples
Green diseased coffee berries with active CBD lesions were collected from the Gurage zone three major coffee producing districts i.e., Cheha, Enemorina ener and Ezya districts. Gurage Zone is found in Southern Nations Nationalities and Peoples Regional State, located between 7.8° - 8.5° latitude and 37.5° -38.7° longitude [10]. From each districts three peasant associations were selected and a total of 33 coffee specimens were collected from randomly selected coffee farms. A total of fifteen to twenty green coffee berries from each farm with active CBD lesions were collected. Samples were picked using disinfected forceps, packed in perforated sterile plastic bags and transported to Plant Pathology Laboratory of Jimma Agricultural Research Center and maintained at 4°C for further studies.
Isolation and identification of the pathogen
The pathogens were isolated from infected coffee berries by following the methods described by Tefestewold [9]. The collected berries were cut into pieces with margin of diseased and healthy tissues using sterilized surgical blade. Then samples were surface-disinfected with 5% sodium hypo-chlorite solution for 2 minutes and then rinsed 5 times in sterilized water for 2 minutes. The sterilized samples were dried in laminar flow hood and then, five fragments (cut pieces) of each sample was taken and placed onto Petri dishes containing PDA supplemented with 0.04% streptomycin and incubated at 25°C for 3 to 5 days. The growing edges of any fungal hyphae (mycelial tip) developing from the tissues were sub-cultured aseptically to PDA and inoculated for 7–10 days at 25°C. After morphological and microscopic identification (conidial morphology, conidial lengths, colony growth rate, colony shape and colony colors) from 33 samples 13 representative pure cultures mono conidial C. kahawae isolates were preserved in 50% PDA slant method at 4°C for later use. Phoma spp., Aspergillus spp., Penicilium spp. and Fusarium spp. were grown on culture of some fragment samples and removed it and sterilize the petri dish to avoid contamination.
Cultural and morphological characterizations
Pure culture of 14 representatives C. kahawae isolates (for Cheha 4, for Ezya 5, Enemorina Ener 4 and 1 Gera isolate (hot spot area for CBD)) were isolated from infected green coffee berries. The plates were examined cultural, morphological and pathogenic characteristics of thepathogenisolates were studied following the methods and procedures used [9,11,12].
Cultural appearances: Ranges of cultural variation of 14 representative isolates were examined by culturing on PDA containing 0.04% streptomycin and incubated at 25°C in three replications in CRD design for all characters. An isolate was examined for a colony (mycelial) radial growth, colony color, colony shape and aerial mycelial growth characters. Mycelial (colony) radial growth (mm) of each isolate was measured from the reverse side of the Petri-dishes daily with ruler for 10 days starting from the 3rd day of incubation. The colony (mycelia) color on the upper side and types of pigments on the reverse side of the Petri-dish for each isolate was determined on PDA every 3 days using RGB color chart [13]. Hence, cultures were monitored for 12 days. Vigor of aerial mycelium growth types of each isolate was observed on upper side of a plate after 10 days of being cultured on PDA. Then, it was examined and recorded as dense (regular), irregular (scarce) or very scarce culture types. The colony form of each isolates was observed on reverse side of the plate on 8 days of being cultured on PDA. Then, it examined and recorded as round, irregular, filamentous, rhizoid or curled types of culture forms.
Morphological characteristics: Isolates was cultured on PDA medium in three replications for 10 days and then conidial size (length and width) were measured on 30 randomly selected conidia per replication per isolate. Length and width of conidia were measured with ocular micrometer ( µm), at 400x magnification of compound microscope. Sporulation capacity of each isolates was determined from 10 days old culture of the isolates on PDA was washed by flooding with 10 ml sterilized distilled water, rubbed with sterilized scalpel and transferred to 50 ml sterilized beaker and thoroughly stirred for 15 minutes with magnetic stirrer to extract the spores from the interwoven mycelia. Finally, the mycelia were filtered into another sterilized beaker through double layer cheese clothes. The number of conidia per ml were counted using haemocytometer.
Pathogenicity test of C. kahawae isolates
The 14 representativeisolates were evaluated for their pathogenic ability (virulence) on a detached green berry of susceptible variety (370) by following the methods of van der Vossen et al., and Bayetta [14,15]. Fifteen weeks old from date of flowering of the expanding coffee berries from bottom, middle and top of the coffee tree were randomly collected [16]. Berries were surfaced sterilized with 5% sodium hypochlorite solution for 2 minutes and rinsed three times with sterile distilled water for 2 minutes each and dried using sterile cotton cloth. The wounded stalk end of the berries was removed with a sterile scalpel to avoid contamination with saprophytic fungi. Eighteen berries per isolates were placed in 3 rows in plastic box on sterilized tissue paper for inoculation, in CRD design in three replications per isolates.
Inoculum preparation and Inoculation: Ten days old mycelia colonies culture of each isolate was washed by flooding with 10 ml sterilized distilled water, rubbed with sterilized scalpel and then transferred to 50 ml sterilized beaker to harvest conidia. The suspension of each isolate was stirred with magnetic stirrer for 15 minutes and filtered through double layers of cheese clothes. After repeating the procedure the spore concentration of each suspension was adjusted to 2 × 106 conidia/ml and 20 µl of conidia suspension was deposited on the berries using a pipette while shaking time to time when drawing the inoculums [17,18]. As a control (check) 20 µl distilled sterilized water was poured on the berries. Boxes were sealed to provide saturated humid conditions necessary for disease development. Regular opening after every three days was done for 10 minute to allow for aeration of the berries. The data on infection collected every three days starting from 3rd days post inoculation when CBD symptoms were visible. After 14 days, data on disease intensity (PSI), expressed as pathogenicity level of each isolates were recorded using a scales of 0 to 6 [19]. After scoring each coffee berry individually, average infection percentage (AIP) on each isolates across the replicates was calculated as follows:
AIP =Σ [Ir1 + Ir2 + Ir3+...Irn]/N
Where, I is the sum of disease score; n is the number of replication; Irn is the sum of disease score in replication n; Nis the total number of berries scored in the replications.
Data analysis
All the data were subjected to analyses of variance (ANOVA) using SAS program version 9.3 software [20]. Fishers least significant different (LSD) mean separation tests were performed for comparison of isolate characters means that showed significant difference. The relationships among pathogen characteristics were determined by Pearson correlation analysis using the SAS software (Proc procedure).
Results and Discussion
Cultural and morphological characteristics of C. kahawae isolates
There was highly significant (p < 0.001) difference among isolates in their radial colony growth rate (Table 1). Mean radial colony (mycelial) growth rate was ranged from 2.67 ± 0.26 to 4.08 ± 0.26 mm/24 hrs in isolates of CA1 (Cheha districts) and EZA (Ezha districts), respectively (Table 1). Over all mean of radial growth rate of 3.11 ± 0.26 mm/24 hrs was recorded and this results indicated a faster mean growth rate as compared to Hindorf [11,21], i.e., 1.9 ± 0.5 and slower mm/24hrs for the average mycelia growth rate of CBD isolates at 22°C incubation on 2% Oxoid MEA. However, this study result was comparable with Waller et al., and Arega et al. the colony growth rate of 2-4 and 0.6 - 5.5 mm/24 hrs, respectively [22,23]. As C. kahawae species are slow growing nature in mycelial growth rate in culture medium, this may use as distinguishing criterion of CBD pathogen from other Colletotrichum species (like C. gloesporioides, C. acutatum) and could serves as indicator of variability within the species [24].
| Isolate code | Form | Texture | Color | colony growth (mm/day) | |
|---|---|---|---|---|---|
| Upper | Reverse | ||||
| EK1 | Irregular | Dense | Gray white | Light gold rod | 3.05 ± 0.26c |
| CW | Irregular | Dense | Ghost white | Light rod | 3.04 ± 0.26c |
| EZS1 | Curled | Dense | Cottony white | Lemon chiffon | 2.99 ± 0.26c |
| EKO | Irregular | Scarce | Gray white | Lemon chiffon | 3.06 ± 0.26c |
| EZS3 | Irregular | Dense | Dark gray white | Light gold rod | 3.12 ± 0.26bc |
| EZA | Irregular | Dense | Gray white | Pale gold rod | 2.67 ± 0.26d |
| EZD | Curled | Scarce | Gray white | Pale gold rod | 3.36 ± 0.26b |
| CA1 | Irregular | Scarce | Dark gray white | Light gold rod | 4.08 ± 0.26a |
| CS | Curled | Dense | Floral white | Light gold rod | 3.01 ± 0.26c |
| CA2 | Irregular | Scarce | Gray white | Pale gold rod | 3.05 ± 0.26c |
| EK2 | Irregular | Dense | Dark gray white | Antique white | 2.88 ± 0.26cd |
| GC | Irregular | Scarce | Dark gray white | Navajo white | 3.33 ± 0.26b |
| EB | Irregular | Scarce | Ghost white | Corn silk | 2.91 ± 0.26cd |
| EZS2 | Irregular | Scarce | Cottony white | Lemon chiffon | 2.97 ± 0.26c |
| Mean 3.11 | |||||
| LSD 0.26 | |||||
| CV (%) 4.99 | |||||
Notes: EK1; EK2; EKO and EB (Enemorina Ener district isolates), CW; CA1; CA2 and CS (Cheha district isolates), EZS1; EZS2; EZS3; EZA and EZD (Ezya District isolates) and GC (Gera isolate)
Table 1: Cultural characteristics of C. kahawae isolates of Gurage districts.
Based on visual observation of the upper side of culture plates of colony appearance (aerial mycelial growth), dense, irregular (scarce) and very scarce types of colony (texture) were identified. Half of the isolates showed dense types of aerial mycelia growth and the rest scare types on PDA media (Table 1). Seventeen C. kahawae isolates showed 47.1%, 11.8 and 5.8% dense, irregular and very scarce aerial mycelia growth on both PDA and MEA media, respectively, whereas the rest 35.3% isolates showed inconsistent aerial mycelia growth [23]. From 35 Colletotrichum spp. isolates 60.0%, 31.4% and 8.6% on PDA indicated dense, irregular (scarce) and very scarce types of aerial mycelial growth, respectively [9].
Different colony colors were observed on both sides of the culture plates. Five groups of mycelial color were observed in upper side of plate‟s viz.; Gray white (35.71%), Dark gray white (28.57%), Ghost white (14.28%), Cottony white (14.28%) and floral white (7.14%) (Table 1). The reverse side of the culture plates also showed; light golden rod (28.57%), pale golden rod (21.42%), lemon chiffon (21.42%), Navajo white (7.14%), antique white (7.14%) and corn silk (7.14%) colony pigmentation (Table 1). Diverse colony colors have been previously reported on both sides of a culture plates. Light gray, dark gray, gray and white mycelia types of colony color were also observed from Hararghe C. kahawae isolates [24,25]. Abdi and Abu also indicated pale yellowish to pinkish with dense whitish-grey aerial mycelium and a few bright orange conidial masses on the tips of the active growing hyphae on MEA media [19]. Diverse colony colors of the pathogens were observed due to by using different growth media and the characteristics‟ the pathogens. In general, the mycelial colony color of the isolates are whitish at the 3-5 incubation days; light gray in 6-7 and then 8-10 incubation days changed to dark gray; a distinctive characteristic colony color of C. kahawae isolates.
The colony form of the Gurage C. kahawae isolates showed two types of colony form, which was most of the isolates was irregular and some isolates showed curled colony shape. These colony forms were the characteristics the filamentous fungi that the Colletotrichumspp. belongs.
Morphological characteristics C. kahawae isolate: There was highly significant (p< 0.001) difference among isolates in their conidia size (Table 2). All C. kahawae isolates had variable conidia length and width, which ranged between 9.03 to 10.49 ± 0.54 µm and 5.0 to 6.04 ± 0.22 µm, respectively. The average conidial length and width of isolates were 9.87 ± 0.54 µm and 5.38 ± 0.22 µm recorded, respectively (Table 2). Isolate EZA had the largest mean conidial length (10.49 ± 0.54 µm) and the smallest mean conidial length was recorded from isolate EZD (9.24 ± 0.54). While the widest conidial width was recorded on isolate EZS2 (6.04 ± 0.22), and the narrowest mean conidial width was from isolates EB (5.00 ± 0.22) (Table 2). In this study, all isolates showed variable conidial length and width similar previous observations by different authors. Tefestewold reported of variable mean conidia length (13.5 -19.3 µm) and width (2.9-5.2 µm) on PDA [8]. Kilambo et al. also recorded the conidia length ranged from 8 to 18 mm and width ranged from 2 to 6 mm and showed an overlap of conidia size between isolates thus, making it difficult to distinguish the strains of C. kahawae by conidia size [26]. Talhinhas et al.indicated variability in conidia size within and between strains when studying the diversity of Colletotrichum species in olive anthracnose and concluded that it is difficult to distinguish fungal strains using spore size [27].
| Isolate code | Conidia size (μm) | Conidia production | |
|---|---|---|---|
| Width | Length | (x10,000/ml) | |
| EZS2 | 6.04 ± 0.22a | 9.77± 0.54bcd | 277.33 ± 27.04g |
| CA2 | 5.64 ± 0.22b | 9.86 ± 0.54bc | 209.79 ± 27.04h |
| GC | 5.55 ± 0.22bc | 9.44 ± 0.54cde | 395.11 ± 27.04bc |
| CA1 | 5.47 ± 0.22bcd | 10.22 ± 0.54ab | 380.45 ± 27.04cd |
| EZS3 | 5.44 ± 0.22bcd | 9.03 ± 0.54e | 392.17 ± 27.04bc |
| EZS1 | 5.36 ± 0.22cde | 10.14 ± 0.54ab | 256.00 ± 27.04g |
| EK2 | 5.34 ± 0.22cde | 9.72 ± 0.54bcd | 355.75 ± 27.04de |
| EK1 | 5.33 ± 0.22cde | 10.22 ± 0.54ab | 182.25 ± 27.04i |
| EKO | 5.31 ± 0.22de | 10.06 ± 0.54ab | 305.97 ± 27.04f |
| EZD | 5.29 ± 0.22de | 9.24 ± 0.54de | 432.92 ± 27.04a |
| EZA | 5.26 ± 0.22de | 10.49 ± 0.54a | 250.83 ± 27.04g |
| CS | 5.25 ± 0.22de | 10.08 ± 0.54ab | 344.06 ± 27.04e |
| CW | 5.14 ± 0.22ef | 9.83 ± 0.54bc | 416.33 ± 27.04ab |
| EB | 5.00 ± 0.22f | 10.06 ± 0.54ab | 340.25 ± 27.04e |
| Mean | 5.38 | 9.87 | 324.23 |
| LSD | 0.22 | 0.54 | 27.04 |
| CV (%) | 2.49 | 3.25 | 5.84 |
Notes: EK1; EK2; EKO and EB (Enemorina Ener district isolates), CW; CA1; CA2 and CS (Cheha district isolates), EZS1; EZS2; EZS3; EZA and EZD (Ezha District isolates) and GC (Gera isolate).
Table 2: Mean conidia size and conidia production of C. kahawae isolate of Gurage zone.
Sporulation capacity of Gurage C. kahawae isolates has been evaluated on 10 days old cultures that revealed highly significant (P<0.001) differences among isolates (Table 2). Conidia production ranged between 182.25 to 432.92 × 104 conidia/ml of isolate EK1 and EZD, respectively. Hence, isolate EZD produced highly significant amount of conidia (432.92 ± 27.04 × 104) followed by isolates CW, GC and EZS3 but was not statistically different. While, isolate EK1 (182.25 ± 27.04 × 104) was produced the smallest amount of conidia which was highly significant difference among all isolates (Table 2). The high conidia production was the characteristics of the virulent pathogen of the C. kahawae isolates that produce enough inoculum sources for disease development. Tefestewold observed (1.2-5.2) × 105 conidia /ml and (6.84- 17.20) × 106 conidia /ml production from six isolates of C.kahawae on PDA medium and GCA (green coffee seed extract agar) [8]. Isolates of Colletotrichum species also produced an average number of conidia that ranged between 2.4 × 105to 1.3 × 107 on PDA [9].
Virulence determination of C. kahawae isolate of Gurage zone
The result revealed that all isolates were pathogenic to variety 370 and showed distinct and highly significant (p < 0.001) variations in the level of aggressiveness (Figure 1). The highest level of berry infection was recorded in isolate EZD with 68.06% infection from Ezha districts but statistically not significantly different from GC, CW, EB and CS isolates. The lowest berry infection level were recorded on isolate EZS2 (45.83%) but statistically not significantly different from EK2, CA2, EKO, EK1, CA1, EZA and EZS1 isolate (Figure 1). The isolate EZD produce highest amount conidia production and more virulent isolates as compare to the other isolates. C. kahawae strain can exhibited high virulence because of high sporulation capacity and germination of conidia in the host tissues [28,29]. The aggressiveness of the pathogen can be considered as quantitative measure of the level of the disease reached over time. This indicates that the most aggressive pathogen reached at a specific disease level faster than the less aggressive one. The situation can be measured via latent period, spore production, infection, lesion size and disease severity [30].
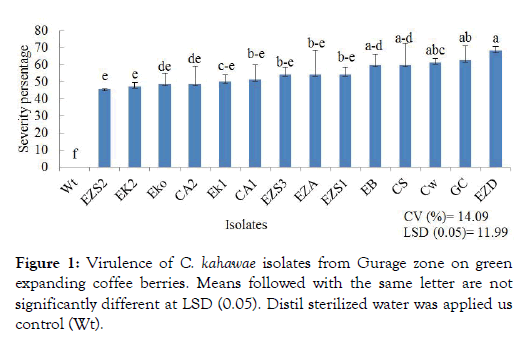
Figure 1: Virulence of C. kahawae isolates from Gurage zone on green expanding coffee berries. Means followed with the same letter are not significantly different at LSD (0.05). Distil sterilized water was applied us control (Wt).
Correlation between virulence and morpho-cultural characteristics: Pearson correlation analysis revealed that highly significant (P<0.001) and strong positive correlation of virulence with the conidia production of isolates (r=0.63) (Table 3). The isolate EZD produce high amount of conidia which infected coffee berry more severely than the remaining isolates. A related finding was also reported earlier by Varzea et al.[28]. Kilimbo et al. also indicated that the conidia productions were weak positively correlated to virulence of isolates (r=0.15) [17]. Pearson correlation result revealed that virulence of the isolates was positive but non-significant correlation with conidia growth rate of the isolates (r=0.14) (Table 3). Positive correlation was found between enlargement of lesion size and sporulation capacity, lesion size and percent berry infection, as well as sporulation capacity and percent berry infection [17]. But this result was not similar to Kilimbo et al. results, in which the lesion size was positively correlated to percent berry infection [17].
| Growth rate | Conidia | Width | Length | Virulence | |
|---|---|---|---|---|---|
| Growth rate | 1.00 | 0.42ns | 0.17ns | -0.15ns | 0.14ns |
| Conidia | 1.00 | -0.24ns | -0.59* | 0.63** | |
| Width | 1.00 | -0.23ns | -0.49* | ||
| Length | 1.00 | -0.34ns | |||
| Virulence | 1.00 |
Table 3: Pearson correlation coefficients of morpho-cultural characteristics and virulence of the 14 C. kahawae isolate of Gurage zone.
In this study the conidia size of the isolates were highly variable within and among the C. kahawae isolates. In fact, since there was high variation between the same isolate of the pathogen on its conidial size, there was insignificant relationship between pathogenicity (virulence) and conidial size [23]. The variability in fungal pathogenicity and the close relationship between sporulation and virulence could provide a useful information base for screening coffee germplasm collections for resistance to the pathogen and subsequent breeding programs for durable resistance through the selection of highly sporulated and virulent fungal isolates [17]. Hence, the virulent isolate EZD found in this study should be used as screening isolate in coffee CBD resistance evaluation.
Conclusion and Recommendations
Thirteen representative C. kahawae isolates of Gurage zone of major coffee producing areas of Ethiopia and one isolates from the CBD hot spot area of Gera were studied based on their cultural, morphological and pathological criteria. The study revealed the existence of variations in cultural, morphological and pathogenicity characteristics among C. kahawae isolates. All C. kahawae isolates collected from Gurage zone were pathogenic to susceptible Arabica coffee variety 370. However, isolates showed highly significant variation among them on their level of aggressiveness. The difference in virulence and aggressiveness implies that care should be taken and for further development of resistant varieties aggressive isolates should be used for a successful screening of coffee germplasms before the release of new developed cultivars.
REFERENCES
- Lashermes P, Combes MC, Ansaldi C, Gichuru E, Noir S. Analysis of alien introgression in coffee tree (Coffea arabica L.). Mol Breeding. 2011;27(2):223-232.
- Tefera A. Ethiopia: Coffee Annual Report. GAIN Report Number ET1514 (USDA Foreign Agricultural Service. Ethiopia. J Plant Pathol Microbes. 2015;6:302.
- Tadesse K, Mekdim D, Minten B. Coffee Income, Food Security and Diet Diversity of Small holder Coffee Growers in Ethiopia. EDRI Working Paper. 2015;15:39.
- ICO (International coffee organization). Coffee market report in the international trade, challenges and opportunities facing the sector. 2018;1-8
- Batista D, Silva DN, Vieira A, Cabral A, Pires AS, Loureiro A, et al. Legitimacy and implications of reducing Colletotrichum kahawae to subspecies in plant pathology. Frontiers in Plant Science. 2017;7:2051.
- Derso E, Waller JM. Variation among Colletotrichum isolates from diseased coffee berries in Ethiopia. Crop Protect. 2003;22(3):561-565.
- Garedew W, Lemessa F, Pinard F. Assessment of berry drop due to coffee berry disease and non CBD factors in arabica coffee under farmer’s fields of Southwestern Ethiopia. Crop Protect. 2017;98:276-282.
- Tefesetewold B. Studies of Colletotrichum population on Coffea arabica L. in Ethiopia and evaluations of the reactions of coffee germplasm. PhD Dissertation, University of Bonn, Germany. 1995;231.
- Alemu K, Adugna G, Lemessa F, Muleta D. Variation among colletotrichum isolates associated with coffee berry disease in Ethiopia. Cogent Biol. 2020;6(1):1740537.
- Abrar S, Fetta N, Ashenafi A, Negussie M, Gebre E. Quality status evaluation of Gurage coffee (Coffea arabica L.), Southern Ethiopia. J Biol Agri Healthcare. 2015;5(7):206-112.
- Hindorf H. Colletotrichum Population of Coffea arabica L. in Kenia. J Phytopathol. 1973;77(2):97-116.
- Biratu T, Hulluka M. Colletotrichum species associated with coffee berry disease in Harerge. Ethiopian J Agri Sci. 1989.
- Rayner W. A Mycological Colour Chart: In Commonwealth mycological Institute, Kew, Surrey, UK. 1970.
- Van der Vossen M, Cook A, Murakaru W. Breeding for resistance to coffee berry disease caused by Colletotrichum coffeanum Noack (Sensu Hindorf) in Coffea arabica LI Methods of preselection for resistance. Euphytca. 1976;25:733-745.
- Bayetta B. Arabica coffee breeding for yield and resistance to coffee Berry disease (Colletotrichum kahawae), Doctoral Dissertation. Imperial College at Wye University of London, UK. 2001;272
- Pinard F, Omondi CO, Cilas C. Detached berries inoculation for characterization of coffee resistance to coffee berry disease. J Plant Pathol. 2012;1:517-523.
- Kilambo L. Virulence of Colletotrichum kahawae strains and their effect on resistant Arabica coffee varieties in Tanzania (Doctoral dissertation, Sokoine University of Agriculture). 2008.
- Kamau AP. Characterization of coffee genotypes derived from crossing Rume Sudan and SL 28 coffee varieties against coffee berry disease (CBD) causal pathogen (Colletotrichum kahawae) (Doctoral dissertation). 2015.
- Mohammed A, Jambo A. Importance and characterization of coffee berry disease (Colletotrichum kahawae) in Borena and Guji Zones, Southern Ethiopia. J Plant Pathol Microbiol. 2015;6(09).
- Sas SA. STAT 9.3 User’s guide. Cary, NC: SAS Institute Inc. 2011.
- Hindorf H. Colletotrichum spp. isolated from Coffea arabica L. in Kenya. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. J Plant Dis Protect. 1970;1:328-331.
- Waller JM, Bridge PD, Black R, Hakiza G. Characterization of the coffee berry disease pathogen, Colletotrichum kahawae sp. nov. Mycological Res. 1993;97:989-994.
- Zeru A, Assefa F, Adugna G, Hindorf H. Variation of Colletotrichum kahawae isolates from diseased cherries of montane rainforest coffee in Ethiopia. In: 22nd International Conference on Coffee Science, ASIC 2008, Campinas, SP, Brazil, 14-19 September, 2008 2009;1341-1350. Association Scientifique Internationale du Café (ASIC).
- Abera A. Morphological characteristics of Colletotrichum species associated with mango (Mangifera indica L.) in Southwest Ethiopia. 2016.
- Berhanu T. Coffee berry disease (Colletotrichum kahawae): Status, pathogenic variability and reactions of coffee landraces in Hararghe, Eastern Ethiopia. Int J Plant Breed Crop Sci. 2014;2(1):38-42.
- Kilimbo D, Guerra L, Mabagala R, Varzea V, Haddad F, Loureiro A, et al. Characterization of Colletotrichum kahawae Strains in Tanzania. Int J Microbiol Res. 2013;5(2):382-389.
- Talhinhas P, Sreenivasaprasad S, Neves-Martins J, Oliveira H. Molecular and phenotypic analyses reveal association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Appl Environ Microbiol. 2005;71(6):2987-2998.
- Varzea P, Rodriques J, Silva C. Loss of resistance in interspecific tetraploid coffee varieties to some pathotypes of Hemileia vastatrix. In: International Synposium on Durable resistance: A key to sustainable agriculture, Ede-Wageningen, Holanda. 2002;34.
- Varzea P, Rodriques J, Silva C, Pedro P, Marques M. High virulence of a Colletotrichum kahawae isolate from Cameroon as compared with other isolates from other regions. In: Proceedings of the 18th International Conference on Coffee Science (ASIC), Helsink, Finland. 1999;516-519
- Pires S, Azinheira G, Cabral A, Cytogenomic characterization of Colletotrichum kahawae, the causal agent of coffee berry disease, reveals diversity in mini-chromosome profiles and genome size expansion. Plant Pathol. 2016;65:968-977.
Citation: Amare D, Hailu B, Daba G (2021) Cultural, Morphological and Pathogenic Variability of Colletorichuma kahawae Isolate of Gurage Zone. J Plant Pathol Microbiol. 12:532.
Copyright: © 2021 Amare D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.