Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 6
Cultural, Morphological and Pathogenic Variability among Isolates of Fusarium oxysporum f. sp. capsici Causing Wilt of Hot Pepper in Central Rift Valley, Ethiopia
Endriyas Gabrekiristos1*, Daniel Teshome2 and Getachew Ayana12Jimma University College of Agriculture and Veterinary Medicine, P.O. Box 307, Jimma, Ethiopia
Received: 27-Feb-2020 Published: 10-Jun-2020, DOI: 10.35248/2157-7471.20.11.499
Abstract
Fusarium wilt, caused by Fusarium oxysporum f. sp. capsici (FOC) is one of the major pathogens that constrained productivity of hot pepper in Ethiopia. The present study was conducted to characterize FOC isolates and evaluate the pathogenic variability of FOC isolates from Central Rift Valley of Ethiopia. Collection of diseased Fusarium wilt samples were carried out in Alaba, Adama, Adami Tullu Jiddo Kombolcha, Dugda, Mareko and Meskan districts, during the 2018 main cropping season. FOC isolates were characterized based on morphological features and pathogenicity test under greenhouse conditions. Regarding the characterization of FOC isolates, from the collected 70 root and stem samples, 49 were identified as F. oxysporum based on macroscopic (colony color, shape and margin) as well as microscopic characteristics (production of microconidia, macroconidia and chlamydospores). Of these, except 4AA2 (isolated from Alaba district), all were found pathogenic to the susceptible Mareko Fana variety, confirming the identity of the 48 isolates as FOC. However, Isolate 4DGK was identified as the most virulent isolate with 100% wilt incidence and 4.89 vascular discoloration index. Therefore 4DGk were identified as the master isolate for further study. The macroscopic and microscopic features of 4DGK isolate on potato dextrose agar are pink (color), filamentous (shape and margin), flat (elevation) and produce macroconidia with 1, 3 and 5 cell, microconidia and chlamydospore. However, 4AA2 was white (color), round (shape), raised (elevation) and entire (margin) macroscopically and produce macroconidia with single cell, microconidia and chlamydospore. Therefore, for the effective development of pepper variety resistant to Fusarium wilt should using virulent isolates like 4DKG together with other mixed isolates, in order to test a disease interaction and select for durable resistant genotypes.
Keywords
Fusarium wilt; Isolate; Hot pepper; Pathogenicity; Virulent; Ethiopia
Introduction
Hot pepper is one of the most important cash crops to Ethiopian smallholder farmers and an important agricultural commodity which contributes to export earnings [1]. Pepper is produced in most parts of Ethiopia, among which the southern Rift Valley regions are the major production areas [2].
However, a number of biotic and abiotic stresses are a constraint in hot pepper production. Among the biotic factors, wilt causing pathogens are becoming the leading problems in major hot pepper + producing countries of the world [3]. In Ethiopia, Fusarium wilt, caused by Fusarium oxysporum f. sp. Capsici, is reemerging and reported from major hot pepper producing regions. Recently, the disease is becoming more aggressive and forcing farmers to shift to the cultivation of other crop species in the country [4]. In the Plant Protection Research Strategy of Ethiopia, almost two decades ago, Fusarium wilt was not a top priority disease compared to pepper motile virus [5]. But Tameru et al. reported that the disease is becoming more aggressive and forcing farmers shift this crop with cultivation of other crop [4].
Recent research report by Assefa et al. indicates 86.4% paper wilt incidence was due to fusarium wilt in Ethiopia [6]. Estimated yield loss due to fusarium wilt in one of the major pepper growing areas of Ethiopia ranging between 68 and 71% [7].
The importance of Fusarium wilt varies depending on host susceptibility, virulence level of the pathogen, soil type and temperature [8]. According to Sanogo and Assefa et al. high temperature, moisture and clay loam soil type were found to play a crucial role in Fusarium wilt disease development; implying that the significance of Fusarium wilt varies even from field to field within a farmers’ association [6,9]. This could suggest the need for detail study of the morphological and virulence level of the pathogen for specific areas which is a key requirement for the development of management strategies.
Previous studies indicated that variability of the pathogen in their morphological characteristics [10], and morphological variability was described for differences in shape, size, septation and sporulation characteristics of macro-and micro-conidia of the pathogen isolates [11]. But, studies towards characteristic features of the pathogen were not received adequate attention in the country. Cognizant of the above facts, this study presented the morphological characteristic features and pathogenic level of Fusarium oxysporum f. sp. capsici isolates collected from the Central Rift Valley of Ethiopia.
Materials and Methods
Characterization of Fusarium oxysporum isolates
Sampling, isolation and purification of F. oxysporum isolates: For this assay, which involved characterization of F. oxysporum isolates via morphological traits and pathogenicity tests, both stem and root samples of diseased hot pepper plants showing wilt symptoms were collected from six districts (Table 1). The districts were selected based on their history and potential of hot pepper production in Ethiopia. Accordingly, five stem/root samples from plants showing the typical symptoms of Fusarium wilt were collected from each field and placed in plastic bags, and transported to Melkassa Agricultural Research Center (MARC) phytopathology laboratory. The samples were preserved at 4°C until isolation in the laboratory.
Table 1: List of districts, Farmers associations and Code in Central Rift valley of Ethiopia, 2018.
| S. No. | District | Farmers association | Code | Number of sample |
|---|---|---|---|---|
| 1 | Alaba | Ansha-2 | AA2 | 5 |
| Ansha-1 | AA1 | 4 | ||
| Alam Tena | AAT | 4 | ||
| 2 | Meskan | Bate Futo | MBF | 5 |
| Samen Dida | MSD | 5 | ||
| Bache Gulchan | MBG | 4 | ||
| 3 | Mareko | Dida Mindore | MDM | 5 |
| Dida Halibo | MDH | 5 | ||
| Jara Damaka | MJD | 5 | ||
| 4 | Dugda | Bekele Girrisa | DBG | 6 |
| Graba Korke Adi | DGK | 5 | ||
| Dodota Dambel | DDD | 5 | ||
| 5 | Adama | Melkassa | MRC | 7 |
| 6 | ATJK | Eddo Gojola | ATEG | 5 |
| Total | 6 | 14 | 14 | 70 |
Note: ATJK (Adami Tullu Jiddo Kombolcha)
Isolation procedures followed, segments of root/stem samples showing lesions characteristics of fusarium wilt infection were thoroughly washed under running tap water, cut into small pieces (2-3 mm) with lesion having half healthy and half diseased tissue and surface sterilized with 2% sodium hypochlorite solution for 30 seconds and subsequently washed 3 times in sterile distilled water. Samples were dried on filter paper, transferred to potato dextrose agar (PDA) plates (9 cm diameter) supplemented with streptomycin (30 μg/l) to avoid bacterial contaminations and subsequently incubated for 2 days at 28°C in dark. Two days later, typical fusarium colonies were transferred into new PDA plates following the hyphal tip method [12] and incubated for 5 days to get pure culture. Each isolates were replicated three times.
Morphological characterization: Colony characters of color, shape, margin, elevation and radial growth were visually examined from the upper and reverse sides of 3rd, 5th and 7th days old pure cultures. For further verification, vegetative and reproductive (macroconidia, microconidia and chlamydospore) structures of the isolates were studied under compound microscope (40x magnification) using the descriptions in the atlas of Fusarium [13]. The radial growth of the isolates was measured at the odd dates starting from culture date as stated above.
Characterization via pathogenicity test: Pathogenicity analysis of the isolates morphologically identified as F. oxysporum f. sp. capsici was conducted under greenhouse conditions using the susceptible variety Mareko Fana [14]. Inocula of the individual isolates were prepared on PDA from 10 days old cultures. Conidia were harvested to 15ml beaker by adding 10 ml of sterile distilled water (SDW) in each Petri plate. From the filtered culture, conidia were resuspended in SDW and the final conidial density was adjusted to 1 × 106 spore/ml using a haemocytometer.
Raising of seedlings, inoculation and disease assessment: Accordingly, roots of 4 weeks old seedlings, raised in the greenhouse at 28°C on a tray using sterilized sand and top soil (1:2), were trimmed with a sterile scissor and submerged for 30 minutes into tubes containing 9 ml of the spore suspension (9 seedlings/tube). Inoculation was performed following the standard cut-root dip inoculation technique of Herman and Perl-Treves and Karimi et al. Roots of uninoculated plants were similarly cut and dipped into SDW for the same period of time [15,16]. Both inoculated and uninoculated seedlings were transplanted to 26 × 22 cm2 pots filled with 2:1 ratio mixture of sterilized top soil and sand [17]. The pots were arranged in a completely randomized design (CRD) with three replications. Starting from two weeks after inoculation, wilt incidence was evaluated based on appearance of typical symptoms of Fusarium wilt. Moreover, vascular disease severity expressed as vascular discoloration was also evaluated immediately after uprooting of inoculated plants following procedures of Ulloa et al. using the disease index of 0 to 5 scales and root length was also measured [18]. To further verify the pathogenicity of each isolate, reisolation of the pathogen from diseased plants was performed to confirm the identity of the pathogen.
Data analysis
The data on colony characteristics and microscopic studies were analyzed by descriptive statistics. The fusarium wilt incidence, vascular disease index and root length of the pathogenecity study was subjected to analysis of variance using one way ANOVA by SAS software version 9.3. Differences among treatment means were separated using the Least Significant Difference (LSD) test at the alpha level of 5%.
Results and Discussion
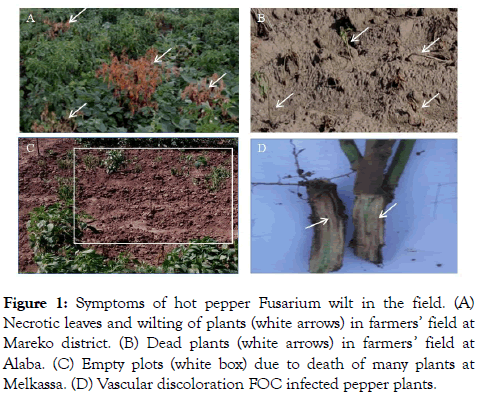
Characterization of Fusarium oxysporum isolates Symptoms of hot pepper Fusarium wilt in the field: In pepper plants, FOC invades xylem vessels through xylem pits and grows within the vascular tissues (Figure 1). HPFW inhibits the upward movement of plant nutrients due to blockage of the xylem vessels by the formation of vascular occlusions which also causes wilting and plant death [19].
Figure 1: Symptoms of hot pepper Fusarium wilt in the field. (A) Necrotic leaves and wilting of plants (white arrows) in farmers’ field at Mareko district. (B) Dead plants (white arrows) in farmers’ field at Alaba. (C) Empty plots (white box) due to death of many plants at Melkassa. (D) Vascular discoloration FOC infected pepper plants.
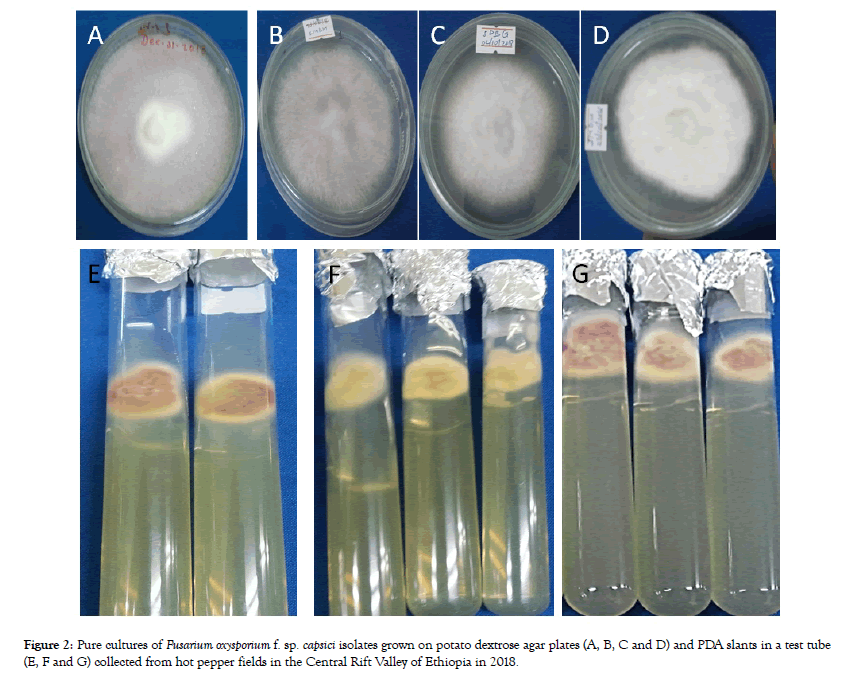
Culture characterization of Fusarium oxysporum isolates: Based on the basic colony characteristics on PDA, out of the 70 collected samples, 49 isolates were identified as Fusarium oxysporum f. sp. capsici, the causative agent of HPFW (Figure 2).
Figure 2: Pure cultures of Fusarium oxysporium f. sp. capsici isolates grown on potato dextrose agar plates (A, B, C and D) and PDA slants in a test tube (E, F and G) collected from hot pepper fields in the Central Rift Valley of Ethiopia in 2018.
Mycelial growth, color, shape, elevation and margin of colony were evaluated at 3rd, 5th and 7th date after incubation (DAI) for macroscopic examination of the isolates (Table 2).
Table 2: Colony characteristics of cultures identified as Fusarium oxysporum f. sp. capsici isolates on PDA plates at 7 days after incubation.
| No. | Isolate | Radial | Color | Shape | Elevation | Margin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth (cm) | Pink | White | Filamentous | Round | Flat | Raised | Filamentous | Entire | ||
| 1 | 1AA2 | 3.1 | + | - | + | - | + | - | + | - |
| 2 | 3AA2 | 3.2 | + | - | + | - | + | - | - | + |
| 3 | 4AA2 | 4.5 | - | + | - | + | - | + | - | + |
| 4 | 5AA2 | 3.8 | + | - | + | - | - | + | + | - |
| 5 | 3AA1 | 2.9 | + | - | - | + | - | + | - | + |
| 6 | 1AAT | 3.2 | + | - | - | + | - | + | - | + |
| 7 | 2AAT | 3.5 | + | - | - | + | - | + | + | - |
| 8 | 3AAT | 3.5 | + | - | + | - | - | + | + | - |
| 9 | 4AAT | 3.2 | + | - | + | - | - | + | + | - |
| 10 | 2MBF | 3.0 | + | - | + | - | - | + | + | - |
| 11 | 5MBF | 3.5 | - | + | - | + | - | + | - | + |
| 12 | 1MSD | 3.2 | - | + | - | + | - | + | - | + |
| 13 | 3MSD | 3.4 | - | + | - | + | - | + | - | + |
| 14 | 1MBG | 3.0 | + | - | + | - | - | + | + | - |
| 15 | 2MBG | 3.0 | + | - | - | + | - | + | + | - |
| 16 | 3MBG | 3.5 | + | - | + | - | - | + | + | - |
| 17 | 5MBG | 3.2 | - | + | - | + | - | + | - | + |
| 18 | 1MDM | 3.2 | + | - | + | - | - | + | - | + |
| 19 | 2MDM | 2.5 | + | - | + | - | - | + | + | - |
| 20 | 3MDM | 3.2 | + | - | + | - | + | - | + | - |
| 21 | 4MDM | 3.8 | + | - | + | - | - | + | + | - |
| 22 | 5MDM | 3.2 | + | - | + | - | + | - | + | - |
| 23 | 1MDH | 3.8 | + | - | - | + | - | + | - | + |
| 24 | 3MDH | 3.2 | - | + | - | + | - | + | - | + |
| 25 | 1MJD | 3.5 | + | - | - | + | - | + | - | + |
| 26 | 2MJD | 3.2 | + | - | - | + | - | + | - | + |
| 27 | 3MJD | 2.5 | + | - | + | - | - | + | + | - |
| 28 | 4MJD | 2.8 | + | - | + | - | - | + | + | - |
| 29 | 1MRC | 3.2 | + | - | - | + | - | + | - | + |
| 30 | 2MRC | 2.6 | + | - | + | - | + | - | - | + |
| 31 | 3MRC | 3.2 | + | - | - | + | - | + | - | + |
| 32 | 4MRC | 2.4 | + | - | + | - | - | + | + | - |
| 33 | 5MRC | 3.2 | + | - | - | + | - | + | - | + |
| 34 | 1ATEG | 3.8 | + | - | - | + | - | + | - | + |
| 35 | 2ATEG | 3.0 | - | + | - | + | + | - | - | + |
| 36 | 1DBG | 3.2 | + | - | + | - | + | - | + | - |
| 37 | 2DBG | 3.2 | + | - | + | - | + | - | + | - |
| 38 | 4DBG | 2.9 | - | + | + | - | - | + | - | + |
| 39 | 5DBG | 3.0 | + | - | + | - | - | + | + | - |
| 40 | 1DGK | 3.0 | - | + | - | + | - | + | + | - |
| 41 | 2DGK | 3.5 | + | - | - | + | - | + | - | + |
| 42 | 3DGK | 3.2 | + | - | - | + | - | + | - | + |
| 43 | 4DGK | 3.0 | + | - | + | - | + | - | + | - |
| 44 | 5DGK | 3.6 | - | + | + | - | - | + | - | + |
| 45 | 1DDD | 2.8 | - | + | + | - | - | + | - | + |
| 46 | 2DDD | 3.0 | - | + | + | - | - | + | - | + |
| 47 | 3DDD | 2.6 | - | + | + | - | - | + | - | + |
| 48 | 4DDD | 3.0 | + | - | + | - | + | - | + | - |
| 49 | 5DDD | 3.5 | - | + | + | - | - | + | - | + |
+=Present and-=Absent of the colony characteristic studied on PDA medium.
Based on the fungal radial growth, isolates were grouped as sluggish grower (2.4-2.9 cm), intermediate grower (3-3.5 cm) and fast grower (3.6-4.5 cm). Accordingly, isolate 4MRC, 2MDM, 3MJD, 2MRC, 3DDD, 4MJD, 1DDD, 3AA1 and 4DBG were found to be slow growers. The fastest growing isolates were 5DGK, 5AA2, 4MDM, 1MDH, 1ATEG and 4AA2. The remaining 31 isolates were grouped under intermediate growth category (Table 2).
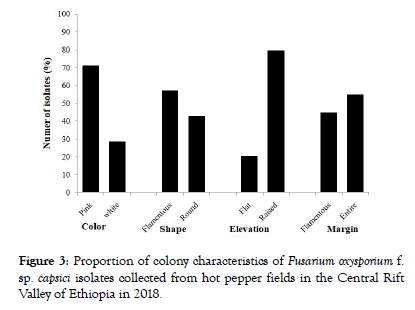
Of the 49 FOC isolates, 35 (71.4%) of them had pink colony color and the remaining 14 (28.6%) isolates had white colony color (Figure 3 and Table 2). Regarding colony shape, 21 isolates were round shaped and 28 were filamentous. The differential color of the F. oxysporum isolates may be assigned to the presence of specific pigment viz., javanicin, bostrycoidin, solanione and lycopersin [20]. The elevations of the colony were flat type (10 isolates) and 39 isolates had raised colony elevation (Figure 3).
Figure 3: Proportion of colony characteristics of Fusarium oxysporium f. sp. capsici isolates collected from hot pepper fields in the Central Rift Valley of Ethiopia in 2018.
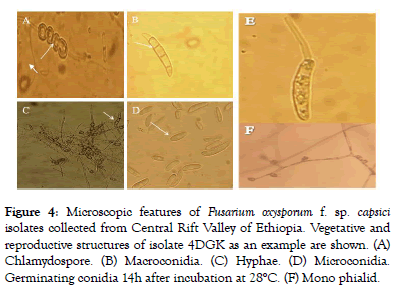
Morphological characterization of Fusarium oxysporum isolates: After characterization by colony features, the morphological features like presence or absence of Chlamydospore and microconidia, number of septa in macroconidia and formation of microconidia on phialid were examined as in Figure 4.
Figure 4: Microscopic features of Fusarium oxysporum f. sp. capsici isolates collected from Central Rift Valley of Ethiopia. Vegetative and reproductive structures of isolate 4DGK as an example are shown. (A) Chlamydospore. (B) Macroconidia. (C) Hyphae. (D) Microconidia. Germinating conidia 14h after incubation at 28°C. (F) Mono phialid.
Hyphae of the fungus were more or less branched and septated. Most macroconidia were sickle shaped and some of them were straight shaped with 3-5 septa. Similar microscopic features were also reported by Leslie and Summerell and Samson et al. who found that the macroconidia in F. oxysporum were fusoid, thinwalled, relatively slender with the widest part in the middle and pointed at both ends [21].
Variations in number of septations in the macroconidia were also observed. Average number of septa most frequently occurred varied from 1 to 5. The most frequent conidial septations observed in most (29) of the isolates were in the range of 1 to 3, and 1 to 5 conidial septations in 8 isolates and 12 isolates did not produce septa until 10th date (Table 3). Jaywant reported that FOC isolates were frequently 1-3 and less frequently 1-5 septa per conidia [22]. Moreover, all isolates produced chlamydospore in single or in pairs and microconidia in mono philiads. The germination of FOC isolates was also examined by putting the isolate in sterilized distilled water for 14h and successful germination of the spores were observed 7 days after incubation (Figure 4).
Table 3: Microscopic features of Fusarium oxysporum f. sp. capsici isolates collected from diseased hot pepper plants in the Central Rift Valley of Ethiopia, during the 2018 cropping season.
| No. | Isolate | Conidia | No. of septa in | Chlamydospore | ||
|---|---|---|---|---|---|---|
| Micro | Macro | Macroconidia | Yes | No | ||
| 1 | 1AA2 | + | + | 1, 2 and 3 | + | |
| 2 | 3AA2 | + | + | 1, 3, 4 and 5 | + | - |
| 3 | 4AA2 | + | + | 1 | + | - |
| 4 | 5AA2 | + | - | - | + | - |
| 5 | 3AA1 | + | - | - | + | - |
| 6 | 1AAT | + | - | - | + | - |
| 7 | 2AAT | + | - | - | + | - |
| 8 | 3AAT | + | - | - | + | - |
| 9 | 4AAT | + | - | - | + | - |
| 10 | 2MBF | + | + | 1, 2, 3, 4 and 5 | + | - |
| 11 | 5MBF | + | + | 1 | + | - |
| 12 | 1MSD | + | + | 1 | + | - |
| 13 | 3MSD | + | + | 1 | + | - |
| 14 | 1MBG | + | + | 1, 3 and 4 | + | - |
| 15 | 2MBG | + | + | 1, 3 and 4 | + | - |
| 16 | 3MBG | + | + | 1, 3 and 5 | + | - |
| 17 | 5MBG | + | + | 1, 3 and 4 | + | - |
| 18 | 1MDM | + | + | 1, 2 and 3 | + | - |
| 19 | 2MDM | + | + | 1 | + | - |
| 20 | 3MDM | + | + | 1 and 3 | + | - |
| 21 | 4MDM | + | + | 1 and 2 | + | - |
| 22 | 5MDM | + | + | 1, 2 and 3 | + | - |
| 23 | 1MDH | + | - | - | + | - |
| 24 | 3MDH | + | - | - | + | - |
| 25 | 1MJD | + | + | 1 and 3 | + | - |
| 26 | 2MJD | + | - | - | + | - |
| 27 | 3MJD | + | + | 1 and 2 | + | - |
| 28 | 4MJD | + | + | 1, 2 and 3 | + | - |
| 29 | 1MRC | + | + | 1, 2 and 3 | + | - |
| 30 | 2MRC | + | + | 1, 2 and 3 | + | - |
| 31 | 3MRC | + | + | 1, 2 and 3 | + | - |
| 32 | 4MRC | + | + | 1, 2 and 3 | + | - |
| 33 | 5MRC | + | + | 1, 2 and 3 | + | - |
| 34 | 1ATEG | + | + | 1, 2 and 3 | + | - |
| 35 | 2ATEG | + | + | 1 and 2 | + | - |
| 36 | 1DBG | + | + | 1 and 2 | + | - |
| 37 | 2DBG | + | + | 1, 2 and 3 | + | - |
| 38 | 4DBG | + | - | - | + | - |
| 39 | 5DBG | + | - | - | + | - |
| 40 | 1DGK | + | + | 1 and 2 | + | - |
| 41 | 2DGK | + | + | 1,2 and 3 | + | - |
| 42 | 3DGK | + | + | 1,3 and 5 | + | - |
| 43 | 4DGK | + | + | 1,3 and 5 | + | - |
| 44 | 5DGK | + | - | - | + | - |
| 45 | 1DDD | + | + | 1 and 2 | + | - |
| 46 | 2DDD | + | + | 1,2 and 3 | + | - |
| 47 | 3DDD | + | + | 1,2 and 3 | + | - |
| 48 | 4DDD | + | + | 1 and 2 | + | - |
| 49 | 5DDD | + | + | 1 and 3 | + | - |
FOC: Fusarium oxysporium; +: present; -: Absent
Pathogenicity of Fusarium oxysporum isolates: Results of wilt incidence analysis showed that most of the isolates induced wilting at 20 days after inoculation. Depending on the level of wilt incidence, isolates were grouped into five pathogenic classes, non-pathogenic, less pathogenic, moderately pathogenic, pathogenic and highly pathogenic. Accordingly; 1, 3, 21, 12 and 12 isolates were identified as a virulant, less virulent, moderately virulent, virulent and highly virulent, respectively (Table 4).
Table 4: Evaluation of pathogenicity of Fusarium oxysporum f. sp. capsici isolates collected from hot pepper fields in the Central Rift Valley of Ethiopia, during the 2018 cropping season.
| No. | Isolates | Source (Isolated from Kebele) |
Wilt incidence (%) 50 DAI | Virulence level considering wilt incidence |
Vascular disease index |
Root length (cm) |
|---|---|---|---|---|---|---|
| 1 | 3MDH | Dida Halibo | 0.0 | Avr | 0.22 | 25.78 |
| 2 | 3DDD | Dodota Dembal | 44.4 | ** | 2.00 | 25.44 |
| 3 | 2MBF | Bate Futo | 66.7 | *** | 2.67 | 18.67 |
| 4 | 1AA2 | Ansha 2 | 66.7 | *** | 3.44 | 22.78 |
| 5 | 5DBG | Bekele Girrisa | 66.7 | *** | 3.22 | 16.89 |
| 6 | 1MBG | Bache Gulchana | 55.6 | *** | 2.7 | 22.89 |
| 7 | 3DGK | Giraba Korke Adi | 77.8 | **** | 3.33 | 14.56 |
| 8 | 4MRC | Melkassa | 33.3 | ** | 1.56 | 20.78 |
| 9 | 2MBG | Bache Gulchana | 55.6 | *** | 2.67 | 15.11 |
| 10 | 1DGK | Giraba Korke Adi | 33.3 | ** | 2.22 | 18.89 |
| 11 | 3MBG | Bache Gulchana | 77.8 | **** | 3.11 | 17.33 |
| 12 | 1MDH | Dida Halibo | 22.2 | ** | 1.67 | 22.56 |
| 13 | 2MRC | Melkassa | 33.3 | ** | 1.00 | 18.00 |
| 14 | 3MDM | Dida Midore | 88.9 | **** | 4.11 | 6.11 |
| 15 | 2DGK | Giraba Korke Adi | 33.3 | ** | 3.11 | 13.11 |
| 16 | 5DGK | Giraba Korke Adi | 44.4 | ** | 2.44 | 22.11 |
| 17 | 1AAT | Alem Tena | 33.3 | ** | 2.00 | 19.78 |
| 18 | 5DDD | Dodota Dembal | 33.3 | ** | 1.11 | 25.78 |
| 19 | 4MJD | Jarra Demaka | 66.7 | *** | 3.00 | 18.00 |
| 20 | 1MJD | Jarra Demaka | 77.8 | **** | 3.56 | 20.78 |
| 21 | 3AAT | Alem Tena | 88.9 | **** | 2.11 | 19.22 |
| 22 | 1ATEG | Eddo Gojola | 77.8 | **** | 3.22 | 21.00 |
| 23 | 1MDM | Dida Midore | 77.8 | **** | 2.89 | 17.67 |
| 24 | 4DBG | Bakele Girrisa | 44.4 | ** | 1.22 | 19.56 |
| 25 | 3MRC | Melkassa | 33.3 | ** | 1.11 | 22.33 |
| 26 | 5MDM | Dida Midore | 55.6 | *** | 2.78 | 16.44 |
| 27 | 2DBG | Bakele Girrisa | 33.3 | ** | 2.11 | 23.22 |
| 28 | 5AA2 | Ansha 2 | 33.3 | ** | 2.89 | 15.67 |
| 29 | 1MSD | Samen Dida | 22.2 | ** | 1.11 | 24.00 |
| 30 | 2ATEG | Eddo Gojola | 44.4 | ** | 1.89 | 20.22 |
| 31 | 4DGK | Giraba Korke Adi | 100.0 | **** | 4.89 | 5.78 |
| 32 | 2MJD | Jarra Demaka | 77.8 | **** | 2.67 | 14.89 |
| 33 | 3MSD | Samen Dida | 11.1 | * | 0.44 | 28.11 |
| 34 | 1MRC | Melkassa | 88.9 | **** | 3.11 | 17.33 |
| 35 | 5MRC | Melkassa | 33.3 | ** | 1.89 | 20.11 |
| 36 | 2AAT | Alem Tena | 66.7 | *** | 3.11 | 20.78 |
| 37 | 5MBF | Bate Futo | 55.6 | *** | 1.22 | 22.78 |
| 38 | 1DDD | Dodota Dembal | 33.3 | ** | 1.67 | 23.44 |
| 39 | 2MDM | Dida Midore | 44.4 | ** | 3.22 | 17.78 |
| 40 | 4DDD | Dodota Dembal | 11.1 | * | 2.00 | 24.33 |
| 41 | 3AA1 | Ansha 1 | 77.8 | **** | 3.56 | 15.44 |
| 42 | 2DDD | Dodota Dembal | 66.7 | *** | 2.44 | 23.89 |
| 43 | 3AA2 | Ansha 2 | 22.2 | ** | 1.89 | 19.00 |
| 44 | CCC | Control | 0.0 | - | 0.00 | 29.89 |
| 45 | 3MJD | Jarra Demaka | 33.3 | ** | 1.56 | 21.00 |
| 46 | 4AAT | Alem Tena | 66.7 | *** | 3.33 | 12.44 |
| 47 | 1DBG | Bakele Girrisa | 55.5 | *** | 3.33 | 18.67 |
| 48 | 4AA2 | Ansha 2 | 0.0 | Avr | 0.00 | 29.22 |
| 49 | 5MBG | Bache Gulchana | 33.3 | ** | 3.56 | 15.33 |
| 50 | 4MDM | Dida Midore | 88.9 | **** | 4.00 | 13.89 |
| CV (%) | - | - | 16.75 | 5.74 | ||
| LSD (5%) | - | - | 0.64 | 1.82 |
DAI: Days After Inoculation, None-patho: None pathogenic. *: Less pathogenic (1-20). **: Moderately pathogenic (21-50). ***: Pathogenic (51-70). ****: Highly pathogenic (71-100). CCC: Control.
Pathogen variability in terms of levels of pathogenicity is a very common phenomenon within the same pathogen species as several factors influence the virulence level of phytopathogens [23]. Interestingly, Dugda district (Giraba Korke Adi kebele) was found as the major source of the most pathogenic isolates that induced 100% disease incidence.
Since evaluating the incidence of the disease from foliage part is not sufficient and it has to be supported by other evidences, effect of the isolates on the level of vascular discoloration was evaluated according to [24]. As Fusarium wilt of pepper is a vascular pathogen, the severity of the disease was higher on the xylem vesicles than foliage part [25]. This is verified in the present vascular discoloration data that revealed some isolates as virulent which have been grouped a virulant when their foliage disease (wilting) was assessed. This suggest that evaluating only the foliage part for systemic disease like fusarium wilt may mislead the pathogen’s character, as verified in isolate 3MDH. This isolate results no wilt incidence upon evaluating the foliage part but results in a disease index of 0.22 when evaluated for vascular discoloration (Table 4). According to this evaluation, almost all (48) isolates were found to be pathogenic to hot pepper (Mareko Fana variety) and induced the typical vascular discoloration symptom on xylem vesicles. Of these, the Dugda isolates 3DGK, 4DGK 1DBG, the Mareko isolates 3MDM, 4MDM and 1MJD, the Alaba isolates 3AA1, 1AA2 and 4AAT and the Meskan isolate 5MBG were identified as the highly aggressive isolates.
According to Ulloa et al. devised scale (0 to 5) where 0=No discoloration, 1=Light discoloration evident as spotty areas in the cross section of the stem, 2=More continuous discoloration covering an area between one quarter and one half of the crosssection stem but light in color, 3=Vascular discoloration (moderate in color) evident in a band encircling almost the entire stem cross-section, 4=Vascular discoloration darker in color than in 1 or 2, and evident across most of the vascular tissue in a cross section of the stem, and 5=Plant severely damaged, vascular discoloration evident throughout crosssection of the stem, isolates 4DGK, 3MDM and 4MDM which recorded 4.89, 4.11 and 4.00 vascular disease index values respectively, were as the most aggressive isolates [18].
When isolates were evaluated for their effect of root growth, significant differences were observed. In the present study, as expected the highest root length was recorded on the uninfected control plants (29.89 cm). Interestingly 60 days after inoculation, plants inoculated with isolate 4AA2 and 3MSD had 29.22 cm and 28.1 cm, respectively, almost similar to the control plants, indicating less/no effect on the root. On the other hand, the lowest root length was recorded in plants inoculated with 4DGK (5.78 cm) and 3MDM (6.1 cm) (Table 4). Mamta et al. reported that root length, among other parameter describes the effect of the pathogen which has direct correlation with wilt [23]. Because, once the pathogen colonizes the xylem tissue which is nutrient poor region to obtain all factors required for growth, reproduction, and survival, the plant starts to decline in growth of different plant cells and tissues [26].
Isolate 4AA2, were not pathogenic to the host and this isolate was unique from others by having fast radial growth, white in colony color and Macroconida having one septa (Tables 3 and 4). Based on Fusarium oxysporum f. sp. capsici identification key, this isolates share common features like production of white colony color, microconidia, Chlamydospore and macroconidia but the radial growth of the isolate was faster when compared with 48 FOC isolates. The significant occurrence of hot pepper fusarium wilt causing pathogen is approved both in morphological characterization and pathogenecity tests; but this study more precisely identified the virulent isolates in the pathogenicity test, suggesting that characterizing isolates based on macroscopic and microscopic features is not enough, unless it is supported by other elegant methods such as pathogenecity and molecular analyses.
Conclusion
Fusarium wilt caused by Fusarium oxysporum f. sp. capsici (FOC) is the most emerging pathogen by causing qualitative and quantitative loss of hot pepper. The present study was undertaken in order to identify and characterization of FOC isolates based on morphological identity and pathogenic variability. Regarding the diversity of FOC isolates in the study area, based on cultural and morphological characteristics, 49 isolates were identified as hot pepper Fusarium oxysporum. The colony colour of 35 isolates were pink and 14 of them were white and among those F. oxysporum isolates, 21 isolates revealed round shaped colony and 28 isolate revealed filamentous. The elevation of the colony was flat type in 10 isolates, 39 isolates had raised/elevate colony growth with entire colony margin (i.e., 27 isolates) and filamentous (22 isolates). Most macroconidia were sickle shaped and some were straight shaped with 3-5 septa/conidia in most isolates. In all isolates, hypha was branched and septated. All isolates produce chlamydospore and microconidia which have no septation. Interestingly, the above mentioned morphological characters were similar with that of most previously described (literatures) of Fusarium oxysporum isolates in hot pepper.
The study on the pathogenicity of FOC isolates also revealed that almost all isolates were pathogenic to the susceptible Mareko Fana variety, of course with different level of aggressiveness as evidenced by the variation on the effect on root growth and intensity of vascular discoloration. In this regard, Dugda (Giraba Korke Adi kebele) was identified as the source of the most pathogenic isolate 4DGK which caused 100% wilt incidence/plant death, severe reduction (80.7%) of root growth and the highest level of vascular disease index (4.89) which is equivalent to 97.8% disease severity. 4AA2 isolate from Alaba was not pathogenic to Mareko Fana variety but the isolate shares similar morphological identity.
Studies on molecular characterization should have to be conducted to identify pathogenic isolates in Ethiopia. Varietal screening should be intensively conducted by using 4DGK isolate, which is identified as the most pathogenic isolate. Morphologically identified isolates with their respective virulence level should have to be evaluated in different soil textural classes. Since the pathogen is soil born and persistent, integrated management options should be developed to keep soil health.
Acknowledgment
I would like to thank Melkassa Agricultural Research Center (Horticulture and Plant protection staff member) and Korean project on international Agriculture for the support in budget for undertaking the survey and laboratory work. Similarly, I would like to acknowledge Plant science and Horticulture Staff members of Jimma University, college of Agriculture and Veterinary medicine for their contributions.
REFERENCES
- Beyene T, David P. Ensuring small scale producers in Ethiopia to achieve sustainable and fair access to pepper market. Uganda Journal of Agriculture. 2007;3(2):113-119.
- Tameru A. Characterization of virus of pepper (Capsicum spp.) and sweet potato (Ipomoea batatas) from Ethiopia. PhD thesis, University of Bonn, Germany. 2004;150.
- Naqvi SAMH. Diseases of fruit and vegetables. In: Diseases of pepper and their management (Eds. Roberts PD, Adkins S, Pernezny K and Jones JB). Kluwer Academic Publishers, The Netherlands. 2004;2:333-387.
- Tameru A, Hamacher J, Dehne HW. The increase in importance of Ethiopian Pepper mottle Virus (EPMV) in the rift valley part of Ethiopia; time to create Awareness among researchers an extension workers, Paper presented at Deutsches Tropentage, Gottingen, Germany. 2003.
- EARO. Ethiopian Agricultural Research Organization. Ethiopian Agricultural Research organization (EARO). Annual report for 2000, Addis Ababa, Ethiopia. 2002.
- Assefa M, Dawit W, Lencho A. Hunduma T. Assessment of wilt intensity and identification of causal fungal and bacterial pathogens on hot pepper (capsicum annuum L.) in Bako Tibbe and Nonno districts of West Shewa Zone, Ethiopia. International Journal of Phytopathology. 2015;4(1):21-28.
- Soboka TB, Fininsa C, Gorfu D. Integrated Approach and Plant Extract Management Options against Pepper Wilt (Fusarium oxysporum Var. Vasinfectum) at Bako, Western Ethiopia, MSc. Thesis in Plant Pathology, Haramya University, Ethiopia. 2012.
- Goldberg NP. Verticillium Wilt of Chile Peppers College of Agricultural, Consumer and Environmental Sciences on the World Wide. 2010.
- Sanogo S. Chile pepper and the threat of wilt diseases. Plant Health Progress. 2003.
- Kaushal A. Studies on Fusarium Wilt of Bell Pepper (Capsicum annuum L.). Master of Science (Agriculture) Plant Pathology Department of Plant Pathology College of Horticulture. 2016.
- Bahar M, Shahab H. Analysis of Iranian isolates of Fusarium solani using morphological, pathogenicity and microsatellite DNA marker characterization. African Journal of Biotechnology. 2012;11(2):474-482.
- Tuite K. Review article on B.G. Hewitt’s Georgian: A Structural Reference Grammar (London Oriental and African Language Library, 2. Amsterdam: Benjamin’s 1995. Functions of Language. 1996;3(2):258-260
- Leslie JF, Summerell BA. The Fusarium Laboratory Manual. Black well Publishing, 2006, Iowa, USA.
- Kassahun S, Tariku H, Mekonnen A. Characterization and evaluation of hot pepper (Capsicum annuum L.) cultivars against bacterial wilt disease (Ralstonia solanacearum). Pyrex Journal of Microbiology and Biotechnology Research. 2016;2(3):22-29.
- Herman R, Perl-Treves R. Characterization and inheritance of a new source of resistance to Fusarium oxysporum f. sp. Melonis Race 1.2 in Cucumis. Plant Disease. 2007;91:1180-1186.
- Karimi R, Owuoche JO, Silim SN. Inheritance of fusarium wilts resistance in pigeon pea [Cajanus cajan (L.) Millspaugh]. Indian Journal Genetics. 2010;70(3):271-276.
- Dubey SC, Suresh M, Singh B. Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of Chickpea wilt. Biological Control. 2007;40:118-127.
- Ulloa M. Hutmacher RB, Davis RM, Wright SD, Percy R, Marsh B. Breeding for Fusarium wilt race 4 resistance in cotton under field and greenhouse conditions. The Journal of Cotton Science. 2006;10:114-127.
- Stover RH. Banana root diseases caused by Fusarium oxysporum f. sp. cubense, Pseudomonas solanacearum and Radopholus similis: A comparative study of life cycles in relation to control. In Root diseases and soil-borne pathogens, (Eds. Toussoun TA, Bega RV and Nelson PE). Berkeley: University California Press. 1970;197-200
- Booth C. The Genus Fusarium. Common wealth Mycological Institute, Kew Surrey, England. 1971;237
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. Food and Indoor Fungi. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands. 2010.
- Jaywant K. Pathogenic Variability and Management of Fusarium wilt of Chilli (Capsicum annuum L.) PhD Thesis in plant pathology in College of Agriculture CCS Haryana Agricultural University Hisar. 2016;95.
- Mamta J. Screening of Resistant Varieties and Antagonistic Fusarium oxysporum for Biocontrol of Fusarium Wilt of Chilli. Journal of Plant Pathology and Microbiology. 2012;3:1-5
- Ferniah RS, Daryono BS, Kasiamdari RS, Priyatmojo A. Characterization and pathogenicity of Fusarium oxysporum as the causal agent of Fusarium wilt in chilli (Capsicum annuum L.). Microbiology Indonesia. 2014;8(3):121-126.
- Abada KA, Ahmed MA. Management Fusarium wilt of sweet pepper by Bacillus strains. American Journal of Life Sciences. 2014;2:19-25.
- Yadeta KA, Thomma BP. The xylem as battle ground for plant hosts and vascular wilt pathogens, Frontiers in Plant Science. 2013;4(10):94-97.
Citation: Gabrekiristos E, Teshome D, Ayana G (2020) Cultural, Morphological and Pathogenic Variability amongIsolates of Fusarium oxysporum F. Sp. Capsici Causing Wilt of Hot Pepper in Central Rift Valley, Ethiopia. Plant Pathol Microbiol. 11: 499. doi: 10.35248/2157-7471.20.11.499.
Copyright: © 2020 Gabrekiristos E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.