Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 7
Cryodepleted Plasma Exchange Improves Survival in Thrombotic Thrombocytopenic Purpura: A Systematic Review and Meta-Analysis
Andrea O. Sugay1, Daniel Kerage1 and Denise E. Jackson1,2*2Thrombosis and Vascular Diseases Laboratory, School of Health and Biomedical Sciences, STEM College, RMIT University, Bundoora, Victoria, Australia
Received: 13-Nov-2024, Manuscript No. JBDT-24-27549; Editor assigned: 15-Nov-2024, Pre QC No. JBDT-24-27549 (PQ); Reviewed: 29-Nov-2024, QC No. JBDT-24-27549; Revised: 06-Dec-2024, Manuscript No. JBDT-24-27549 (R); Published: 13-Dec-2024, DOI: 10.4172/2155-9864.24.15.604
Abstract
Objectives: Thrombotic Thrombocytopenic Purpura (TTP) is a rare disease Associated with a Deficiency in a Disintegrin-like Metalloproteinase with Thrombospondin Motif Type 1 Member 13 (ADAMTS13) leading to Unregulated Ultra-Large von Willebrand Factor (ULVWF), which forms platelet-rich thrombi in the microvasculature. The first-line treatment for TTP is Therapeutic Plasma Exchange (TPE) with Fresh Frozen Plasma (FFP) or Cryodepleted Plasma (CDP). CDP has been hypothesised to perform better because it contains minimal levels of ULVWF. This study aimed to explore whether CDP, rather than FFP, improves outcomes in patients with TTP.
Methods: A systematic review and meta-analysis were performed according to the Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) guidelines. A comprehensive search and screening of the PubMed, Scopus, Ovid and Embase databases resulted in seven eligible articles.
Results: The current study revealed an increased odds of survival (Odds Ratio (OR)=3.44; 95% Confidence Interval (CI) 1.55-7.66; p=0.002) and a decreased odds of mortality (OR=0.29; 95% CI 0.13-0.65; p=0.002) for patients treated with CDP compared to FFP. Analysis of the differences in the number of TPE sessions and volume of TPE fluid required, as well as the rates of response and relapse did not reach statistical significance.
Conclusion: This study reveals favourable odds of survival for TTP patients treated with TPE using CDP over FFP. There is insufficient evidence to support CDP’s superior performance over FFP in other regards. Acceptance of this conclusion is limited by confounding variables and the risks of bias associated with the reviewed studies.
Keywords
Plasma; Platelets; Anaemia; Blood flow; Therapeutic plasma; Thrombocytopenia
Introduction
TTP is a rare disease that manifests as intravascular haemolysis, resulting in anaemia with marked thrombocytopenia, neurological issues and renal impairment. It is Associated with a Deficiency in a Disintegrin-like Metalloproteinase with Thrombospondin Motif Type 1 Member 13 (ADAMTS13) [1]. VWF proteins exist in circulation as Ultra-Large VWF (ULVWF) multimers which can recruit and bind platelets to form the platelet plug [1-3]. ADAMTS13 regulates VWF by cleaving ULVWF multimers to avoid undesired occlusion of vessels by thrombi. ULWVF is secreted in an inactive, globular formation; however, it undergoes a conformational change under high-shear conditions to expand along the direction of blood flow while bound to collagen in the vascular lumen [1-3]. This conformational change exposes the A1 and A2 domains in VWF which contain the platelet binding regions and ADAMTS13 Tyr-Met cleavage regions respectively [2,4-6]. VWF-platelet binding is enhanced in areas of high shear stress and ULVWF multimers are haemostatically more effective than cleaved VWF [2-6].
Pathogenesis of thrombotic thrombocytopenic purpura
In ADAMTS13 deficient individuals, ULVWF multimers remain uncleaved and unregulated and are hence more effective in binding platelets, forming thrombi capable of occluding blood vessels. Red cells are damaged while trying to cross VWF-platelet thrombi, thus explaining the presence of schistocytes and symptoms of haemolysis in patients with TTP [1,3,4]. As a result, occlusions in TTP mainly affect organs associated with high shear stress of blood flow such as the heart, brain, gastrointestinal tract and kidney. Sequestration of platelets by ULVWF precipitates the profound thrombocytopenia found in TTP [1,3-5]. This pathophysiology and the presence of platelet-and-VWF-rich fibrin-poor thrombi, differentiates TTP from other microangiopathic haemolytic anaemias. TTP can be classified as congenital or acquired [1,3,5]. Congenital TTP is characterised by a persistent <10% ADAMTS13 activity caused by pathogenic mutations in the ADAMTS 13 gene. Acquired immune TTP involves inhibitory antibodies towards the activity of ADAMTS13, or antibodies which bind to ADAMTS13 to significantly increase its clearance from the circulation [1,2,6].
Although a deficiency in ADAMTS13 is core to the pathophysiology of TTP, studies suggest that it is not always the sole activator of an acute TTP episode [1,4,6-8]. Induction of the complement pathway is also thought to contribute to TTP manifestation; mediated by the classical pathway-activating IgG antibodies directed towards ADAMTS13 in acquired TTP and the ability for ULVWF to act as a site for alternative complement pathway activation [3,4,6,9].
Therapeutic Plasma Exchange (TPE)
The most common initial strategy for the treatment of acute TTP is Therapeutic Plasma Exchange. TPE involves removing 1-1.5 patient plasma volumes and replacing it with some form of fluid; commonly derived from donated human plasma [4-6,10]. The emergence of TPE in the treatment of TTP has allowed mortality rates to decline from 90% to between 10% and 20% [11]. Current guidelines recommend commencing TPE immediately after the identification of a TTP episode [1,4,5,12,13]. TPE is believed to reverse acute TTP episodes by replenishing ADAMTS13 while diluting or removing anti-ADAMTS13 antibodies and ULVWF multimers [10]. Most TPE procedures are performed with FFP or CDP as the replacement fluid [4,7,12,14]. FFP is known to replenish all known coagulation factors and plasma constituents and is prepared by freezing whole single-donor plasma within eight hours of collection. When FFP is thawed slowly, cryoprecipitate forms, enriched in clotting factors and fibrinogen [11]. The slowly thawed FFP is centrifuged to allow the separation of CDP from insoluble cryoprecipitate [15]. The optimal number of TPE sessions or therapy duration has not been prescribed for the treatment of TTP as TPE is generally performed daily until a clinical response is achieved. The primary aim of TPE is not only to induce a clinical response, defined as platelet normalisation (>150 × 109/L) with no clinical signs of organ injury or acute TTP symptoms for two consecutive days, rather than solely correcting ADAMTS13 levels or activity [4].
Scope of the review
Considering the pathogenesis of TTP, the ideal fluid product for TPE would theoretically be one with high ADAMTS13 activity and minimal ULVWF levels [16]. The presence of ULVWF has previously been associated with recurring TTP episodes and an increased likelihood of platelet-rich thrombus formation. It is due to these reasons that TPE with CDP has been hypothesised to be more effective when treating TTP compared to FFP as, while ADAMTS13 activity is preserved in both FFP and CDP, CDP contains lower levels of ULVWF which remains in the cryoprecipitate fraction of plasma [17-19]. Some studies have shown improvement in TPE response when switching from FFP to CDP for refractory TTP patients [10-14]. However, the possibility that these results are due to confounding factors such as additional sessions of TPE has not been excluded [11,20,21]. Currently, there is little evidence that TPE with CDP has a significant advantage over FFP in the treatment of TTP and both fluids are considered suitable. Hence, a systematic review and meta-analysis of the current data may be informative in comparing the performance of the two fluids in treating TTP with TPE.
The aim of this systematic review and meta-analysis was to address the primary research question, constructed following the PICO framework: Is cryodepleted plasma (intervention) superior to fresh frozen plasma (comparator) in improving treatment outcomes through therapeutic plasma exchange (outcome) in patients with thrombotic thrombocytopenic purpura (population)?
Materials and Methods
Study design
This systematic review was conducted following the Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) guidelines [22].
Search strategy
A comprehensive search of the PubMed, Scopus, Ovid and Embase databases was performed. Searches were not restricted by publication date or article type [23]. Major search terms included “clinical fresh frozen plasma”, “cryodepleted plasma”, “cryoprecipitate-poor plasma”, “thrombotic thrombocytopenic purpura”, along with the abbreviations “FFP”, “CDP” and “TTP” in different combinations using Boolean operators such as “AND” and “OR” ensure a thorough retrieval strategy. References from these searches were imported onto the EndNoteTM 21 reference management tool for eligibility screening.
Eligibility criteria/Eligible articles
Peer-reviewed original research articles were considered for review [24]. Articles were considered significantly eligible if they were available in English and the full text was accessible using an RMIT University account. Abstracts, case studies, posters, book chapters, letters and other systematic reviews were deemed ineligible.
Participants
Studies were included if the sample size was greater than five human participants and participant demographics included patients with a confirmed diagnosis of TTP, regardless of participant age or gender. Studies that did not specify the type of thrombotic microangiopathy in the participants were excluded.
Interventions and comparators
Articles were deemed eligible for review if participants were allocated to two TPE groups for comparison: Treatment with FFP or CDP. Studies in which all participants were treated with only one type of plasma exchange fluid, or treated with a mix of fluids for plasma exchange were excluded. Studies in which CDP was compared with other fluids were not included in this review.
Outcome measures
To be eligible for meta-analysis, studies must have reported post-TPE outcomes for both intervention and comparator groups.
Assessment of methodological quality
The quality of the studies included in this systematic review was assessed following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Data extraction
Following full-text screening, eligible articles were read to extract the following data: Primary author, year of publication, study design, study period, country, sample size and parameters reported [25]. While studies measured several parameters of interest, only the following data was extracted for meta-analysis: Rate of survival, total number of TPE sessions performed, total volume of TPE fluid administered, clinical response to TPE and incidence of relapse.
Statistical analysis
Extracted data were manually input into the Cochrane Collaboration’s Review Manager (RevMan) computer program version 7.2.0. For two-way proportion analysis, the data type was set to “dichotomous” and the Mantel-Haenszel statistical method was applied [26]. An Odds Ratio (OR) effect measure with a random effects model was used. The RevMan tool calculated the cumulative OR and 95% Confidence Interval (CI). To analyze continuous data, an Inverse Variance (IV) statistical method was adopted. A Mean Difference (MD) effect measure was applied using a random effects model. In this case, the RevMan tool calculated the cumulative MD and 95% CI. In both types of analysis, the RevMan tool performed a test for Overall effect (Z) and Heterogeneity (I2). Statistical significance was set at a p-value of <0.05.
Risk of bias
Version 2 of the Cochrane tool for assessing Risk of Bias (RoB 2) was used in this systematic review and meta-analysis. Signalling questions for the RoB 2 tool provided by Sterne, et al. were used to summarise the risk that the observed study results are biased by confounding variables [27].
Results
Study selection
Database searches resulted in 4,280 significantly relevant articles. Ten non-academic records and 1,333 duplicates were automatically identified using EndNote™ 21 software and removed, leaving a total of 2,947 records for assessment. Articles were further excluded based on title and abstract (n=1,707) leaving a total of 1,212 articles for eligibility assessment. Articles were excluded for the following reasons: Abstracts and conferences (n=389); case reports (n=652); book chapters and reviews (n=117); no author listed (n=28). Following full-text screening, a final total of seven articles were deemed eligible for systematic review and meta-analysis (Figure 1).
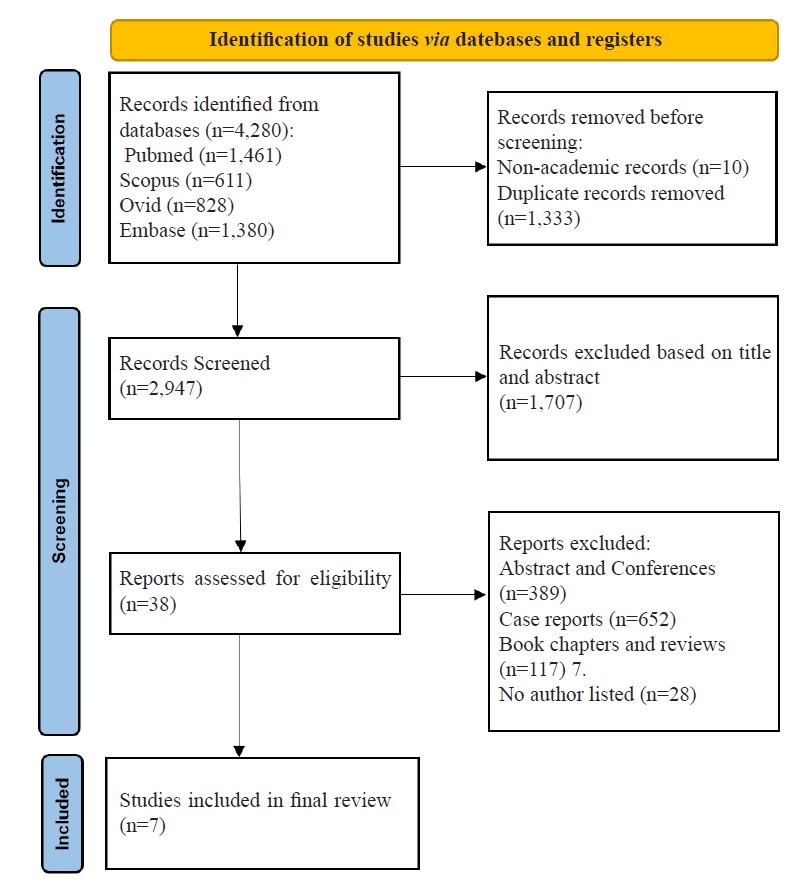
Figure 1: PRISMA flowchart. Outline of the process of searching databases for potentially eligible articles, removing articles prior to screening, initial article exclusion criteria, and final article screening with additional exclusion criteria. This process resulted in seven eligible articles for systematic review and meta-analysis.
Study characteristics
The characteristics of the seven articles included in this review are summarised in (Table 1). Four studies adopted a retrospective design, gathering data from the USA, Turkey and Brazil between 1982 and 2012 [13,21,28-32]. Three studies were conducted in either the USA or Canada and adopted a prospective study design, with only one study specifying its study period to be between 1993 and 1995. The most common parameter measured across six of the included studies was survival and mortality rates between FFP-treated and CDP-treated participants. This was followed by the number of TPE sessions, which was measured in four studies; however, one study did not state the standard deviation for the number of TPE session data and was therefore excluded from the meta-analysis for this parameter. Therapy response rate, relapse rate and total volume of TPE fluid administered were reported in three studies each. Three studies reported laboratory parameters pre and post-TPE, but only the CDP group’s parameters were measured in the study conducted by Rock et al. [28]. As a result, meta-analysis could not be performed for this parameter because of insufficient data. Other parameters of interest such as time to platelet normalisation, length of hospital stay, laboratory parameters pre and post-TPE and adverse reactions were not measured by enough studies to procure sufficient data for meta-analysis.
| Primary author | Year | Study design | Study period | Country | Sample size | Parameters reported | ||
|---|---|---|---|---|---|---|---|---|
| Treated with FFP | Treated with CDP | Total | ||||||
| Altuntas et al. [29] | 2007 | Retrospective | 1997-2005 | Turkey | 40 | 12 | 52 | Survival/mortality, number of TPE sessions, therapy response rate; time to platelet normalisation |
| Owens et al. [21] | 1995 | Retrospective | 1985-1993 | USA | 19 | 18 | 37 | Survival/mortality, number of TPE sessions, total volume of TPE fluid, length of hospital stay |
| Reutter et al. [30] | 2001 | Retrospective | 1982-1999 | USA | 21 | 20 | 41 | Number of TPE sessions, total volume of TPE fluid, adverse reactions, relapses |
| Rock et al. [28] | 1996 | Prospective | N/M | Canada | 9 | 40 | 49 | Survival/mortality, therapy response rate, relapses, laboratory parameters pre- and post-TPE b |
| Rock et al. [31] | 2005 | Prospective | N/M | Canada | 24 | 28 | 52 | Survival/mortality, therapy response rate, laboratory parameters pre- and post-TPE, VWF antigen and FVIII levels, ADAMTS13 levels and activity, presence of antibodies |
| Stefanello et al. [32] | 2014 | Retrospective | 2007-2012 | Brazil | 9 | 5 | 14 | Survival/mortality, number of TPE sessionsa, total volume of TPE fluid; adverse reactions; relapses |
| Zeigler et al. [13] | 2001 | Prospective | 1993-1995 | USA | 13 | 14 | 27 | Survival/mortality, time to platelet normalisation, relapses, laboratory parameters pre- and post-TPE |
Note: N/M: Not Mentioned; a: Standard deviation of number of TPE sessions was not stated; b: Laboratory parameters measured only for the CDP group, not the control FFP group.
Table 1: Characteristics of eligible studies included in the systematic review and meta-analysis of cryodepleted plasma and fresh frozen plasma as therapeutic plasma exchange fluids, resulting from exclusion following the Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) guidelines
Methodological quality assessment
Assessment of the scientific reporting of the studies included in this review using the STROBE checklist is summarised in (Table 2). All included studies outlined a clear title and abstract, as well as an introduction explaining the scientific background of the study. However, not all articles clearly state the rationale and objectives of the study. All studies outlined their study designs, variables, data collection and inclusion criteria for participating patients. Statistical methods were clearly stated in 5 out of the seven articles. In two studies, the statistical test used was named; however, other details regarding the statistical analysis, such as the definition of statistical significance, were not described. Most articles had a thorough report of participant characteristics, study outcomes and major results in their results sections. However, two studies failed to describe the participant characteristics. All studies fulfilled the criteria for interpreting the results in the discussion, but not all discussed the limitations and significant sources of bias for their studies.
| Primary author | Altuntas et al. [29] | Owens et al. [21] | Stefanello et al. [32] | Rock et al. [31] | Zeigler et al. [13] | Rock et al. [28] | Reutter et al. [30] |
|---|---|---|---|---|---|---|---|
| Year | 2007 | 1995 | 2014 | 2005 | 2001 | 1996 | 2001 |
| Title and abstract | |||||||
| Clear title and abstract with study design indicated | Y | Y | Y | Y | Y | Y | Y |
| Introduction | |||||||
| Explains scientific background | Y | Y | Y | Y | Y | Y | Y |
| States rationale for study and study objectives | Na | Yc | Y | Y | Y | Y | Y |
| Methods | |||||||
| Detailed study design, setting, variables, data collection presented | Y | Y | Y | Y | Y | Y | Y |
| Eligibility criteria for participants described | Y | Y | Y | Y | Y | Y | Y |
| Statistical methods described | Y | Nd | Ye | Y | Y | Nd | Y |
| Results | |||||||
| Gives characteristics of study participants | Y | Y | Y | Nf | Y | Y | Ng |
| Clearly reports study outcomes and main results | Y | Y | Y | Y | Y | Y | Y |
| Discussion | |||||||
| Summarises and interprets key results | Y | Y | Y | Y | Y | Y | Y |
| Discusses limitations or sources of bias of study | Nb | Y | Y | Y | Nb | Y | Nb |
Note: Y: Criteria fulfilled; N: Criteria not fulfilled; a: Rationale for study alluded to, but unclear, Study objectives unclear; b: Limitations, sources of bias, and generalisability of study not discussed; c: Rationale for study detailed, but study objective not clearly stated; d: Statistical test stated, but details on method of analysis and definition of statistical significance not outlined; e: Statistical method used is not detailed in methods, but is included in table of results; f: Study participant characteristics (e.g., age, gender, severity of TTP) not outlined, but baseline laboratory characteristics included as part of results; g: Study participant characteristics not described.
Table 2: Evaluation of methodological and scientific reporting quality following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.
Meta-analysis
Data extracted from the eligible studies for meta-analysis are included in Table 3 as either a proportionate value or mean and standard deviation, as appropriate (Table 3). For studies in which a median number was reported instead of the mean, an estimation of the mean and standard deviation formula was used for approximation [33]. This formula was proposed as a method to approximate mean and standard deviation to widen the significant available studies for meta-analysis. These data were used to perform meta-analysis resulting in forest plots for the rate of survival and mortality, number of TPE sessions performed to reach a clinical response, total volume of TPE fluid administered, clinical response to TPE and incidence of relapse, as outlined (Figure 2) [33].
| Primary author | Year | Sample size (N) | Survival/N | Number of TPE sessions | Volume of TPE fluid administered | Response to TPE/N | Relapses/N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFP | CDP | FFP | CDP | FFP | CDP | FFP | CDP | FFP | CDP | FFP | CDP | ||
| Altuntas et al. [29] | 2007 | 40 | 12 | 26/40 | 11/12 | 14 (± 10.20)b | 15.5 (± 13.46)b | - | - | 21/40 | 10/12 | - | - |
| Owens et al. [21] | 1995 | 19 | 18 | 8/19 | 13/18 | 12 (± 8) | 14 (± 10) | 37.4 (± 30.1) | 50.2 (± 35.9) | - | - | - | - |
| Reutter et al. [30] | 2001 | 21 | 20 | - | - | 7.9 (± 4.2) | 7 (± 3.2) | 25.5 (± 12) | 24.2 (± 10.6) | - | - | 9/21 | 8/20 |
| Rock et al. [28] | 1996 | 9 | 40 | 6/9 | 38/40 | - | - | - | - | 4/9 | 30/40 | 2/9 | 4/40 |
| Rock et al. [31] | 2005 | 24 | 28 | 22/24 | 27/28 | - | - | - | - | 20/24 | 22/28 | - | - |
| Stefanello et al. [32] | 2014 | 9 | 5 | 7/9 | 5/5 | - | - | 30.15 (27.45)b | 58.86 (± 46.1)b | - | - | 2/9 | 1/5 |
| Zeigler et al. [13] | 2001 | 13 | 14 | 10/13 | 11/14 | - | - | - | - | - | - | 4/10 | 6/11 |
Note: a: Mean (± SD); b: Original papers reported data in median and range or interquartile range, transformation of data to mean and standard deviation was performed using estimation formulas as proposed by Wan et al. [33].
Table 3: Data extracted from eligible studies for the meta-analysis comparing cryodepleted plasma to fresh frozen plasma.
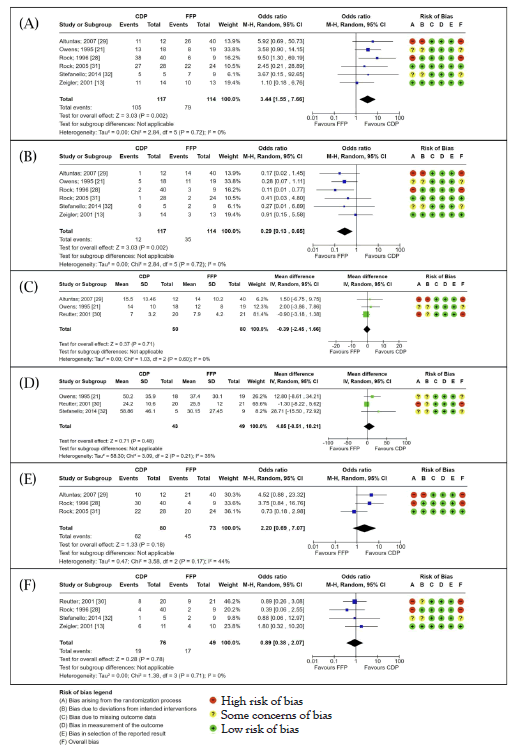
Figure 2: Forest plots. Results from the meta-analyses comparing the outcomes of TPE with CDP or FFP for patients with (TTP). Note: A) Survival rate; B) Mortality rate; C) Total number of TPE sessions; D) Total volume of TPE fluid administered; E) Rate of clinical response; F) Rate of relapse following TPE; Proportional data was analysed using the Mantel-Haenszel statistical method with an OR effect measure and random effects model. Continuous data was analysed using an Inverse Variance (IV) statistical method with a Mean Difference (MD) effect measure and random effects model. Both types of analysis presented a 95% CI and statistical significance was evaluated with a test for overall effect (Z) and p-value. Heterogeneity was assessed using the I2 value. Risk of bias assessment for individual studies is included alongside each forest plot.
Survival and mortality
Two-way proportion meta-analysis of the rates of survival for TTP patients showed statistically significant increased survival rates for patients treated with TPE using CDP over FFP with an OR of 3.44 (95% CI: 1.55-7.66; p=0.002; I2=0%). A similarly statistically significant outcome was achieved from the meta-analysis of mortality rates showing that FFP-treated patients were more likely to experience mortality following TPE with an OR of 0.29 (95% CI: 0.13-0.65; p=0.002; I2=0%).
Total number of therapeutic plasma exchange sessions and volume of plasma exchange fluid
Mean difference meta-analysis of the total number of TPE sessions required for TTP patients to reach a clinical response observed a slight increase in patients treated with FFP over those treated with CDP (MD: -0.39; 95% CI: -2.45-1.66; p=0.60; I2=0%). The meta-analysis of the total volume of TPE fluid required for treatment revealed an increased amount of fluid administered when treating with CDP (MD 4.85; 95%CI: -8.51-72.92; p=0.48). Neither result reached statistical significance and the studies included in the meta-analysis on the total volume of TPE fluid exhibited moderate heterogeneity (I2=35%).
Rates of clinical response and relapse
Two-way proportion meta-analysis on patient clinical response rate to TPE using CDP or FFP indicated a higher rate of clinical response in patients treated with CDP with an OR of 2.20 (95% CI: 0.690- 7.07; p=0.18) [34]. This result was not statistically significant and the included study data exhibited moderate heterogeneity (I2=44%). Two- way proportion analysis also showed a slightly increased rate of relapse in patients treated using FFP with an OR of 0.89 (95% CI: 0.38-2.07; p=0.78; I2=0%). This result was not statistically significant.
Risk of bias
Results from the risk of bias analysis using the RoB 2 tool are included alongside each forest plot in Figure 2. Any study that took a retrospective study design, or did not indicate that participants were randomly allocated into a CDP-treated or FFP-treated group was deemed to have bias arising from the randomisation process. Studies that indicated the use of therapies in addition to TPE were considered to have a high risk of bias due to deviations from the intended interventions. While some studies did not report the use of additional therapies to TPE, some concerns of bias due to deviations from intended interventions were also indicated due to their retrospective study design. Only two of the included studies were assessed to have a low overall risk of bias.
Discussion
Survival and mortality
The results of this systematic review and meta-analysis suggest that the use of CDP when administering TPE improves the odds of survival and lowers the odds of mortality in patients with TTP. This is supported by the statistical significance in the meta-analysis of survival and mortality rates comparing CDP and FFP (Figures 2A and 2B). A previous study conducted by Byrnes et al., showed that seven patients refractory to TPE using FFP and subsequently treated with CDP exhibited rapid improvement and eventual resolution of TTP [19]. Even so, not all patients experienced an immediate response. Delayed recovery was associated with more established microthrombi. The results of the current review are consistent with the theoretical assumption that the use of CDP can prevent overloading patients with ULVWF which is a culprit in the formation of platelet-rich thrombi during acute TTP episodes, leading to life-threatening symptoms of haemolysis and organ damage [19].
While CDP and FFP exhibit similar levels of ADAMTS13, a previous study demonstrated that ADAMTS13 in CDP is primarily unbound, whereas in FFP it is bound to ULVWF [10,11,13,14,35]. This distinction supports the preferential use of CDP, as unbound ADAMTS13 in CDP may be more effective in degrading ULVWF than the bound form in FFP [35,36]. TPE with CDP offers three major advantages when compared to FFP: a) ADAMTS13 levels and activity comparable to that of FFP with reduced odds of mortality and increased odds of survival; b) the presence of unbound ADAMTS13, as opposed to the bound form found in FFP and c) minimal levels of ULVWF [36]. The proposed mechanisms and major differences between CPP and FFP in their therapeutic effects are illustrated in (Figure 3) [17,36-38].
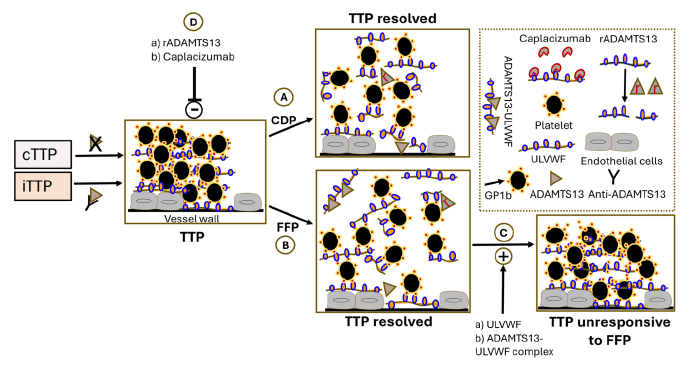
Figure 3: Potential mechanisms through which CDP and FFP exert their therapeutic actions in the treatment of TTP. TTP occurs due to a severe deficiency of the ADAMTS13 enzyme, which can result from genetic mutations (causing congenital TTP or cTTP) or from the development of autoantibodies (leading to immune-mediated TTP or iTTP). Treatment with CDP (A) or FFP (B) helps resolve TTP by replenishing ADAMTS13 levels [7,8,17]. However, FFP carries a complication: It contains ULVWF multimers and ADAMTS13 bound to ULVWF (C), both of which are associated with an increased risk of TTP relapses and reduced responsiveness to FFP therapy. To address this, future treatment strategies should consider using CDP in combination with rADAMTS13 or Caplacizumab (D) to improve therapeutic outcomes and prevent relapses in TTP patients [36]. The dotted square is a key to the diagram [45].
Administration of plasma exchange
Despite a statistically significant increase in the odds of survival when using CDP compared to FFP, the evidence in the current study does not support the superior performance of CDP in other aspects associated with TPE. As CDP requires additional steps for preparation compared with FFP, it is important to explore the differences in the number of TPE sessions and the required volume of fluid associated with CDP or FFP usage to discuss their efficiency. Results suggest that patients treated with FFP require more sessions of TPE to achieve a clinical response, but in total require a smaller volume of fluid for TPE than for CDP (Figures 2C and 2D). However, as neither result showed statistical significance, this suggests that the differences between CDP and FFP requirements are marginal or that there is inadequate evidence to support the observed trend.
Comparison of the efficiency of CDP and FFP when used for TPE is limited by the inadequate availability of study data. In the current review, only three available studies were included in the meta-analysis of each parameter. The lack of achievement of a statistically significant MD was likely affected by the contradictory outcomes between studies, as well as their small sample sizes and large spread (SD) of data. This suspicion is supported for comparing the differences in volume of TPE fluid required, as moderate heterogeneity was observed during the meta-analysis (I2=35%) indicating variation in study outcomes which likely affected the statistical significance. It is important to acknowledge the effect that differences in treatment protocols between studies may have on study outcomes, especially because all studies included in the meta-analysis of number of TPE sessions and volume of TPE fluid had a retrospective design. Variations in the number of TPE sessions and volume of TPE fluid prescribed by physicians may be affected by TTP type and severity, patient weight and co-existing conditions and may not have solely depended on the performance of CDP or FFP during treatment alone. Patients with recurrent episodes of TTP have also been shown to require more TPE sessions to achieve clinical remission [35].
Rates of clinical response and relapse
The meta-analysis in the current review measuring the clinical response rate to TPE showed increased odds of achieving a clinical response for TTP patients when treated with CDP instead of FFP (Figure 2E). However, this result did not reach statistical significance, likely due to moderate heterogeneity (I2=44%) between the study outcomes. Analysis of the TTP relapse rate revealed increased odds of relapse in FFP-treated patients, but the difference was not statistically significant (Figure 2F). These results may again indicate a marginal difference between the performance of CDP and FFP when used for TPE to treat TTP, but is most likely due to limitations in available data, as discussed previously.
Complications arise when drawing conclusions from the meta-analyses on response and relapse in the current review as the included studies did not distinguish between outcomes from different presentations of TTP. Response to TPE may vary between patients with congenital TTP (cTTP) and acquired TTP (aTTP). As the rationale behind TPE is the removal and dilution of anti-ADAMTS13 antibodies, this treatment may be indicated for patients with aTTP. Treatment of cTTP involves the replenishment of ADAMTS13 which could be through TPE, but is more commonly achieved using FFP infusion [4,7,12,14]. None of the studies included in the meta-analysis of TPE response rates distinguished outcomes between patients with cTTP or aTTP, which raises concerns about the type of TTP being a factor in TPE response [39-41]. Furthermore, TTP severity, the presence of co-existing conditions and the administration of additional therapies such as immunosuppressants may also affect the rate of clinical response to TPE as well as the rate of TTP relapse following TPE. For example, the inclusion of pregnant patients in a study sample introduces additional complications, as pregnancy has been identified as a trigger for acute episodes of previously asymptomatic cTTP. In the majority of patients, relapse occurs due to the autoimmune response to ADAMTS13 [31,42,43]. The differentiation of ADAMTS13-reactive B-cell clones from naïve populations has been suggested to lead a role in relapse following TPE and the introduction of immunosuppressive therapy alongside TPE has been shown to limit TTP relapse and exacerbations [6]. The differences in relapse rate for patients with aTTP or cTTP, or for patients with aTTP being treated with or without immunosuppressive therapy, has not been discussed by the studies included in this review [35,44]. This weakens the association between CDP and increased odds of clinical response or decreased odds of relapse following TPE therapy as observed in the respective meta-analyses.
Recombinant ADAMTS13
A novel recombinant ADAMTS13 (rADAMTS13) therapy has recently emerged for the treatment of ADAMTS13-deficient TTP. The use of this therapy may be less effective for patients with aTTP as this form of TTP is associated with the presence of anti-ADAMTS13 antibodies. However, preference for the use of rADAMTS13 instead of TPE, regardless of fluid type, may be indicated for cTTP as the aim of treatment is to replenish ADAMTS13 activity. Currently there is only one completed phase 3 trial comparing the use of rADAMTS13 to TPE, with other clinical trials still in progress [40]. While there is currently insufficient data to review the efficacy of rADAMTS13 compared to present TTP standards of care, this is an area of interest to explore in the coming years when discussing the choice of treatment for TTP [45-47].
Limitations
As TTP is a rare disease, sufficient data on parameters related to its treatment are difficult to obtain for a meta-analysis. This is further driven by a lack of randomised controlled studies, with all studies included in this systematic review and meta-analysis having an observational study design and most studies collecting data retrospectively [48-50]. Differences within participant demographics such as patient age, racial differences and pregnancy can confound results and complicate comparisons between studies. As a result, small sample sizes and differences in methods has led to high variability in study outcomes which limits the achievement of statistically significant results and therefore the ability to draw an acceptable conclusion from these results [51-53]. One study assessed rates of mortality and survival after 13 days. Although this shorter assessment period may have been influenced by the nature of the patients under investigation, this timeframe confounds a comparison with studies that evaluated outcomes over a 30-day period [13].
Evaluation of the studies included in this review using the RoB 2 tool showed a moderate-to-high risk of bias for most of the included studies, particularly arising from the randomisation process [54,55]. In retrospective or non-randomised studies, a conscious choice of TPE fluid for TTP patients was made by treating physicians rather than by random allocation. This decision may have been influenced by the participants’ clinical presentation and posed a risk of affecting TPE response. Furthermore, concerns surrounding bias due to deviations from intended interventions limit the applicability of the results from this review as patient outcomes from TPE may not be influenced by fluid choice alone, but by these additional variables.
Summary
There was a statistically significant increase in the odds of survival in patients with thrombotic thrombocytopenic purpura associated with the use of cryodepleted plasma instead of fresh frozen plasma for therapeutic plasma exchange. Evidence supporting the superior efficiency and lasting efficacy of therapeutic plasma exchange with cryodepleted plasma instead of fresh frozen plasma is limited by the number of available randomised controlled studies. Clinicians should consider the type and severity of the patient’s thrombotic thrombocytopenic purpura when making treatment decisions.
Conclusion
The outcome of this systematic review and meta-analysis showed a statistically significant increase in the odds of survival and decreased odds of mortality in patients with TTP treated with TPE using CDP. However, there is inconclusive evidence that CDP outperforms FFP in terms of TPE efficiency, clinical response and relapse rate. These, along with the patient’s clinical presentation and the type and severity of TTP, are important factors clinicians must consider when deciding which fluid to administer during TPE. Nevertheless, the findings of the current review suggest that prioritising CDP over FFP in the treatment of TTP warrants serious consideration. However, the complete elimination of FFP is not justified, as it can serve as a valuable resource in emergency situations where CDP is unavailable. Moving forward, randomised controlled studies with larger sample sizes comparing the performance of CDP to FFP used in TPE could be performed to assess whether the outcomes align with the results of the present review. Future studies may also consider exploring other factors related to TTP manifestations, such as routine laboratory parameters related to microangiopathic haemolysis, platelet count and ADAMTS13 and VWF assays to add depth to the investigation of TPE fluid comparison and performance. As other novel treatments for TTP, such as rADAMTS13, emerge, further investigations into their efficacy and benefits compared to current standards of care will be warranted.
Authors Contributions
Andrea O. Sugay and Daniel Kerage conducted the systematic review and meta-analysis. Daniel Kerage created figures 1 and 3. AOS conducted the risk of bias assessment, created the tables and figure 2 and prepared the final report. Denise E. Jackson supervised and edited the final document.
References
- Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(3):323-335.
[Crossref] [Google Scholar] [PubMed]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488-494.
[Crossref] [Google Scholar] [PubMed]
- Gomez-Segui I, Pascual Izquierdo C, Mingot Castellano ME, de la Rubia Comos J. An update on the pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev Hematol. 2023;16(1):17-32.
[Crossref] [Google Scholar] [PubMed]
- Sukumar S, Lämmle B, Cataland SR. Thrombotic thrombocytopenic purpura: Pathophysiology, diagnosis, and management. J Clin Med. 2021;10(3):24.
- Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130(10):1181-1188.
[Crossref] [Google Scholar] [PubMed]
- Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91:1-19.
[Crossref] [Google Scholar] [PubMed]
- Tsai HM, Lian ECY. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585-1594.
[Crossref] [Google Scholar] [PubMed]
- Scheiflinger F, Knöbl P, Trattner B, Plaimauer B, Mohr G, Dockal M, et al. Nonneutralizing IgM and IgG antibodies to von Willebrand factor–cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura. Blood. 2003;102(9):3241-3243.
[Crossref] [Google Scholar] [PubMed]
- Wu H, Jay L, Lin S, Han C, Yang S, Cataland SR, et al. Interrelationship between ADAMTS13 activity, von Willebrand factor, and complement activation in remission from immune‐mediated trhrombotic thrombocytopenic purpura. Br J Haematol. 2020;189:e18-e20.
[Crossref] [Google Scholar] [PubMed]
- Blombery P, Kivivali L, Pepperell D, McQuilten Z, Engelbrecht S, Polizzotto MN, et al. Diagnosis and management of Thrombotic Thrombocytopenic Purpura (TTP) in Australia: Findings from the first 5 years of the Australian TTP/thrombotic microangiopathy registry. Intern Med J. 2016;46(1):71-79.
[Google Scholar] [PubMed]
- McLeod BC. Therapeutic apheresis: Use of human serum albumin, fresh frozen plasma and cryosupernatant plasma in therapeutic plasma exchange. Best Pract Res Clin Haematol. 2006;19(1):157-167.
[Crossref] [Google Scholar] [PubMed]
- Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015 Jan 14;66(1):211-25.
[Crossref] [Google Scholar] [PubMed]
- Zeigler ZR, Shadduck RK, Gryn JF, Rintels PB, George JN, Besa EC, et al. Cryoprecipitate poor plasma does not improve early response in primary adult Thrombotic Thrombocytopenic Purpura (TTP). J Clin Apher. 2001;16(1):19-22.
[Crossref] [Google Scholar] [PubMed]
- van Marle AC, Joubert J, Meiring SM. Comparison of ADAMTS13 and von Willebrand factor levels and activities, and plasminogen levels, in plasma products currently available for the treatment of thrombotic thrombocytopenic purpura in South Africa. Transfus Apher Sci. 2019;58(1):72-78.
[Crossref] [Google Scholar] [PubMed]
- Nascimento B, Goodnough L, Levy J. Cryoprecipitate therapy. Br J Anaesth. 2014;113(6):922-934.
[Crossref] [Google Scholar] [PubMed]
- Cuker A, Cataland SR, Coppo P, de la Rubia J, Friedman KD, George JN, et al. Redefining outcomes in immune TTP: An international working group consensus report. Blood. 2021 Apr 8;137(14):1855-61.
[Crossref] [Google Scholar] [PubMed]
- Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, Azocar J, et al. Unusually large plasma factor VIII: von willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307(23):1432-1435.
[Crossref] [Google Scholar] [PubMed]
- Moake JL, McPherson PD. Abnormalities of von Willebrand factor multimers in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. Am J Med. 1989;87(3):9-15.
[Crossref] [Google Scholar] [PubMed]
- Byrnes JJ, Moake JL, Klug P, Periman P. Effectiveness of the cryosupernatant fraction of plasma in the treatment of refractory thrombotic thrombocytopenic purpura. Am J Hematol. 1990;34(3):169-174.
[Crossref] [Google Scholar] [PubMed]
- Obrador GT, Zeigler ZR, Shadduck RK, Rosenfeld CS, Hanrahan JB. Effectiveness of cryosupernatant therapy in refractory and chronic relapsing thrombotic thrombocytopenic purpura. Am J Hematol. 1993;42(2):217-220.
[Crossref] [Google Scholar] [PubMed]
- Owens MR, Sweeney JD, Tahhan RH, Fortkolt P. Influence of type of exchange fluid on survival in therapeutic apheresis for thrombotic thrombocytopenic purpura. J Clin Apher. 1995;10(4):178-182.
[Crossref] [Google Scholar] [PubMed]
- Eriksen MB, Frandsen TF. The impact of Patient, Intervention, Comparison, Outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J Med Library Ass: JMLA. 2018;106(4):420.
[Crossref] [Google Scholar] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:9.
- The EndNote Team. EndNote (21) program Philadelphia, PA:Clarivate. 2013.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499.
- Review Manager (RevMan). (7.2.0) Program. The Cochrane Collaboration. 2024.
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:8.
- Rock G, Shumak KH, Sutton DM, Buskard NA, Nair RC. Cryosupernatant as replacement fluid for plasma exchange in thrombotic thrombocytopenic purpura. Members of the Canadian Apheresis Group. Br J Haematol. 1996;94(2):383-386.
[Crossref] [Google Scholar] [PubMed]
- Altuntas F, Aydogdu I, Kabukcu S, Kocyigit I, Cikim K, Sari I, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: A retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
[Crossref] [Google Scholar] [PubMed]
- Reutter JC, Sanders KF, Brecher ME, Jones HG, Bandarenko N. Incidence of allergic reactions with fresh frozen plasma or cryo-supernatant plasma in the treatment of thrombotic thrombocytopenic purpura. J Clin Apher. 2001;16(3):134-138.
[Crossref] [Google Scholar] [PubMed]
- Rock G, Anderson D, Clark W, Leblond P, Palmer D, Sternbach M, et al. Does cryosupernatant plasma improve outcome in thrombotic thrombocytopenic purpura? No answer yet. Br J Haematol. 2005;129(1):79-86.
[Crossref] [Google Scholar] [PubMed]
- Stefanello B, de Paula EV, Orsi FA, Marques JFC, Roveri EG, Colella MP, et al. Safety and efficacy of cryoprecipitate-poor plasma as a replacement fluid for therapeutic plasma exchange in thrombotic thrombocytopenic purpura: A single center retrospective evaluation. J Clin Apher. 2014;29(6):311-315.
[Crossref] [Google Scholar] [PubMed]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
[Crossref] [Google Scholar] [PubMed]
- Zlowodzki M, Poolman RW, Kerkhoffs GM, Tornetta Iii P, Bhandari M. On behalf of the international evidence-based orthopedic surgery working G. How to interpret a meta-analysis and judge its value as a guide for clinical practice. Acta Orthop. 2007;78(5):598-609.
[Crossref] [Google Scholar] [PubMed]
- Scully M, Cohen H, Cavenagh J, Benjamin S, Starke R, Killick S, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS‐13. Br J Haematol. 2006;136(3):451-461.
[Crossref] [Google Scholar] [PubMed]
- Hori Y, Hayakawa M, Isonishi A, Soejima K, Matsumoto M, Fujimura Y. ADAMTS13 unbound to larger von Willebrand factor multimers in cryosupernatant: Implications for selection of plasma preparations for thrombotic thrombocytopenic purpura treatment. Transfusion. 2013;53(12):3192-3202.
[Crossref] [Google Scholar] [PubMed]
- Scott EA, Puca KE, Pietz BC, Duchateau BK, Friedman KD. Comparison and stability of ADAMTS13 activity in therapeutic plasma products. Transfusion. 2007;47(1):120-125.
[Crossref] [Google Scholar] [PubMed]
- Yarranton H, Machin SJ. An update on the pathogenesis and management of acquired thrombotic thrombocytopenic purpura. Curr Opin Neurol. 2003;16(3):367-373.
[Crossref] [Google Scholar] [PubMed]
- Alwan F, Vendramin C, Liesner R, Clark A, Lester W, Dutt T, et al. Characterization and treatment of congenital thrombotic thrombocytopenic purpura. J Americ Society Hematol. 2019;133(15):1644-1651.
[Crossref] [Google Scholar] [PubMed]
- Kremer Hovinga JA, George JN. Hereditary thrombotic thrombocytopenic purpura. N Engl J Med. 2019 Oct 23;381(17):1653-62.
[Crossref] [Google Scholar] [PubMed]
- van Dorland HA, Taleghani MM, Sakai K, Friedman KD, George JN, Hrachovinova I, et al. The international hereditary thrombotic thrombocytopenic purpura registry: Key findings at enrollment until 2017. Haematologica. 2019;104(10):2107-2115.
[Crossref] [Google Scholar] [PubMed]
- Moatti-Cohen M, Garrec C, Wolf M, Boisseau P, Galicier L, Azoulay E, et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. J Americ Society Hematol. 2012;119(24):5888-5897.
[Crossref] [Google Scholar] [PubMed]
- Mariotte E, Azoulay E, Galicier L, Rondeau E, Zouiti F, Boisseau P, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): A cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3(5):e237-e245.
[Crossref] [Google Scholar] [PubMed]
- Shin JS, Subhan MO, Cambridge G, Guo Y, de Groot R, Scully M, et al. Alterations in B-and circulating T-follicular helper cell subsets in immune thrombotic thrombocytopenic purpura. Blood Advance. 2022;6(12):3792-3802.
[Crossref] [Google Scholar] [PubMed]
- Scully M, Antun A, Cataland SR, Coppo P, Dossier C, Biebuyck N, et al. Recombinant ADAMTS13 in Congenital Thrombotic Thrombocytopenic Purpura. N Engl J Med. 2024;390(17):1584-1596.
[Crossref] [Google Scholar] [PubMed]
- Scully M, Ortel TL, Yu Z, Waliullah M, Zhang P, Patel M, et al. Recombinant ADAMTS13 for the treatment of acute TTP events in patients with congenital thrombotic thrombocytopenic purpura: Results from the phase 3 randomized, controlled, crossover study and the phase 3b continuation study. Blood. 2023;142:692.
- Scott M. Recombinant ADAMTS13 for immune thrombotic thrombocytopenic purpura: A way to rescue refractory patients–and perhaps one day avoid plasma exchange altogether? The Hematol. 2024;21(5):1690-1698.
- Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor–cleaving protease and its identification as a new member of the metalloproteinase family. J Americ Society Hematol. 2001;98(6):1662-1666.
[Crossref] [Google Scholar] [PubMed]
- Furlan M, Robles R, Solenthaler M, Lämmle B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood. 1998;91(8):2839-2846.
[Crossref] [Google Scholar] [PubMed]
- Stubbs MJ, Giles K, Scully M. Recombinant ADAMTS13 in severe neonatal thrombotic thrombocytopenic purpura. N Engl J Med. 2022;387(25):2391-2392.
[Crossref] [Google Scholar] [PubMed]
- Asmis LM, Serra A, Krafft A, Licht A, Leisinger E, Henschkowski-Serra J, et al. Recombinant ADAMTS13 for Hereditary Thrombotic Thrombocytopenic Purpura. N Engl J Med. 2022;387(25):2356-2361.
[Crossref] [Google Scholar] [PubMed]
- Bendapudi PK, Foy BH, Mueller SB, Liu J, Feingold LM, Burke KE, et al. Recombinant ADAMTS13 for immune thrombotic thrombocytopenic purpura. N Engl J Med. 2024;390(18):1690-1698.
[Crossref] [Google Scholar] [PubMed]
- Cid J, Lozano M. Caplacizumab as frontline therapy in addition to standard treatment in iTTP. Blood Advances. 2023;7(10):2129-2131.
[Crossref] [Google Scholar] [PubMed]
- Volker LA, Kaufeld J, Balduin G, Merkel L, Kühne L, Eichenauer DA, et al. Impact of first-line use of caplacizumab on treatment outcomes in immune thrombotic thrombocytopenic purpura. J Thromb Haemost. 2023;21(3):559-572.
[Crossref] [Google Scholar] [PubMed]
- Djulbegovic M, Tong J, Xu A, Yang J, Chen Y, Cuker A, et al. Adding caplacizumab to standard of care in thrombotic thrombocytopenic purpura: A systematic review and meta-analysis. Blood Advance. 2023;7(10):2132-2142.
[Crossref] [Google Scholar] [PubMed]
Citation: Sugay AO, Kerage D, Jackson DE (2024). Cryodepleted Plasma Exchange Improves Survival in Thrombotic Thrombocytopenic Purpura: A Systematic Review and Meta-Analysis. J Blood Disord Transfus. 15:604.
Copyright: © 2024 Sugay AO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

