Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 12, Issue 4
COVID-19: Trial on SARS-COV-2 Vaccines
Anis Daou*Received: 24-Jun-2021 Published: 16-Jul-2021, DOI: 10.35248/2157-7560.21.12.458
Abstract
The discovery of vaccines has had positive effects in decreasing the incidence of worldwide infectious diseases. Despite these achievements, a decline in vaccine confidence threatens to reverse these accomplishments to date. Restoring confidence is therefore paramount towards promoting timely and effective intervention for diseases. Most recently, Severe Acute Respiratory Syndrome Corona-Virus (SARS-CoV-2) has consumed and crippled the world’s population, impacting the health of hundreds of millions. Vaccine hesitancy continues to be pervasive amongst the worldwide public who harbor significant doubt towards both Pharmaceutical and Governmental authorities. This article takes a detailed look into the history and mechanisms of the four most commonly used vaccines for SARS-CoV-2 – namely Pfizer/BioNTech; AstraZeneca/Oxford; Sputnik and Moderna – providing critical analysis of published data, dissecting and appraising the clinical trials in detail and combining all those findings into this review. This article also addresses common misconceptions about these vaccines and other innoculations in general.
Keywords
SARS-CoV-2; COVID-19; Vaccines; Pfizer/BioNTech; AstraZeneca/Oxford; Sputnik
Introduction
Whilst consistently successful in eradicating multiple diseases, vaccines in recent years have evoked unanticipated skepticism towards mass immunization campaigns as a whole. This lack of trust is especially prevalent in the more economically developed countries. The rebellion against vaccines boils down to significant unawareness of the drug development model for compounds which is ironically responsible for the mass incident reduction of former deadly diseases only a couple of generations ago, that have now become virtually eradicated. Even though this mass reduction of casualties is indebted to vaccines boosting immune systems, it’s become an increasing challenge in recent years to convince the younger generation to accept vaccinations – a generation too young to have experienced Measles, Mumps or even Polio. Vaccinations have greatly increased life expectancy, with the average age around the world increasing year on year. The non-vaccination of children and adults can risk non-immunized individuals being diagnosed with a disease that health services are ill-equipped to treat immediately, due to their sparse prevalence. SARS-CoV-2 has had a uniquely major impact on our planet in this regard for which the vaccines have finally been rolled out after rigorous and extensive clinical trials as well as obtaining the Food and Drug Administration (FDA) approval. This review will look into detail at the top four distributed vaccines (Pfizer/BioNTech, AstraZeneca/Oxford, Sputnik and Moderna vaccines), offering analysis into their safety and efficacy during clinical trials, which should demystify misconceptions through what experts in the field themselves state regarding the vaccines.
Literature Review
When we get vaccinated, we aren’t just protecting ourselves, but also those around us. Some people like those who are seriously ill, are advised not to get certain vaccines – so they depend on the rest of us to be vaccinated and help reduce the spread of disease.’ — World Health Organization (WHO) [1].
Vaccinations have a relatively short history when measured against the thousands of years in which human beings have sought to tackle and battle plagues and pestilence. Only over the last few centuries have vaccinations been instrumental in protecting humans against disease. As the saying goes: ‘prevention is better than cure’ – hence prevention being the most timely and effective medical intervention in any therapeutic era [2]. Through the mid-twentieth century, the practice of vaccinating large populations had become the norm. Yet despite its relative infancy, it cannot be emphasized enough how much the contribution of vaccines has liberated nations from multiple outbreaks. Since their inception in 1798, they have helped control and eradicate a number of major dis-eases; these include Smallpox, Tetanus, the Flu, Polio, Measles, Mumps, Rubella and Hepatitis B amongst others [3]. Figure 1 below shows how Polio was eliminated around the world in this regard. Vaccines have measurably improved the status of public health worldwide–case in point, Smallpox has been eradicated [4], cases of Poliomyelitis have been reduced by 99% and Rubella and Congenital Rubella Syndrome have been reduced to almost non-existence [5,6].
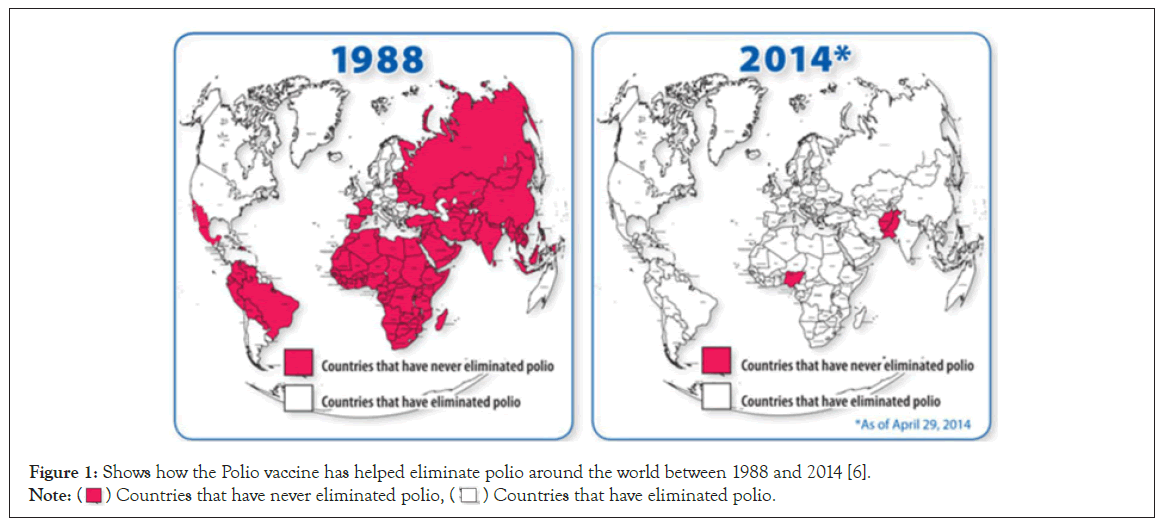
Figure 1: Shows how the Polio vaccine has helped eliminate polio around the world between 1988 and 2014 [6].
Note:  Countries that have never eliminated polio,
Countries that have never eliminated polio,  Countries that have eliminated polio.
Countries that have eliminated polio.
Why are vaccines important?
Vaccines provide a safe, effective and simple way in protecting people from potentially harmful diseases even before encountering them. They help improve the capabilities of the body’s natural immune system by advancing its mechanisms to build resistance against the target disease and they train your system to create antibodies defence mechanisms produced to fight foreign particles. Once achieved, the system strengthens to acquiring the capability to fight off the target ailment.
‘Vaccines only contain killed or weakened form of germs like viruses or bacteria; they do not cause the disease or put you at risk of its complications.’— World Health Organization (WHO) [1].
From birth, humans are in constant exposure to the presence of numerous foreign particles in the air. Most are not harmful, many are beneficial and some can cause physiological distress. The body’s immune system is readily equipped to fight against these through triggering a series of responses to neutralize these particles and limit their harmful effects. Exposure and recovery to an infectious disease can provide lifelong immunity, so we may not contract this illness again (for example Chicken-Pox), this is due to the immune system’s heuristic nature to remember the foreign particle in the future. However, there are some diseases our immune system is not capable of coding a response to, and it is these that can then lead to some serious complications – and sometimes death. The vaccines aim to achieve this immunity from the latter before the patient ever obtains the ailment, hence preventing symptoms arising from the disease in question [1].
When we vaccinate, the immune systems ‘memory’ is activated. A weakened microbe or a fragment of the microbial particle is injected into the body. The immune system is then activated without us feeling any side effects to that ailment. Vaccinations can provide a lifelong protection against the target condition, whilst for others the effect diminishes after a few years and ‘booster’ doses are required. Below are mechanisms of how a vaccine induces a response from the immune system and how we build immunity [1]. The vaccine:
• Harmful microorganism enters the body,
• The immune system recognizes this and produces antibodies to fight the bacteria or virus
• The immune system builds up immunity through the production of memory B-cells and T cells,
• If you are then exposed to the microorganism again, the immune system will already know what the ideal response for the microorganism is and produce an immune response prior to becoming unwell.
Herd immunity
Herd immunity is when the majority of the population (around 80%) are vaccinated against a target disease, there will be few people left who have not taken the vaccine due to health reasons. The vaccine not only protects its recipients but also others around them too ill to receive the vaccine [7].
Herd immunity is a crucial concept that is commonly misunderstood. Not all dis-eases are transmitted through personperson interactions. Tetanus is a common example of this; as it’s an environmental bacterium that cannot be transmitted from person to person therefore, the minority that are unimmunized are not protected no matter how big the immunized majority may be.
When a person is vaccinated, it reduces their risk of infection as was explained earlier. This makes the person less likely to transmit the virus or bacterium to others. As larger quantities in the community are vaccinated, fewer people remain vulnerable, hence significantly decreasing the possibility for an infected person to pass the pathogen to another. This ultimately results in lower pathogen circulation, effectively protecting communities [1].
Vaccination classifications
Vaccines fall into two main categories
• Live attenuated vaccines
• Inactivated vaccines
Live attenuated vaccines: Live attenuated vaccines are those derived from the disease-causing microorgan-isms. These microorganisms are weakened in a laboratory usually through repeated cell culturing. A common example of an attenuated virus is the Measles vaccine. Firstly, the Measles virus was extracted from a suffering child. In the decade to follow, through a process known as serial passaging (i.e., continuous splitting of cells using a liquid medium that feeds and multiplies the cells in order to weaken their capacity); the wild virus eventually became an attenuated virus, capable of inducing an immune response without side effects being felt. For the immunity to enhance, these live attenuated viruses must replicate in the vaccinated person. When the replication does occur, they are usually not strong enough to cause disease within the vaccinated person–just a milder response–which is referred to as an adverse reaction. The immune response produced by this type of vaccine is identical to that caused from a natural infection. Live attenuated vaccines produce immunity for a large number of the population after the first dose, however if this is not the case, a second dose may be administered. Sufferers with an immunodeficiency such as Leukemia or Human Immunodeficiency Virus (HIV) are most likely advised against taking this type of vaccine as it can cause a fatal reaction through uncontrolled replication of the viral envelope present within the vaccine [3,8,9]. These types of vaccines are heat and light sensitive, correct storage is paramount. Below are some types of these types of vaccines:
• Measles
• Mumps
• Rubella
• Yellow fever
• Rotavirus
Inactivated vaccine: The second type known as the inactivated vaccine consists of growing the virus in a culture media, then inactivating it chemically or thermally. For fractional vaccines, a microorganism is further treated for purification purposes. Inactivated viruses are not live and cannot replicate. The entire antigen dose is administered; they do not cause disease from the infection, even in an immunocompromised person. Inactivated vaccines always require multiple doses. Generally, the first dose does not provide protective immunity, but primes the immune system. This is then followed by a second dose, whereby the protective immune response is developed similar to that of a natural response (Table 1). The efficacy of an inactivated virus diminishes over time, this results in periodic supplemental increase in dose. Currently the list of inactivated vaccines consists of [10]:
| Live Attenuated Vaccines | Inactivated Vaccines |
|---|---|
| Attenuated (weakened) form of the "wild" virus or bacterium | Cannot replicate |
| Must replicate to produce an immune response | Less affected by circulating antibody than live vaccines |
| Immune response virtually identical to natural infection | Always require multiple doses |
| Immune response virtually identical to natural infection | Immune response mostly humoral |
| Usually produce immunity with one dose | Anti-body titer diminish with time |
| Severe reactions possible | May require periodic supplemental booster doses |
| Interference from circulating antibody | Whole-cell vaccines |
| Fragile-must be stored and handled carefully | viral: polio, hepatitis A, rabies, influenza* |
| Viral: measles, mumps, rubella, vaccinia, varicella, zoster, yellow fever, rotavirus, intranasal influenza, oral polio | bacterial: pertussis, typhoid, cholera, plague |
| Bacterial: BCG 0, oral typhoid | Fractional vaccines |
| Except those administered orally | Subunits: hepatitis B, influenza, acellular pertussis, human papillomavirus, anthrax |
| Not available on the United States | Toxoids: diphtheria, tetanus not available in the United States |
Table 1: Brief description of a live attenuated virus and an inactivatedimg class="img-responsive" src="https://www.walshmedicalmedia.com/articles-images-2021/ virus.as described by the CDC [10].
• Polio
• Hepatitis A and B
• Rabies
• Typhoid
• Cholera
• Plague
Clinical trial phases and their purpose brief
Daou 2021 recently published an extensive look into the processes vaccine under-takes from the drug discovery and development stage right through to the clinical stages [11]. Briefly, clinical trials consist of four phases. All vaccines must undergo rigorous and extensive testing, this ensures that they are safe and can be distributed for public use. The first stage of testing is on animals, this is to identify its safety and potential to prevent disease. The animals used are selected carefully; this includes identifying the correct type of animal that can be closest in immune response to that of a human. This phase is also known as Phase 0.
In Phase I of clinical trials, the vaccine is administered into a small number of human volunteers. This assesses its safety profile and confirms to whether or not an immune response is elicited. This stage also can verify correct dosage concentrations.
In Phase II, the vaccine is given to a larger number of volunteers, usually in the hundreds, who are closely monitored for any harmful adverse effects. In addition, its immune response capabilities are assessed. Participants in this phase are meant to mimic the target population (such as age and sex) for whom this vaccine will be delivered to. To assess its effectivity, this stage provides (i) some participants with the vaccine and (ii) some with the placebo. This allows comparisons and conclusions to be drawn about the vaccine [12].
Phase III is when the vaccine is given to thousands of volunteers. In this phase, some sufferers are also included into the study (in addition to the healthy volunteers). This allows investigators to draw out a larger conclusion in terms of effectivity to suffering volunteer’s vs. side effects that may be caused to healthy volunteers. Not all volunteers are given the active vaccine, some are given the placebo. This phase is the most important and significant, not only due to the size of the population tested, but this is also the stage at which a drug will go into either production or mass immunization. This phase can further clarify safety and efficacy of vaccine [12].
Once the results of the clinical trials become available, a series of steps are required, including a review on the efficacy and safety profiles of the drug. Following the introduction of the vaccine, close monitoring is continuously undertaken to review all results and detect unexpected adverse effects and assesses effectiveness in a normal setting [12].
Severe Acute Respiratory Syndrome-CoronaVirinae-2 (SARS-CoV-2)
The SARS-CoV-2 viral structure carries similar traits to its predecessors, Severe Acute Respiratory Syndrome-CoronaVirinae-1 (SAR-CoV-1) and Middle East Respiratory Syndrome-Corona Virinae (MERS-CoV), all of which belong to the same viral family. Briefly, SARS-CoV-2 has a single strand RNA envelope (sRNA). The virus is a linear, positive sense RNA which has a large genome and ranges between 50 to 150 nm in size. The Coronavirinae family that SARS-CoV-2 belongs to was initially found in the early 1960’s [13,14]. The shape of the virus is spherical, surrounded with envelopes on the outer lining containing nculeo-capsids and nucleoproteins, consequently these make up the genomic structure of viral RNA. Since early identification of this viral family, the spike proteins that abide on the outer structure of the virus have been linked to their capably in attaching to the host cell [15]. This is due to the trimer spike protein, containing hemagglutinin esterases, membrane and envelope proteins, which are also essential to the binding of host cells. The virus infects humans at varying levels and this depends on many factors which include any underlying health conditions making them especially suseptible, such as (i) cardiovasular conditions, (ii) the viral colony a person may have been exposed to, and (iii) the type of viral mutation that they may encounter. Animals also have varying responses built on immune systems more capable and better equiped to combat the virus. Therefore, the spread of the virus between animals is less frequent when compared to human transmission [11].
SARS-CoV-2 targets the respiratory, hepatic, gastro-intestinal and neurological systems [14]. The virus consists of an envelope containing helical nucleo-capsids and nucleo-proteins (N). Embedded in the envelope is a 2 nm trimer of spike glycoproteins (S), this is the protein that attaches itself to the Angiotensin Converting Enzyme-2 (ACE-2). The virus also contains an integral membrane (M) and an envelope protein (E). SARS-CoV-2 contains an additional membrane not previously identified on the previous coronavirinae counterparts, it has an additional membrane named hemagluttinin esterases, which is 5-7 nm long (Figure 2) [16]. New mutations can appear due to the large genomic potential, high prevalence and wide distribution within the animal kingdom [17-19].
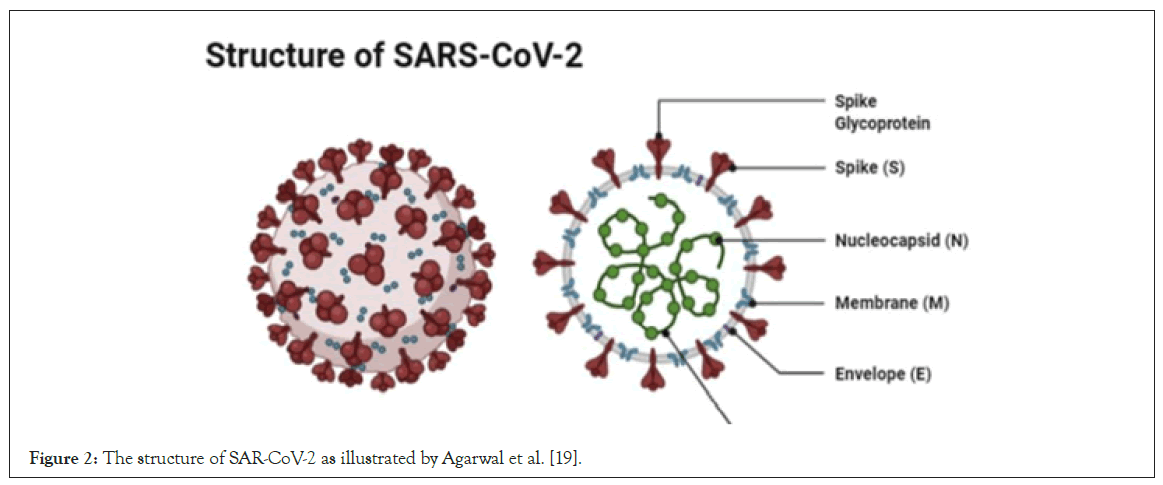
Figure 2: The structure of SAR-CoV-2 as illustrated by Agarwal et al. [19].
As was previously mentioned, SARS-CoV-2 binds to the ACE-2 via the spike protein. The spike protein consists of the receptor binding and membrane binding subunits. The receptor binding subunit facilitates binding whilst the membrane fusing subunit allows the virus to fuse into the host cell [20]. Prior to fusion, the protease enzyme, TMPRSS2, activates the spike protein. It is the combination of fusion and activation that is required for infection to occur [21]. Once within the cell, they translate small parts of the virus onto non-structural proteins, these results in the formation of the RA Dependent RNA polymerase enzyme. The enzyme induces the restructuring of the endoplasmic reticulum of the host cell. Resulting in continuous replication and transcription of the SARSCoV-2 virus [22], the viral proteins, in addition to the sRNA, are assembled in the endoplasmic reticulum and golgi apparatus of the cell. These accumulated structures then release and spread within the body, attacking target sites and multiple systems within the body [11,23].
Detection of the virus is done through Reverse Transcription- Polymerase Chain Reaction (RT-PCR). The test detects the RNA of the virus. This test targets two components of the virus, the Open Reading Frame Gene (ORFG) and the viral nucleo-capsid region. The test operates through reverse transcripting the RNA of the virus, this results in the production of the complementary DNA (cDNA) [22]. Once the cDNA is produced, a dye helps the identification of fluorescent signals, resulting in a curve produced by the RT-PCR and presenting a quantitative analysis for the presence of the virus [22,24].
Objectives to the review
This review will look at the four most FDA approve, and most commonly applied vaccines being used worldwide for mass vaccination. It will look at each of the vaccines briefly, describing the early phase findings of these four vaccines, and then take a detailed look at the design of the phase III clinical trials–starting from how they were set up, and leading to the findings in regards to the safety and efficacy data of these inoculations. Once this is done, we will look at some common misconceptions before giving an overall conclusion, discussing the findings, safety and efficacy profiles finally resulting in a clinical opinion on regards to (i) whether or not the vaccines are safe and suitable for mass vaccination, and (ii) if this is the light at the end of the tunnel for the pandemic to be over. Table 2 provides a brief overview of the four vaccines that will be analysed in this review.
| Vaccine candidate | Developer | Country | Technology used | Phase III study | Completed phase (Findings) | Clinical trial sites |
|---|---|---|---|---|---|---|
| AZD1222 University of Oxford, |
Oxford AstraZeneca |
UK | Modified chimp adenovirus vector (ChAdOx1) | Phase III (30,000) Interventional; randomized, placebo-controlled study for efficacy, safety, and immunogenicity. Brazil (5000) | Phase I-II (543) Neutralizing antibodies detected after a booster dose was given at day 56. Side effects included pain at the injection site, headache, fever, chills and muscle ache, acetaminophen was allowed for some participants to increase tolerability. | 20 in the UK, São Paulo |
| BNT162b2 | BioNTech Fosun Pharma Pfizer | Germany, United States of America | mRNA | Phase III (44,820) Randomized, placebo-controlled | Phase I-II (45) Immune system was found to generate neutralizing antibody response peaking 7 days after a booster dose. side effects included pain at the injection site, fatigue, headache, chills, muscle pains, joint pain and fever | 152 in the USA, Argentina, Brazil, South Africa, turkey, and Germany |
| mRNA-1273 | Moderna | United States of America | Lipid nanoparticle dispersion containing mRNA | Phase III (30,420) Interventional; randomized, placebo-controlled study for efficacy, safety, and immunogenicity | Phase I (45) Antibody response was detected after the administration of two-doses; side effects included fever, fatigue, headache, muscle ache, and pain at the injection site | 89 sites in the USA |
| Sputnik V | Gamaleya Research Institute of Epidemiology and Microbiology | Russia | Adenovirus vector vaccine (recombinant adenovirus type 5 and 26 vector) | Phase III (40,000) Randomized double-blind, placebo-controlled to evaluate efficacy, immunogenicity, and safety. | Phase I–II (76) Neutralizing antibody and T cell responses. | 28 sites in Europe, South America and Asia |
Table 2: Below in an at-a-glance analysis of four vaccinations that have passed clinical trials and are distributed for mass immunization for the SARS-CoV-2
virus.
Vaccinations on trial
The vaccine that has caught the media’s attention is the BioNTech/ Pfizer vaccine. The vehicle designed for this is an mRNA-based vaccine; where a synthetic mRNA is injected and translated into a protein by the host. Treatments based around mRNA vehicles are considered safe due to the RNA being transiently expressed and metabolized and does not integrate into the host genome [25]. Another vaccine that carries this vehicle is the Moderna vaccine.
In order to understand how this type of vaccine operates when administered, it is important to grasp the steps that cause a viral infection and its corresponding immune response. Firstly, the mRNA is part of the DNA that sends around a set of instructions; these instructions are significant in the replication and assembly of the new virus, serving as a code for the proteins required for replication. These SARS-CoV-2 vaccines encode for the target spike protein embedded within the envelope of the virus, this protein is the fusion protein that attaches to the angiotensin-converting enzyme (ACE-2). Studies by Kirchdoefer et al. show that immunity to the spike protein was protective of clinical infections [26]. When a virus enters the target cell, fusion proteins undergo structural changes that allow the genetic material to be part of the replication process [27]. A key step to the production of the mRNA vaccine is the introduction of a protein capable of locking onto the spike protein, thus preventing the spike protein from fusing with the ACE-2 enzyme. Henceforth, when the mRNA vaccine is taken, relevant material is present to attack the virus when detected within the system [28].
During a natural infection, when the virus enters, these foreign proteins are then recognized by antigen presenting cells present within the immune system, and they then activate T and B cells, which then protect against the subsequent infection. In an mRNA vaccine, the mRNA is directly introduced to the material that codes for the spike protein. The protein is produced in the antigen presenting cells; therefore making the immune response ready, this is similar to that exhibited in a natural infection. Currently,vaccines that carry this type of vehicle are vaccines for Hepatitis A and B, measles, mumps and rubella. This type of vaccine in theory should provide immunity for many years, due to the generation of the antiviral T cells and memory B-cells [29].
Safety and efficacy of the BioNTech/Pfizer vaccine
• 43,548 participants
• 152 sites worldwide
• 50/50 split between those who received the vaccine and placebo
• 8 cases of COVID-19 among vaccine recipients vs. 163 in placebo recipients after receiving both doses
• 87% of the participants fulfilled the two dose requirement
• Demographic
• 50% female
• 83% Caucasian
• 9% Black/African American
• 28% south American
• 35% obese
• 42% older than 55
• Median age: 52
• Efficacy: 95%
The Phase III trials were conducted between July and November 2020. 87% of participants fulfilled the two dose requirement. From this, investigators were able to compile two months of safety data [30].
Adverse events were analysed for all participants. More vaccine recipients reported adverse events than placebo recipients (27% to 12% respectively). Sixty-four vaccine recipients (0.3%) and six placebo recipients (<0.1%) reported inflammation, few participants showed severe or serious adverse events leading to withdrawal from trial. Four related serious adverse events were reported among vaccine recipients two of which died, as did four placebo recipients. None of the deaths were verified to be related to the vaccine or placebo – nor were they observed to be COVID-19 related [31-34].
Amongst the participants who had no evidence of existing or prior SARS-CoV-2 infection, half took the vaccine and half took the placebo. 7 days after, only 8 cases of COVID-19 were observed and 162 among placebo recipients (a 2000% increase), indicating 95% vaccine efficacy. Among participants with evidence of prior SARSCoV- 2 infection, nine cases of COVID-19 were identified after at least 7 days following the administration of the second vaccine dose, this in comparison to the 169 cases among placebo recipients, equating to 95% vaccine efficacy. Analyses indicated that vaccine efficacy among all groups (with or without underlying conditions) was generally consistent. After the first dose was administered, 39 cases in the vaccine group and 82 cases in the placebo group were detected, indicating a vaccine efficacy of 52%. This suggested that the first dose induced an immune response; therefore early protection (as soon as the 12th day) was detected from the vaccine [34].
Safety and efficacy of the Moderna vaccine
• 30,420 Participants
• 89 sites in the United States of America
• 50/50 split between those who received the vaccine and placebo
• 96% received both doses
• Median time of 63 days, whereby safety and efficacy data was collated
• Demographics
• Average age of participants was 51 years
• Almost half were women
• 25% were 65 years old or older
• 17% were younger and had underlying conditions
• Low grade adverse events occurred in both groups
• More deaths occurred in placebo group as opposed to vaccine group
• Vaccine efficacy of over 95%
• Vaccine efficacy towards symptomatic COVID-19 was 100%
The Phase III trials for the Moderna vaccine were conducted between July and October 2020. A segment of participants were withdrawn from receiving the second dose for various reasons (153 in total)–the most common being the detection of SARS-CoV-2 before the administration of the second dose on day 29 (69 in the placebo group and 45 in the vaccine group). The majority who did receive the second dose (i.e. the 96%) constituted the efficacy data. After the second dose was administered, patients were followed up for a median time of 63 days (range, 0 to 97) [35].
The demographic between the vaccine and placebo group was evenly distributed, allowing the clinical trials to show the effects across the range of participants. In con-junction with the breakdown above, the clinical trials contained a diverse mix proportionally representing the U.S. population. Evidence of SARS-CoV-2 infection was similar in both groups.
Injection site reactions occurred more frequently in the vaccine group than in the placebo group after both the first and second dose. In the vaccine group, low grade injection-site events were reported, these lasted up to three days after each dose. The most commonly reported event at the injection-site was pain. Some reported reactions took over 8 days but they only counted for less than 1%. Injection-site and systemic adverse events were more common among younger participants (<65 years of age) [35].
The frequency of reported adverse events was generally similar between both sets of groups within 28 days after injection. Five deaths occurred (3 in placebo and 2 in vaccine), of which, none were related to the trial. The frequency of a higher grade adverse event in the placebo group and vaccine group did not differ (1.5% ± 0.2). Hypersensitivity reactions were reported at a similar rate in both groups (1.5%). Less than 0.1% of participants in both groups withdrew from the trial due to experiencing adverse side effects. The vaccine group obtained fewer new cases of COVID-19 than those compared to the placebo group, which indicated an immune response had been activated by the vaccine dose. The most common treatment-related adverse events between the two groups were fatigue (1%) and headache (1%). The vaccine cohort indicated a slightly higher rate of adverse events when compared to the placebo cohort (≤ 0.5%), with the vaccine response being similar among all demographics. Safety data is published in the New England Journal of Medicine [35].
Efficacy was tested on a total of 269 COVID-19 cases of which 11 were from the vaccine group and 185 in the placebo group. This equates to 94% vaccine efficacy. After 14 days of administering the first dose, findings showed that there was 11 and 225 cases of COVID-19 in the vaccine and placebo groups respectively, again indicating an efficacy above 95%. Between days 1 and 42, seven counts of COVID-19 were identified in the vaccine group and 65 in the placebo group. Thirty of the placebo participants had severe COVID-19; with none present in the vaccine group – hence indicating vaccine efficacy of 100% towards symptomatic infection. The vaccine efficacy to prevent COVID-19 was consistent across all the clinical trial population. Efficacy data was published in the New England Journal of Medicine [35].
Adenovirus vector vaccines
These vectored vaccines are the second most common COVID-19 vaccine vehicles. The vaccines manufactured using this type of vehicle are the AZD1222 vaccine produced by Oxford/ AstraZeneca and Sputnik (manufactured in Russia by Gamaleya Research Institute of Epidemiology and Microbiology). Similarly to the mRNA vehicle immune response, it is designed to obtain genetic material that encodes for SARS-CoV-2 genes into your cells, and get your cells to illicit the production of viral proteins. The difference is that where the mRNA vaccine uses the mRNA strand to code for the replication and assembly of the new virus, serving as a code for the proteins required for protein replication, the adenovirus-vectored vaccine uses harmless parts of the virus to instigate an immune response, therefore releasing genes that encode for the spike protein present on the viral envelope of the SARS-CoV-2 virus. The adenovirus family consists of hundreds of viruses that are mostly harmless, and may only cause mild symptoms such as the common cold or fever. The genes that are required to exhibit an immune response are placed into the vaccine; these genes are meant to produce a similar immune response that will occur once the SARS-CoV-2 virus is detected within the body. When the adenovirus is injected into the cells, it is translated into mRNA, causing the cells to make the vaccine protein, which then triggers an immune response. The adenovirus vehicle only contains information that produces an immune response but does not contain genes that replicate in a vaccinated person. One of the major advantages of this vehicle is that they are stable, so they would not have to be stored in extremely cold temperature to ensure stability and efficacy [32].
Safety and efficacy of the Oxford/AstraZeneca vaccine
• 30,420 participants
• Undertaken in four different sites
• 50/50 split between those who received the vaccine and placebo
• 90-120 days follow up data was taken for efficacy and safety data
• Demographic
• All participants between 18-55 years
• 65% were female
• 92% were white
• 0.4% were black
• 5.6% were Asian
• 1.4% were other
• Participants who received two standard vaccine doses, efficacy was 62%
• Participants who received an initial low dose followed by a higher dose, efficacy was greater than 90%
• Participants who had a higher time gap between vaccines had a better efficacy
• Vaccine had good safety profile
The Phase III trials for the Oxford/AstraZeneca vaccine were conducted between April and November 2020 in the United Kingdom, South Africa and Brazil. All 30,420 test subjects were 16 years of age or above [36].
After dose #2, three to four months of follow up analysis was undertaken to obtain safety and efficacy data for the vaccine. The results from the sites in Brazil and the United Kingdom were obtained; these studies were done on participants of all ages. The time gap between the two vaccines varied in order to assess the vaccine efficacy from differing intervals between the first and second dose. 53% participants in United Kingdom received a second dose with a minimum 84 day gap after the first and 0.8% received a second dose within 56 days of the first. The median interval between doses ranged between 50-86 days. On the other hand, the majority of participants in Brazil received a second dose within 42 days. One UK participant had an asymptomatic infection 21 days after the first dose of the vaccine. Two other participants who took the placebo had symptomatic reactions after 56 and 150 days respectively. For participants who received the two standard doses of the vaccines, efficacy was 62%, whereas those who received a low dose first (following up with a higher second dose), efficacy was higher at 90%. In the UK, recipients of the two standard doses showed a vaccine efficacy of 59%. When the time taken between doses exceeded 56 days, the vaccine efficacy rose to 66%. In the cohorts from the UK and Brazil, vaccine efficacy was similar, i.e., 53% in less than 42 days between doses and 65% when exceeding 42 days.
Across all four studies, the vaccine’s had a good safety profile. Serious adverse events occurred in 168 participants, of which 47% were in the vaccine group and 53% were in the placebo group. Some adverse events were considered possibly related to either the vaccine or the placebo. The full data is published on the Lancet in behalf of the Oxford trial group [36].
Safety and efficacy of the sputnik vaccine by Gamaleya Research Institute of Epidemiology and Microbiology
• 21,977 participants
• Trials undertaken in Russia
• 68% of participants had taken the two standard doses
• Demographics
• 61% were male
• 98% were white
• 2% other
• Mean age 45 years in both groups
• Average Body mass Index was 26
• Within 21 days of initial vaccine dose, vaccine efficacy was 92%
• When there was more than 21 days between the two doses, vaccine efficacy from moderate to severe COVID-19 was 100%
• If two doses taken with less than 21 days between them, vaccine efficacy was 74%
• Efficacy studies showed that the vaccine induces an immune response in participants
• Vaccine had a good safety profile
The Phase III trials were conducted between September and November 2020 at sites in Russia. The mean age was 45 years in both the vaccine and placebo group. From 21 days after the first dose of the vaccine, 16 COVID-19 cases were confirmed in the vaccine group (accounting for 0.1%) and 62 cases were confirmed in the placebo group (accounting for 0.3%), therefore, the overall vaccine efficacy was 92%. When there was a 21 day gap between the vaccine doses, there were no SARS-CoV-2 cases in recipients of the vac-cine but there were 20 SARS-CoV-2 cases in the placebo group. Henceforth, vaccine efficacy against moderate or severe COVID-19 was 100%. Vaccine efficacy decreased if the days between doses were less than 21 days, the dosage efficacy was calculated to be 74%. 97 confirmed cases of COVID-19 (65% in the vaccine group and 35% in the placebo group) were not included in the data as these cases arose prior to 21 days elapsing from the first vaccine dose. This still proved that vaccine efficacy after the first dose at any time was 73%. Notably, in the vaccine group, most cases of COVID-19 occurred before dose 2. Immunity was shown to be built up after day 16 of the first vaccine dose. This early onset of protection led to the number of cases in the vaccine group increasing at a much lower rate than the placebo group [34].
Efficacy studies showed that the vaccine induces an immune response in participants. Before the first vaccination, no virus neutralizing antibodies were detected in the participants. In the analysis of immune response activity, 456 participants were analyzed for the presence of antibodies for the SARS-CoV-2 spike protein. In the vaccine group, virus-neutralizing antibodies were detected in 98% of samples who took the vaccine. On the other hand, in the placebo group, virus neutralizing antibodies were detected in 15% of the participants. Hence, this indicated an immune response was activated post vaccination. No serious adverse events were recorded from those who took the vaccination during the trial. All data for this trial are published on the Lancet in behalf of the Sputnik Vaccine trial group [36].
Misconceptions about the SARS-COV-2 vaccinesv
Misconception: Unrealistic speed of formulation: The main public concern that questions the vaccine’s safety is due to how quickly the vaccine was manufactured. In a nutshell, most of the building blocks had already been assembled, and here’s now;
SARS-CoV-2 belongs to a family of viruses that has been around for over 50 years. Previous research was undertaken when a similar strand of the virus appeared in the early 2000 (Sever Acute Respiratory Corona Virus 1, SAR-CoV-1), this allowed researchers to identify the structure and composition of the virus. A paper published by Daou [11] showed the history of the Coronovirinae family, it also showed a step by step analysis of active ingredients and how they go from being an ‘agent’ and finally going to the patient. Research found that the spike protein present on the viral envelope attached itself on to the ACE-2 enzyme and spread through the body. Researchers acknowledged that targeting of this spike protein would eventually stop infection for the patient. Vaccines were built to train the immune system to produce suitable proteins to attack the spike protein and successfully terminate the virus prior to symptoms being experienced. The SARS-CoV-2 vaccine not only came in rapid time due to previous research but also due to other factors. Some vaccines such as the Pfizer/BioNTech vaccine has technology that has been researched and worked on for years prior, in research for other vaccines. Some steps overlapped other steps to ensure time was optimized. Additionally the vaccine projects had a greater abundance of investment and resources in order to accelerate rapid results. Furthermore, due to the spread ability and infectivity of COVID-19, it did not take long to see the efficacy of the vaccines on the patients. In addition, companies began producing and manufacturing vaccines whilst in the trial stage, this allowed the immediate roll out if the drug was to be approved and safe for use.
Misconception: Vaccines contain the virus itself: Another common misconception regarding the vaccines is that they contain parts of the virus that can cause a person to be diagnosed with the virus. This cannot be further from the truth. No vaccines contain any live virus; rather vaccines contain part of the virus that is not harmful. The vaccines are designed to initiate an immune response, this is done by instructing the immune system to reproduce a protein similar to the target protein present on the virus envelope, therefore ‘training’ up the immune system to recognize this protein and attack this protein once detected in the body. The protein that helps your immune system recognizes and fight the virus without causing infection of any sort [10]. This has been the standard mechanism of the vast majority of vaccines, where they prime your immune system to recognize and fight off a disease, but to reiterate, they do not actually cause the infection. The only possibility of becoming infected is that pocket of time between taking the injection and the vaccine taking full effect, as was shown in the clinical trials.
Misconception: Vaccine’s alter DNA: Some believe that the vaccine will alter the DNA of the recipient; this claim is false. The mRNA in a vaccine is designed to instruct the cells to produce a protein similar to that present on the virus and not change DNA genomes. The mRNA create an immune response through the production of antibodies [10]. The mRNA does not enter the nucleus of the cell, were the DNA resides and henceforth cannot affect the DNA of an individual. Once the message is sent, the mRNA molecule is destroyed by the body and gets rid of it.
Misconception: Don’t need it if I’m already infected: Another common misconception is that if a person’s already infected with COVID-19, they will not need to take the vaccine. This claim is unfounded; there is little evidence that indicates a person obtains immunity once they are infected with the virus. Many individuals have been infected with multiple cases, with the second or third case causing more complications than the first case. On this basis it is all the more advisable and beneficial to take the vaccine, as this will help booster and improve immunity from the virus, significantly reducing the chances of being re-infected with the virus.
Misconception: Vaccine exempts me from social distancing: It is a common misunderstanding that when one takes a vaccine, they become immune and won’t have to adhere to social distancing requirements. This is unfortunately not true even after the vaccine’s been administered, you will need to maintain the wearing of a mask around others, washing your hands and adhering to social/physical distancing. There are a few reasons for this: firstly, immunity is not built up instantly after you have completed both doses, some studies have shown them to take a few weeks for the body to do so, and secondly, it is to protect others around you who have not taken the vaccine at all. The vaccine helps the recipient build up immunity from severe illness or death from the virus.
Misconception: People with a weak immune system should not be vaccinated: This is not true. It is highly advised for sufferers with underlying conditions to take the vaccination. The only time a person is advised against taking the vaccination is when the person risks hypersensitivity to a specific ingredient within the vaccine this is similar to patients who do not take the flu jab. As was mentioned earlier, the vaccine does not contain any live material so it will not hurt immune-deficient people. Furthermore, it is of greater importance for a person with underlying condition to take the vaccine, as their compromised immune system is susceptible to suffering greater complications from the virus and face hospitalization or even death.
Discussion and Conclusion
The FDA in addition to the World Health Organization (WHO) has a responsibility towards global health. Their role is to preserve the public health through assurances in the correct running of analysis and eventual safety, efficacy of drugs, biological products such as vaccines and medical devices; this also includes the safety of foods, cosmetics and other products that emit radiation.
The WHO is responsible in providing leadership on health concerns worldwide; in addition, they help structure health research by setting protocols and standards that need to be followed. Furthermore, all clinical trials undertaken have to follow strict guidelines – in which the COVID-19 vaccine was no exception. The vaccines have been put through its paces, through strenuous studies and analysis, with all the safety and efficacy data recorded and published. The data is analyzed by external entities who have no vested gain to either pass or fail a trial, their sole aim is to understand and ensure that trials are run correctly, by following standard operating procedure and the vaccine is an effective vehicle that provides reinforcement to our immune system. All clinical trials run worldwide are recorded onto a clinical trials database (clinicaltrials.gov). This website contains information about medical studies that have been done on human subjects.
This review has taken a detailed look at the history of vaccines and how they have successfully eradicated many diseases over the last few generations. Given that, the rollout of the vaccine was rapid and quick; many people have questions towards the process of the vaccine formulation. This review has addressed many of the common doubts and misconceptions through presenting detail of all the clinical trials that were undertaken on four of the most authorized vaccines. It is clear to see that that the vaccines which undertook Phase III of clinical trials are both effective and safe. Results indicated that the vaccinations have specified a reduced possibility of contracting COVID-19, with no recipient of the vaccine indicating a symptomatic state if tested positive for COVID-19. There are many vaccine candidates that were tested and analyzed as potential vaccines in early phases; however, many of them did not show adequate safety and efficacy profiles to reach Phase III of clinical trials. Vaccinations that have reached Phase III studies also showed in earlier phase studies that they were safe and efficacious.
It is also important to state that the vaccines which have been manufactured for mass immunization not only went through detailed testing and analysis, but also are produced by large corporations. These corporations are worth billions and have reputations too big to lose, and most definitely would not want to risk their name and future for one project that may only be relevant for a few years at most. They includes Pfizer, who are worth over $65.5 billion dollars and produce many and multiple other products, AstraZeneca, who are worth $16 billion dollars and Oxford University, which is one of the foremost educational institutes in the world.
It’s for all to see that at least the vaccines analyzed in this review are not only safe and effective, but a genuine light at the end of the tunnel. They have proven to help reduce the presence of COVID-19 in recipients of the vaccination; causing minimal side effects and likely to be a genuine vehicle towards ending the pandemic once and for all.
As the COIVD-19 virus has had a high survival rate and low mortality rate, this whitepaper provides sufficient evidence for you to take the vaccine. Firstly many people with a healthy background and no underlying condition have passed away or been hospitalized due to this virus. Latest figures have shown that almost 2 million people have died due to the virus, this does not account for the millions of others that were hospitalized. Without the vaccine, you are increasing the risk towards a symptomatic infection, which can lead to damage occurring within vital organs in the body, such as the lungs, heart and brain.
Furthermore, sufferers with underlying conditions such as hypertension may also be reluctant to take the vaccines. Recent studies undertaken by Sardu et al. have shown that their medications for treating hypertension do not affect the prognosis in patients with SARS-CoV-2. What this means is that the antiinflammatory and immune therapies could prevent a worse diagnosis in addition to improving the clinical outcomes in patients with hypertension and the SARS-CoV-2 infection. Secondly, another study investigating the effect of type 2 diabetes treatments showed that the prognosis of sufferers who also contract SARS-CoV-2 have indicated more serious symptoms and problems, with higher rates in need of intensive care due to complications within the respiratory system resulting in pulmonary disease. Upon reviewing the clinical trials, which included multiple patients with underlying conditions, the vaccines tested have shown no serious adverse effects to these individuals as of present. Therefore, in addition to healthy individuals, it is equally advisable for high risk category patients to take the vaccine too. This will greatly reduce chances of a symptomatic episode if they were to contract SARSCoV- 2. All pupils who took the vaccine showed a 100% efficacy towards contracting symptomatic episodes, this means that no vaccine recipient showed symptoms upon contracting the virus post inoculation.
In light of the data obtained in this review and through the clinical trials, in addition to the many articles that are published by the scientific community in support of the vaccine, my professional summation is that this review should help alleviate fear amongst the general populations, dispel myths and enlighten the public. The vaccines in this review have shown a consistent profile in terms of efficacy ( ≥ 90%) and no severe adverse effects being felt. The vaccine is no different to previous vaccines, in terms of both mechanism at which it operates to stimulate the immune system and the thorough testing it had to undergo prior to distribution. I therefore conclude it is important to take the COVID-19 vaccinations not only to save yourself from the virus but also to save those around you that are not capable of taking the vaccine, due to medical reasons. The quicker the majority are vaccinated, the quicker we can return to normalcy.
Acknowledgement
I would like to thank Qatar University, College of pharmacy, for giving me the opportunity to research and build on my knowledge. I would also like to thank Aftab Majothi for proof reading and structuring the document.
REFERENCES
- World Health Organization (WHO). Vaccines and immunization: What is vaccination?. 2021.
- Zakir F, Islam F, Jabeen A, Moni SS. Vaccine development: A historical perspective. Biomed Res. 2019;30(3):452-455.
- Plotkin S. History of vaccination. Proceedings of the National Academy of Sciences. 2014;111(34):12283-12287.
- Riedel S. Edward Jenner and the history of smallpox and vaccination. InBaylor University Medical Center Proceedings 2005;18(1):21-25.
- Plotkin SA. The history of rubella and rubella vaccination leading to elimination. Clin Infect Dis.2006;43:S164-S168.
- A brief history of vaccination. (2020).
- World Health Organization (WHO). Vaccines and immunization. 2021.
- Plotkin SA, Plotkin SA. Correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401-409.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055-1065.
- Centers for Disease Control and Prevention (CDC). Immunology and Vaccine-Preventable Diseases: Principals of prevention. 2020.
- Daou A. COVID-19 vaccination: From interesting agent to the patient. Vaccines. 2021;9(2):120.
- FDA U. The drug development process. Step 3: clinical research. 2020.
- Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses. 2015:1-23.
- Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281-286.
- Richman D, Whitley RJ, Hayden FG. Infectious Disease in the Ageing: A Clinical Handbook. 2009;12:1692.
- Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27:1–4.
- Corley J. US government response to COVID-19 was slow. But how does it compare to other countries. The Forbes. 2020.
- Coronavirus Disease (COVID-19) Advice for the Public. 2020.
- Agarwal KM, Mohapatra S, Sharma P, Sharma S, Bhatia D, Mishra A. Study and overview of the novel corona virus disease (COVID-19). Sens Int. 2020:100037.
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237-261.
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2020;52:1–6.
- Snijder EJ, Decroly E, Ziebuhr J. The non-structural proteins directing coronavirus RNA synthesis and processing. Adv Clin Chem. 2016;96:59-126.
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98.
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924.
- Prüβ BM. Current state of the first COVID-19 vaccines. Vaccines. 2021;9(1):30.
- Heald-Sargent T, Gallagher T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses. 2012;4(4):557-580.
- Jardetzky TS, Lamb RA. Activation of paramyxovirus membrane fusion and virus entry. Current opinion in virology. 2014;5:24-33.
- Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567-571.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261-279.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416.
- Shipmen M. How mRNA and adenovirus vaccines work. 2020.
- Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99-111
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Rus-sia. Lancet. 2021;397(10275):671–681
- Sardu C, Maggi P, Messina V, Iuliano P, Sardu A, Iovinella V, et al. Could anti‐hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID‐19 infection? Data from centers of southern Italy. J Am Heart Assoc. 2020;9(17):e016948.
- Sardu C, Gargiulo G, Esposito G, Paolisso G, Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by COVID-19. Cardiov Diab. 2020;19(1):1-4.
Citation: Daou A (2021) COVID-19: Trial on SARS-COV-2 Vaccines. J Vaccines Vaccin. 12:458.
Copyright: © 2021 Daou A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

