Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Convalescent Plasma in COVID-19; Mortality-Safety First Results of the Prospective Multicenter FALP 001-2020 Trial
Raimundo Gazitua1*, Jose Luis Briones1, Carolina Selman2, Franz Villarroel-Espíndola3, Adam Aguirre-Ducler3, Roxana Gonzalez-Steigmaier3, Karina Cereceda3, Mauricio Mahave4, Ricardo Morales5, Fernanda Yarad5, Nicolas Yannez6, María Elvira Balcells7, Luis Rojas7, Bruno Nervi8, Jyh Kae Nien9, Javier Garate10, Carolina Prieto11, Sofía Palma11, Carolina Escobar11, Josefina Bascuñán12, Rodrigo Muñoz13, Mónica Pinto13, Daniela Cardemil13, Marcelo Navarrete14, Soledad Reyes15, Victoria Espinosa15, Betzabé Rubio16, Pedro Ferrer-Rosende16, Jorge Sapunar16, Hugo Marsiglia16 and Christian Caglevic162Department Diagnostic, Arturo López Pérez Foundation Oncological Institute, Santiago, Metropolitan Region, Chile
3Translational Medicine Research Laboratory, Arturo López Pérez Foundation, Santiago, Metropolitan Region, Chile
4Department of Medical Oncology, Arturo López Pérez Foundation Oncological Institute, Santiago, Metropolitan Region, Chile
5Department of Internal Medicine, Arturo López Pérez Foundation Oncological Institute, Santiago, Metropolitan Region, Chile
6Department of Medical Oncology, Regional Hospital of Talca, Maule Region, Chile
7Department of Infectious Diseases, School of Medicine, Pontifical Catholic University of Chile, Santiago, Metropolitan Region, Chile
8Department of Hematology and Oncology, School of Medicine, Pontifical Catholic University of Chile, Santiago, Metropolitan Region, Chile
9Dávila Clinic, Santiago, Metropolitan Region, Chile
10Red Salud Clinic, Santiago, Metropolitan Region, Chile
11Dipreca Hospital, Santiago, Metropolitan Region, Chile
12Hospital del Trabajador, Chilean Security Association, Santiago, Metropolitan Region, Chile
13Department of Infectious Diseases, Magallanes Clinical Hospital, Punta Arenas, Magallanes and Chilean Antarctic Region, Chile
14School of Medicine, University of Magallanes, Punta Arenas, Magallanes and Chilean Antarctic Region, Chile
15Clínica Alemana Temuco, Araucanía Region, Chile
16Department of Cancer Research, Oncological Institute Arturo López Pérez Foundation, Santiago, Metropolitan Region, Chile
Received: 10-Mar-2021 Published: 01-Apr-2021, DOI: 10.35248/2157-7560.21.s12.002
Abstract
Background: The use of Convalescent Plasma (CP) to treat COVID-19 has shown promising results; however, its effectiveness remains uncertain. The purpose of this study was to determine the safety and mortality of CP among patients hospitalized with COVID-19.
Study design and methods: This multicenter, open-label, uncontrolled clinical trial is currently being conducted at nine hospitals in Chile. Patients hospitalized due to COVID-19 with less than 14 days since symptom onset were eligible. Enrolled patients were classified into four groups: Patients with cancer and severe COVID-19. Patients with cancer and non-severe COVID-19. Patients with severe COVID-19 and patients with non-severe COVID-19 only. The intervention involved two 200-cc. CP transfusions with anti-SARS-CoV-2 IgG titers ≥ 1:320 collected from COVID-19-recovered donors
Results: 192 patients hospitalized for COVID-19 received CP transfusions. At the first transfusion, 90.6% fulfilled the criteria for severity, and 41.1% required mechanical ventilation. 11.5% of the patients had cancer. Overall, 7-day and 30-day mortality since the first CP transfusion was 5.7% and 16.1% respectively. There were no differences at either time point in mortality between the four groups. Patients on mechanical ventilation when receiving CP had higher mortality rates than those who were not: 22.8% (95% C.I. 14.1-33.6%) vs. 11.5% (95% C.I. 6.3–18.9%) (p=0.037). Overall, 30-day mortality was higher in patients over 65 than in younger patients: 26.7% (95% C.I. 16.1– 39.7%) (p=0.019). Severe adverse events were reported in four patients (2.1%) with an overall transfusion-related lung injury rate of 1.56%. No CP-related deaths occurred.
Discussion: CP is safe when used in patients with COVID-19 even when also presenting severity criteria or risk factors. Our mortality rate is comparable to reports from larger studies. Controlled clinical trials are required to determine efficacy.
Conclusion: CP is safe when used in the COVID-19 population even for those who present severity criteria and/or risk factors for poor prognosis including cancer. In-depth analyses of the serological and molecular characteristics of CP are needed to evaluate the efficacy of this intervention through controlled clinical trials.
Registration: NCT04384588
Keywords
COVID-19; Vaccine; Convalescent plasma; Diagnosis
Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) was first described in December 2019 in Wuhan, China and quickly spread across the globe, being declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. The first case in South America was reported on February 26 in Brazil [2], while in Chile, the index case was confirmed on March 3, 2020 [3]. Currently, 29% of the cases worldwide are concentrated in Latin America, one of the most affected areas in the world [2], which constitutes a serious health problem considering the complex socioeconomic and health characteristics of this region.
At the time of study start, there was neither a completely effective standard of care nor a proven effective vaccine against COVID-19. Many therapies have been evaluated against SARS-CoV-2 with mixed results. The combination of hydroxychloroquine and azithromycin in an early case series seemed promising [4], but randomized studies were unable to confirm its efficacy, and had an even higher incidence of adverse effects [5-7]. The antiviral drug remdesivir provides clinical improvement yet no decrease in mortality [8,9]. Moreover, antiviral drugs lopinavir/ritonavir were also shown to be ineffective against SARS CoV-2 [10]. Only low doses of dexamethasone decrease the mortality rate in patients with mechanical ventilation and oxygen supplementation requirements [11].
Passive immunotherapy with Convalescent Plasma (CP) from individuals who have already recovered from infection has been safe and effective against previous viral outbreaks including the SARS-CoV-1, MERS-CoV, influenza A (H1N1), and the Ebola virus epidemics [12-17]. The initial reports published on using CP to treat COVID-19 suggest that it confers clinical improvement, decreases viral load, and shortens mechanical ventilation duration [18,19]. The use of CP is safe, with an incidence of related serious adverse events of less than 1% of treated patients, similar to the usual practice of transfusion medicine [20].
In this work, we report on the safety and in-hospital mortality of patients with COVID-19 treated with CP. Because patients with cancer commonly have impaired humoral immunity [21] we included a separate cohort of patients with cancer as part of our compassionate access protocol. The study is a part of the NCT04384588 protocol.
Patients and Methods
This is a multicenter, open-label, uncontrolled clinical trial. For data analysis purposes, the cohort was divided into four arms: A) Patients with cancer who meet the severity criteria; B) Patients with cancer who do not meet the severity criteria but have at least one poor prognostic factor; C) Patients without cancer who meet the WHO severity criteria; and D) Patients without cancer without severity criteria with at least two risk factors for poor prognosis (S1 Appendixes 1a, 1b, and 1c) [20,22-24]. The criteria used to define patients with cancer are listed in S2 Appendix.
A case of COVID-19 was defined as a patient with clinically compatible symptoms [25,26] (S3 Appendix) and an RT-qPCRbased confirmation of SARS-CoV-2 from a nasopharyngeal swab or with a chest CT scan compatible with COVID-19 [27].
This project was developed and executed in accordance with Chilean Law No. 20.120 (“On scientific research in the human being, its genome, and prohibits human clonation” : All scientific research on a human being must have his prior, express, free and informed consent, or, failing that, that of the person who must supply his will in accordance with the law."); Law No. 20,584 ("Regulates the rights and duties that people have in relation to actions related to their health care”); Law No. 19.628 ("On protection of private life”); CIOMS Guidelines, Declaration of Helsinki, Nuremberg code, The Universal Declaration of Human Rights and The International Covenant on Civil and Political Rights.
Ethics
The study was approved by our local ethics committee: Comité Ético Científico Fundación Arturo López Pérez, on April 7th, 2020. Informed consent was obtained from all participants by an authorized delegate in each participating center as follows:
Donors
Written informed consent was provided by all the participating donors. Consent by a legal representative was not needed.
All plasma units were fully anonymized following the guidelines established in local Blood Banks before being accessed by the research team
Plasma recipient
All plasma recipients or the corresponding legal representative provided written informed consent as authorized by the corresponding Ethics Committee. Remote informed consent was authorized by the Local Ethics Committee when legal representatives were unable to physically attend. The remote consent was obtained by a video/phone call followed by digital transfer of the signed form.
This study did not include underage participants.
Patients were enrolled between May 2020 and August 2020. Medical records were accessed from May 2020 to September 2020. The sources of information were the clinical records of the 9 participating institutions: Instituto Oncológico Fundación Arturo López Pérez (FALP) (Santiago), Pontificia Universidad Católica de Chile (Santiago), Clínica Dávila (Santiago), RedSalud (Santiago), Hospital del Trabajador (Santiago), Hospital Dipreca (Santiago), Hospital Clínico de Magallanes (Punta Arenas), Clínica Alemana Temuco (Temuco), Hospital Regional de Talca (Talca).
Study population
Patients were enrolled between May 1 and August 7, 2020 in nine hospitals from four Chilean cities: Instituto Oncológico Fundación Arturo López Pérez (FALP) (Santiago, Metropolitan Region), Pontificia Universidad Católica de Chile (Santiago, Metropolitan Region), Clínica Dávila (Santiago, Metropolitan Region), RedSalud (Santiago, Metropolitan Region), Hospital del Trabajador (Santiago, Metropolitan Region), Hospital Dipreca (Santiago, Metropolitan Region), Hospital Clínico de Magallanes (Punta Arenas, Magellan Region), Clínica Alemana Temuco (Temuco, Araucania region), Hospital Regional de Talca (Talca, Maule Region).
The number of participants was defined, as per design, by the number of available plasma units during a one-year enrollment period. Therefore, sample size and power calculation was not performed. In this article we present an interim analysis and the study is ongoing until planned estimated time or exhaustion of convalescent plasma units.
The trial was registered in clinicaltrials.gov on May 12th 2020: NCT04384588. Clinical trial registration is currently not required by Chilean regulation. Upon decision on trial registration there was a 12 days administrative delay from initial recruitment. Authors confirm that all ongoing and related trials for this drug/ intervention are registered.
Our clinical trial included a total of 192 patients from different hospitals. Forty-one out of those 192 patients (21.3%) also belonged to the NCT04375098 randomised clinical trial and were included in this report through a collaborative network. The NCT04375098 RCT shared inclusion and exclusion criteria and enrolled a total of 58 patients of which only a subset of 41 patients received convalescent plasma and their follow-up was therefore included in the present study. Whereas the aim of the NCT04375098 RCT was to compare the effect of early versus deferred plasma administration on the risk of patient's aggravation, our study evaluates in a large cohort the overall mortality and safety outcomes from a multicenter perspective.
The NCT04375098 study contributed to Arm A with 5 patients, Arm B with 1 patient, Arm C with 26 patients, and Arm D with 9 patients.
Participation criteria
Patients were eligible to receive CP if they were at least 18 years old with a confirmed diagnosis of severe SARS-CoV-2 infection or nonsevere COVID-19 with two or more poor prognostic factors within the first 14 days from symptom onset. Patients with a known allergy to previous plasma infusions, multiple organ dysfunction, active intracerebral hemorrhage, disseminated intravascular coagulation requiring transfusion, acute respiratory distress syndrome, or with active cancer and survival expectancy less than 1 year (S4 Appendix) were excluded.
Plasma collection, processing, and conservation
Plasma was obtained from voluntary donors registered through a website (https://www.donantecovid.cl/) who were diagnosed with COVID-19 by RT-PCR from a nasopharyngeal swab sample or who presented clinically compatible symptoms and tomographic findings. Before the donation, patients had to be asymptomatic for at least 21 days with two negative RT-qPCR tests on two consecutive days or asymptomatic for at least 28 days (Appendix 2). All donors underwent a blood-bank selection survey plus microbiological screening for HIV, hepatitis-B and -C, syphilis, HTLV-I and -II, and Chagas. In addition, A Nucleic Acid Amplification Test (NAAT) was performed for hepatitis-B, -C, and HIV. All women, with or without a history of pregnancy, were tested for anti-HLA antibodies. Anti-SARS-CoV2 antibodies were measured, and only donors with IgG ≥ 1:320 (ELISA Euroimmun®) were selected. Plasma was collected by apheresis (Trima Accel® or Spectra Optia, Terumo®) and the volumes obtained were pooled and divided into doses of 200 mL each. CP units were stored at -40°C.
Convalescent plasma transfusion
Each patient received two 200-mL units 24 hours apart. Units were selected according to blood type and Rh compatibility. The transfusion procedure took 1 to 2 hours, according to the treating physician’s preference. Premedication once administrated with clorpheniramine i.v. 10 mg and acetaminophen 1 gr. was also permitted.
Variables analyzed
This report analyzed the primary outcomes of the FALP project COVID 001-2020 (NCT04384588): In-hospital mortality measured at days 7 and 30 after CP administration and Adverse Event Occurrence according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Statistical analysis
Demographic and epidemiological variables are described in frequency and percentage, while continuous variables by their median and interquartile range. The relationships between the categorical variables were evaluated using the Chi-square test. When the expected frequency for a combination of variables was less than 5, Fischer´s exact test was performed. Overall survival was defined as the time from the first CP transfusion to the time of death or date of the last follow-up. Survival probability was estimated using the Kaplan-Meier method and the difference in survival between the groups was examined using the log-rank test. In addition, the Cox proportional-hazards model was applied to determine possible risk factors associated with mortality. A crude Hazard Ratio (HR) and an HR adjusted for sex, age, ventilation, days of hospitalization, and days of symptom duration until transfusion were estimated along with their 95% Confidence intervals (Cis). All percentage confidence intervals were calculated according to the Clopper- Pearson method. A p-value under 0.05 was considered statistically significant. Statistical analyses were performed with STATA and R program v 3.6.0. (RCore Team, 2020, Vienna, Austria).
Role of funding source
The funders had no role in the design, analysis, or execution of the study nor in the decision to submit the manuscript for publication.
Results
Patient characteristics
Between May 1 and August 7, 2020, 192 patients from nine hospitals in four different regions of Chile enrolled in this study (Figure 1). Table 1 details the clinical and demographic characteristics of these patients. The median follow-up was 62 days (IQR: 34.8 to 88.0) while the median age was 59 years old (IQR: 49.8 to 67.0); 90.6% of the population met the severity criteria, 71.9% were hospitalized in an Intensive Care Unit, and 41.1% received mechanical ventilatory support, which was invasive in 30.7% of cases. The median SOFA score was 3 (IQR: 2 to 5) while the median PaO2/FiO2 was 181 (IQR: 127 to 273) (Table 1). The most common comorbidities were hypertension (59.0%) and diabetes mellitus (56.0%). Cancer was present in 11.5% of the cohort (n=22) (Figure 1).
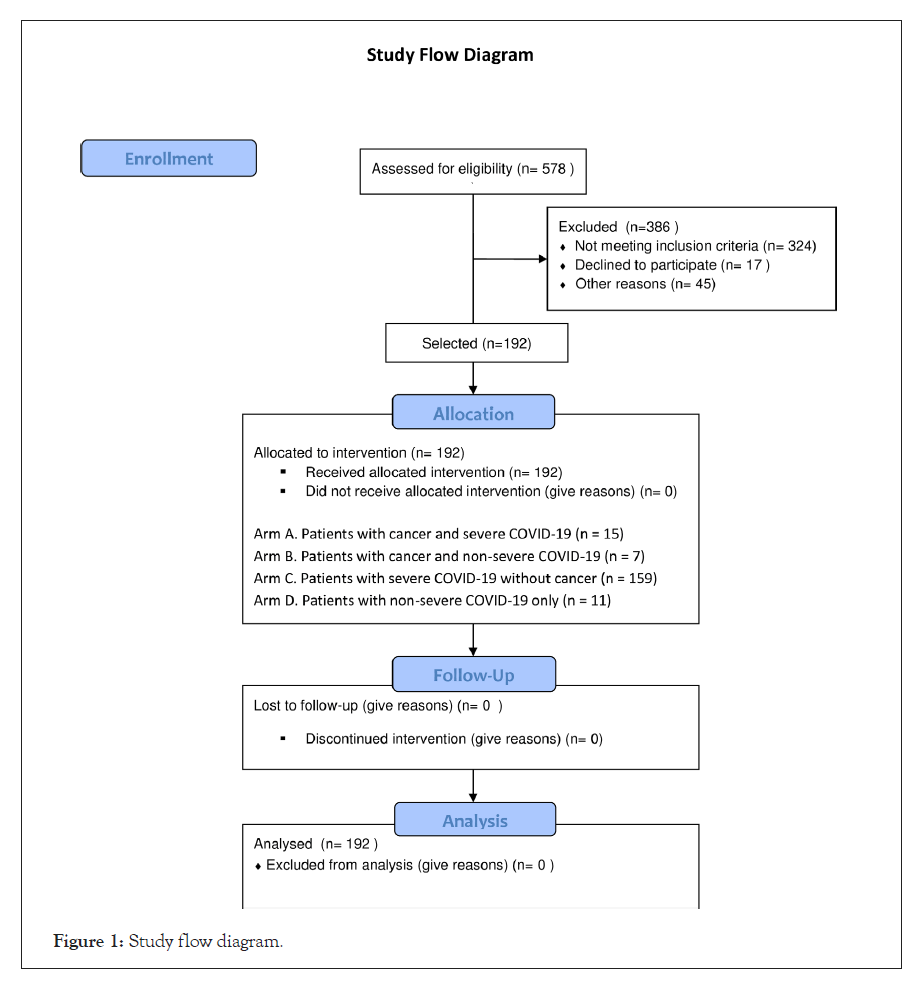
Figure 1: Study flow diagram.
| Age | Values |
|---|---|
| Age – yr (median, IQR) | 59.0 (49.8 - 67.0) |
| Sex | No. (%) |
| Male | 135 (70.3) |
| Female | 57 (29.7) |
| Ethnic group | No. (%) |
| Hispanic or Latino | 180 (93.8) |
| Caucasian | 12 (6.2) |
| Severity | No. (%) |
| Severe | 174 (90.6) |
| Non-severe with RF | 18 (9.4) |
| O2 requirement | No. (%) |
| No oxygen | 20 (10.4) |
| Oxygen | 172 (89.6) |
| Ventilatory support | No. (%) |
| Mechanic ventilation | 79 (41.1) |
| No mechanic ventilation | 113 (58.9) |
| Ventilatory support type | No. (%) |
| Invasive ventilation (IV) | 59 (30.7) |
| Non-invasive ventilation (NIV) | 20 (10.4) |
| O2 support device | No. (%) |
| High-flow nasal cannula oxygenation | 35 (18.2) |
| Oxygen face mask | 11 (5.7) |
| Nasal oxygen cannula with prongs | 47 (24.5) |
| Hospitalization unit (at enrollment) | No. (%) |
| Critical care unit | 138 (71.9) |
| Basic room | 54 (28.1) |
| Comorbidities | No. (%) |
| Hypertension | 59 (30.7) |
| Diabetes | 56 (29.2) |
| Obesity | 41 (21.4) |
| Cancer | 22 (11.5) |
| EPOC | 10 (5.2) |
| Coronary cardiopathy | 5 (2.6) |
| HIV/AIDS | 0 (0) |
| Blood type | No. (%) |
| O | 113 (58.9) |
| A | 58 (30.2) |
| B | 19 (9.9) |
| AB | 2 (1.0) |
| SOFA | |
| Sofa score at enrolment (median, IQR) | 3 (2-5) |
| PAFI | |
| Pa02/ Fi02 (median, IQR ) | 182 (127-273) |
Table 1: Baseline demographics and clinical characteristics.
Outcomes
The mortality rates 7 and 30 days after CP administration were 5.7% and 16.1%, respectively (Figure 2). There were no significant differences in mortality related to the timing of the initial CP administration. Patients who received CP within the first 7 days of symptom onset had a 7-day mortality rate of 9.2% (95% CI, 4.1% to 17.3%) versus. 2.9% (95% CI, 0.6% to 8.1%; p=0.06) for those who received CP between 8 and 14 days. Patients who received CP within the first 7 days of symptom onset had a 30-day mortality rate of 17.2% (95% CI, 10.0% to 26.8%) versus. 15.2% (95% CI, 9.0% to 23.6%; p=0.707) for those who received CP between 8 and 14 days.
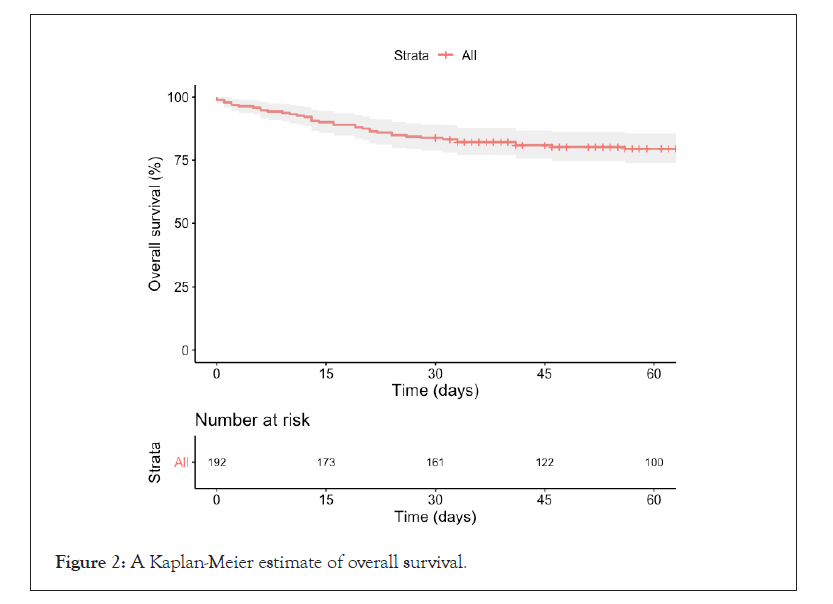
Figure 2: A Kaplan-Meier estimate of overall survival.
Patients who received plasma during the first 3 days of hospitalization did not register any significant differences in terms of 7- or 30-day mortality compared with those transfused after 3 days: 4.7% (95% CI, 1.7% to 9.8%) vs. 7.9% (95% CI, 2.6% to 17.6%; p=0.358), and 14.0% (95% CI, 8.5% to 21.2%) vs. 20.6% (95% CI, 11.5% to 32.7%; p=0.237) respectively. Patients receiving mechanical ventilatory support at the time of CP administration had a higher 30-day mortality compared with patients who did not require mechanical ventilation: 22.8% (95% CI, 14.1% to 33.6%) vs. 11.5% (95% CI, 6.3% to 18.9%; p=0.037) (Table 2). The trial arm with the highest mortality rate was the cancer and severe COVID group with 20.0% after 30 days (n=3). The severe COVID only group had a 17.0% mortality rate. There were no deaths in the cancer and non-severe COVID group while the non-severe COVID only group registered one death (9,1%). There were no statistically significant differences between the different study groups in terms of 30-day mortality (Figure 3).
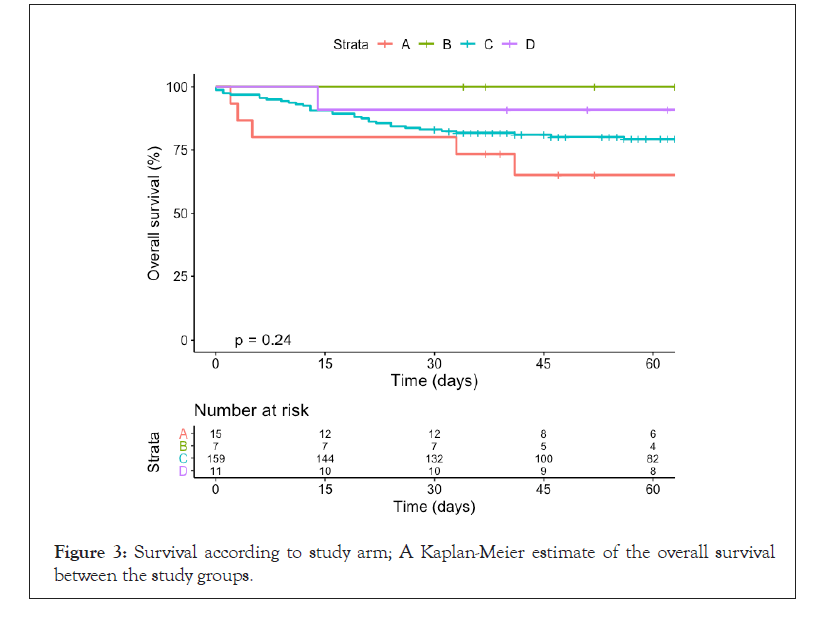
Figure 3: Survival according to study arm; A Kaplan-Meier estimate of the overall survival between the study groups.
| 7-day mortality | 30-day mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample. No | Events. No | Estimate 95% CI | P-value | Sample. No | Events. No | Estimate. 95% CI | P-value | |
| Overall mortality | 192 | 11 | 5.7% (2.9%, 10.0%) | 192 | 31 | 16.1% (11.2%, 22.1%) | ||
| Sex | 0.063 | 0.732 | ||||||
| Female | 57 | 6 | 10.5% (4.0%, 21.5%) | 57 | 10 | 17.5% (8.7%, 29.9%) | ||
| Male | 135 | 5 | 3.7% (1.2%, 8.4%) | 135 | 21 | 15.6% (9.9%, 22.8%) | ||
| Age | 0.071 | 0.019 | ||||||
| 18-44 | 32 | 1 | 3.1% (0.1%, 16.2%) | 32 | 2 | 6.2% (0.8%, 20.8%) | ||
| 45-64 | 100 | 3 | 3.0% (0.6% 8.5%) | 100 | 13 | 13.0% (7.1%, 21.2%) | ||
| 65+ | 60 | 7 | 11.7% (4.8%, 22.6%) | 60 | 16 | 26.7% (16.1%, 39.7%) | ||
| Days to transfusion since symptoms | 0.06 | 0.707 | ||||||
| ≤ 7 days | 87 | 8 | 9.2% (4.1%, 17.3%) | 87 | 15 | 17.2% (10.0%, 26.8%) | ||
| 8+ days | 105 | 3 | 2.9% (0.6%, 8.1%) | 105 | 16 | 15.2% (9.0%, 23.6%) | ||
| Days to transfusion since hospitalization | 0.358 | 0.237 | ||||||
| ≤ 3 days | 129 | 6 | 4.7% (1.7%, 9.8%) | 129 | 18 | 14.0% (8.5%, 21.2%) | ||
| 3+ days | 63 | 5 | 7.9% (2.6%, 17.6%) | 63 | 13 | 20.6% (11.5%, 32.7%) | ||
| Ventilation | 0.74 | 0.037 | ||||||
| No IV/ NIV | 113 | 7 | 6.2% (2.5%, 12.3%) | 113 | 13 | 11.5% (6.3%, 18.9%) | ||
| IV/NIV | 79 | 4 | 5.1% (1.4%, 12.5%) | 79 | 18 | 22.8% (14.1%, 33.6%) | ||
| O2 support device | 0.254 | 0.374 | ||||||
| IV | 59 | 1 | 1.7% (0.0%, 9.1%) | 59 | 13 | 22.0% (12.3%, 34.7%) | ||
| NIV | 20 | 3 | 15.0% (3.2%, 37.9%) | 20 | 5 | 25.0% (8.7%, 49.1%) | ||
| HFNCO | 35 | 2 | 5.7% (0.7%, 19.2%) | 35 | 5 | 14.3% (4.8%, 30.3%) | ||
| O2 face mask | 11 | 0 | 0.0% (0.0%, 28.5%) | 11 | 0 | 0.0% (0.0%, 28.5%) | ||
| NOC | 47 | 4 | 8.5% (2.4%, 20.4%) | 47 | 6 | 12.8% (4.8%, 25.7%) | ||
| No O2 | 20 | 1 | 5.0% (0.1%, 24.9%) | 20 | 2 | 10.0% (1.2%, 31.7%) | ||
| Study Arm | 0.166 | 0.754 | ||||||
| Arm A | 15 | 3 | 20.0% (4.3%, 48.1%) | 15 | 3 | 20.0% (4.3%, 48.1%) | ||
| Arm B | 7 | 0 | 0.0% (0.0%, 41.0%) | 7 | 0 | 0.0% (0.0%, 41.0%) | ||
| Arm C | 159 | 8 | 5.0% (2.2%, 9.7%) | 159 | 27 | 17.0% (11.5%, 23.7%) | ||
| Arm D | 11 | 0 | 0.0% (0.0%, 28.5%) | 11 | 1 | 9.1% (0.2%, 41.3%) | ||
Abbriviations: IV: Invasive Ventilation; NIV: Noninvasive Ventilation; HFNCO: High Flow Nasal Cannula Oxygenation; NOC, Nasal Oxygen Cannula
Note: Arm A, Neoplasm patient w/ severity criteria; Arm B, Neoplasm patient w/o severity criteria w/ risk factors;
Note: Arm C, Non-Neoplasm patient w/ severity criteria; Arm D, Non-Neoplasm patient w/o severity criteria w/ risk factors
Table 2: Seven and thirty-day mortality characteristics.
(A) Patients with cancer and severe COVID-19. (B) Patients with cancer and non-severe COVID-19. (C) Patients with severe COVID-19 without cancer. (D) Patients with non-severe COVID-19 only.
Patients aged over 65 years old showed higher mortality (p=0.019) compared with younger patients: 26.7% vs. 13.0% (45-65 years old) and 6.2% (18-44 years old) (Figure 4). There were no differences in mortality by sex (p=0.326), ABO group (p=0.89), or cancer status (p=0.690). Age over 65 years old and mechanical ventilation at the time of CP administration were the two risk factors significantly associated with mortality (Table 3).
| Crude HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 1 (Ref) | 1 (Ref) | |||
| Male | 0.78 (0.40-1.53) | 0.471 | 0.77 (0.38-1.57) | 0.471 | |
| Age | |||||
| 18-44 | 1 (Ref) | 1 (Ref) | |||
| 45-64 | 2.52 (0.58-11.0) | 0.221 | 2.10 (0.47-9.32) | 0.33 | |
| 65+ | 6.67 (1.56-28.5) | 0.01 | 6.30 (1.45-27.32) | 0.014 | |
| Days to transfusion since symptoms | |||||
| = 7 days | 1 (Ref) | 1 (Ref) | |||
| 8+ days | 1.00 (0.53-1.89) | 0.991 | 0.73 (0.34-1.57) | 0.424 | |
| Days to transfusion since hospitalization | |||||
| =3 days | 1 (Ref) | 1 (Ref) | |||
| 4+ days | 1.60 (0.84-3.04) | 0.155 | 1.31 (0.60-2.82) | 0.496 | |
| Ventilation | |||||
| No IV/NIV | 1 (Ref) | 1 (Ref) | |||
| IV/NIV | 2.30 (1.20-4.40) | 0.012 | 2.72 (1.24-5.54) | 0.006 | |
Abbriviations: IV: Invasive Ventilation; NIV: Noninvasive Ventilation
Table 3: Risk factors associated with mortality. Using a Cox Regression model.
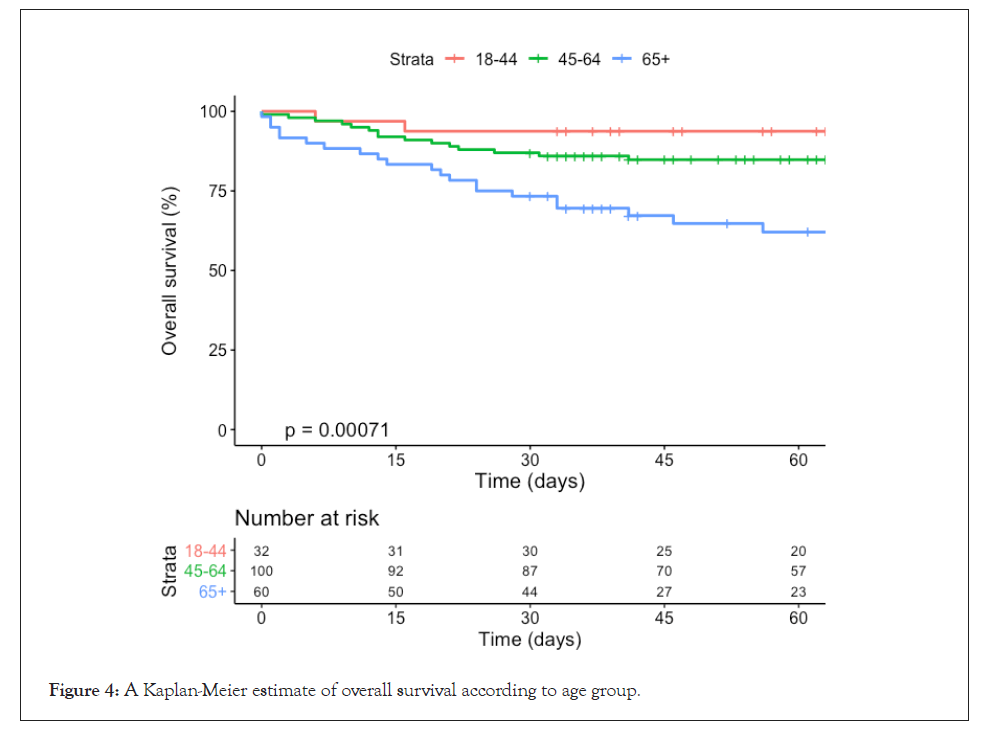
Figure 4: Kaplan-Meier estimate of overall survival according to age group.
Adverse events
We registered a total of 11 CP transfusion-related adverse events (2.9%), four (1.1%) of which were considered to be serious adverse events (SAEs) related to CP transfusion including three cases of transfusion-related acute lung injury (TRALI) and one case of thrombocytopenia. No CP transfusion-related deaths were reported (Table 4). The adverse events are summarized and described in Table 5.
| Adverse event | Any grade | Grade 3, 4 or 5 | SAE | Event/Patient | Event/Transfusion |
|---|---|---|---|---|---|
| TRALI | 3 | 3 | 3 | 1.56% (3/192) | 0.80% (3/373) |
| Thrombocytopenia | 1 | 1 | 1 | 0.52% ( 1/192) | 0.27% ( 1/373) |
| Fever | 5 | 0 | 0 | 2.60% ( 5/192) | 1.34% (5/373) |
| Skin-Rash | 2 | 0 | 0 | 1.04% (2/192) | 0.53% (2/373) |
| Total AE | 11 | 4 | 4 | 5.72% (11/192) | 2.94% ( 11/373) |
| Total SAE | 2.08% (4/192) | 1.07% (4/373) | |||
| Treatment related death | 0% ( 0/192) | 0% ( 0/373) |
Abbriviations: SAE: Serious Adverse Event; TRALI: Transfusion Related Acute Lung Injury; AE: Adverse Events.
Table 4: Adverse events related to the use of convalescent plasma.
| Adverse events | Any Grade | Patients with AE | Events per patient | AE Grade 3, 4 or 5 | Patients with AE Grade 3, 4 or 5 | Events per patient |
|---|---|---|---|---|---|---|
| System | n | n (%) | (range) | n | n (%) | (range) |
| Infectious | 62 | 43 (22.3) | (1-4) | 33 | 24 (12.5) | (1-3) |
| Respiratory | 20 | 18 (9.4) | (1-2) | 6 | 6 (3.1) | 1 |
| Renal | 17 | 14 (7.3) | (1-2) | 10 | 10 (5.2) | 1 |
| Cardiac | 17 | 16 (8.3) | (1-2) | 3 | 3 (1.6) | 1 |
| Gastrointestinal | 16 | 11 (5.7) | (1-2) | 1 | 1 (0.5) | 1 |
| Vascular-Thromboembolism | 11 | 11 (5.7) | 1 | 5 | 5 (2.6) | 1 |
| Neurologic | 8 | 8 (4.2) | 1 | 4 | 4 (2.1) | 1 |
| Fever | 5 | 5 (2.6) | 1 | 0 | 0 (0) | 1 |
| Skin | 4 | 4 (2.1) | 1 | 2 | 2 (1.0) | 1 |
| Hepatic | 3 | 3 (1.6) | 1 | 0 | 0 (0) | 1 |
| Hematologic | 2 | 2 (1.0) | 1 | 1 | 1 (0.5) | 1 |
| Reumatologic | 1 | 1 (0.5) | 1 | 0 | 0 (0) | 1 |
| Total | 166 | 136 (70.8) | (1-4) | 65 | 56 (29.2) | (1-3) |
Abbriviations: AE: Adverse Event
Table 5: Adverse events registered during the study.
Discussion
At the time of writing, this is the first report of in-hospital mortality and treatment safety from a Latin-American multicenter study on patients with COVID-19 who received CP. Although this study did not aim to demonstrate the efficacy of CP, our reported 7-days and 30-days mortality rates are comparable with those reported by the Mayo Clinic in the largest CP study to date, which showed 7-days and 30 day-mortality rates of 10.5% and 24.5%, respectively. Unlike the series published by Joyner et al. our cohort did not display differences in mortality associated with CP administration during the first 3 days of hospitalization compared to patients who were transfused later [22].
Mortality rates reported in patients with cancer and COVID-19 infection range from 30% to 39% [28,29]. Many patients with cancer are immunocompromised as a consequence of their underlying disease and an associated treatment regimen that often includes myelosuppressive chemotherapy, immunosuppressive agents, and radiation, which may increase mortality [30]. In our series, patients with cancer had a 30-day mortality incidence of 13.6%, similar to patients without cancer and lower than reported in other cohort studies of patients with cancer and COVID-19 infection. This low mortality rate may indicate that patients with an impaired humoral and cellular response may benefit from passive antibody administration impeding viral replication and modulating the inflammatory events associated with COVID-19. In contrast to our results, a recent study on the use of CP in patients with cancer reported a mortality rate of 41.7% [31]. One important difference between the two cohorts is the proportion of patients with hematological malignancies; 58.1% was reported by Remblay et al, versus 36.3% from our trial. This particular subgroup seems to have higher mortality rates than patients with solid tumors, which is an interesting issue that warrants additional investigation.
As expected, elderly patients and patients requiring mechanical ventilation had significantly higher mortality rates in our study. These findings are consistent with previous evidence in patients with and without COVID-19 [32,33].
The main endpoint of our study was a safety assessment of the occurrence and type of CP-related adverse events. Overall, 70% of patients reported at least one adverse event. This rate is typical in a cohort of critically ill patients. Only 11 adverse events were related to the CP transfusion, and four of these were identified as SAEs. All 11 adverse events were classified as “possibly related” to the intervention by the treating physician using a four-item Likerttype scale (probably, possibly, remotely, or not related). TRALI was the most frequent SAE (1.5%, n=3). This incidence is within the 0.001 to 2.1% range reported in both the general population and critically ill patients [34,35].
In patients with COVID-19 especially, it is difficult to assign with certainty the occurrence of TRALI or Transfusion-Associated Circulatory Overload (TACO) since, in the context of mostly severe cases, respiratory failure is the predominant dysfunction, and concomitant but potentially unrelated worsening may occur close to the transfusion. As for thrombocytopenia, post-transfusion purpura is rare, with an incidence of less than 0.01%, associated with the detection of antiplatelet antibodies [36]. In the only case of thrombocytopenia, the antiplatelet antibody test was negative. Different mechanisms likely underlie COVID-19-related thrombocytopenia, including direct inhibition of hematopoiesis in bone marrow by SARS-CoV-2, autoimmune destruction induced by the virus, and platelet aggregation and consumption in the lung parenchyma [37].
The present study has some limitations. Since we aimed to assess mortality rates and safety, the absence of a control group prevents drawing conclusions on the benefits of CP treatment. The lack of a control group was grounded on ethical concerns raised at the beginning of the outbreak when the study was designed. Nevertheless, the observed mortality rates are encouraging and additional studies with matched control cases are planned. Another limitation is that the number of patients in the different arms of the study differ substantially. Any inference comparing arms is underpowered.
While donor anti-SARS-CoV-2 spike protein antibody titers were elevated (≥ 1:320), we have not yet investigated their neutralizing activity. Ongoing experiments aim to characterize the neutralizing antibody and cytokine content of CP and identify the factors potentially responsible for its therapeutic benefits in patients with COVID-19 using multivariate analysis. Such studies will generate new hypotheses on CP efficacy in regard to specific types and amounts of neutralizing antibodies and cytokines.
Although CP transfusion has shown promising results against COVID-19 in case series [18,19] and case-control studies [38], its real benefit remains unclear. The first published randomized trial with CP in COVID-19 did not show significant improvement in the clinical condition of patients with severe, life-threatening COVID-19 but the interpretation is limited by the study’s early endpoint. Nevertheless, patients with severe, non-life-threatening COVID-19 improved significantly compared with the control group (91.3% vs. 68.2% (HR, 2.15 [95% CI, 1.07-4.32]; p=0.03) [39]. These findings suggest that CP may be more effective in patients with COVID-19 without life-threatening conditions.
Conclusion
CP is safe when used in the COVID-19 population even for those who present severity criteria and/or risk factors for poor prognosis including cancer. The mortality rate reported here is comparable to those found in larger series and was higher in patients requiring ventilatory support and those over 65 years old. In-depth analyses of the serological and molecular characteristics of CP are needed to evaluate the efficacy of this intervention through controlled clinical trials.
Funding Disclosure
“Fondo de Adopción tecnológica SIEmpre “supported by SOFOFA, Confederation of Production and Commerce and Ministry of Science and Technology, Knowledge and Innovation. Chile.
Conflict of Interest Disclosure
Dr. Nicolas Yáñez reports personals fees from Merck and Pfizer. Dr. Christian Caglevic reports personals fees from Bristol Myers Squibb, MSD, Roche, Boehringer-Ingelheim and Andes Biotechnology, Lilly, Merck Sharpe and Dohme, Medivation, Astra Zeneca, and Astellas Pharma. All other authors have no conflict of interest.
Supporting Information
S1 Appendix; Study terms: Includes description of study arms, severity criteria and risk factors for poor prognosis.
S2 Appendix; Definition of a patient with cancer.
S3 Appendix; Definition of a patient with COVID-19.
S4 Appendix; Participation criteria: Describes inclusion and exclusion criteria.
S5 Table; Contribution of patients included from the NCT04375098 study.
Acknowledgements
All study coordinators in particular to Giselle Godoy, Cecilia Martinez, Macarena Ubal, Mayra Doddis, Isabel Rojo, and Ricardo Palomera. Blood Bank staff from participating hospitals: Marcelo Díaz de Valdes, Jaime Pereira, Mayling Chang, Pablo Sepulveda, Ricardo Hojas, Veronica Soto, Eugenio Reyes, Ana Gonzalez, Celia Gamonal, Marcos Espinoza, Marcos Sandoval, Marcelo Vega, Nordiana Baruzzi and Luz María González Anguita. We’d like to thank the catg.cl laboratory for sampling processing at Punta Arenas, Región de Magallanes y Antártica Chilena. Chile.
REFERENCES
- WHO. Director-General's opening remarks at the media briefing on COVID-19. 2020.
- WHO.Brazil: WHO Coronavirus Disease (COVID-19) Dashboard COVID-19.who.int. 2020.
- CDN. Cdn.digital.gob.cl. 2020.
- Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LC, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate COVID-19. N Engl J Med. 2020;383(21):2041-2052.
- Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. MedRxiv. 2020.
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ. 2020;369.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19: Preliminary report. N Engl J Med. 2020.
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020.
- Group TR. Dexamethasone in hospitalized patients with COVID-19: Preliminary report. N Engl J Med. 2020.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80-90.
- Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44-46.
- Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919-922.
- Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-456.
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfusion. 2016;14(2):152.
- Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis. 1999;179:S18-S23.
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490-9496.
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582-1589.
- Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9).
- Navarrete MA, Heining-Mikesch K, Schüler F, Bertinetti-Lapatki C, Ihorst G, Keppler-Hafkemeyer A, et al. Upfront immunization with autologous recombinant idiotype Fab fragment without prior cytoreduction in indolent B-cell lymphoma. Blood, J Am Soc Hematol. 2011;117(5):1483-1491.
- Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. MedRxiv. 2020.
- Investigational COVID-19 Convalescent Plasma Guidance for Industry. Fda.gov. 2020.
- Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine. 2020;180(7):934-943..
- Consejo Asesor del Ministerio de Salud amplía definición de casos sospechosos y confirmados de COVID-19. Ministerio de Salud – Gobierno de Chile. 2020.
- Coronavirus Disease 2019 (COVID-19) | 2020 Interim Case Definition. Wwwn.cdc.gov. 2020.
- Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological society of north America expert consensus document on reporting chest CT findings related to COVID-19: Endorsed by the society of thoracic Radiology, the American college of Radiology, and RSNA. Radiology: Cardiothoracic Imaging. 2020;2(2):e200152.
- Lee LY, Cazier JB, Starkey T, Briggs SE, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020;21(10):1309-1316.
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337.
- Addeo A, Friedlaender A. Cancer and COVID-19: Unmasking their ties. Cancer Treatment Reviews. 2020:102041.
- Tremblay D, Seah C, Schneider T, Bhalla S, Feld J, Naymagon L et al. Convalescent Plasma for the Treatment of Severe COVID‐19 Infection in Cancer Patients. Cancer Med. 2020.
- Santa Cruz R, Villarejo F, Figueroa A, Cortés-Jofré M, Gagliardi J, Navarrete M. Mortality in critically ill elderly individuals receiving mechanical ventilation. Respir Care. 2019;64(4):473-483.
- Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773.
- Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52:65S-79S.
- Vlaar AP, Binnekade JM, Prins D, Hofstra JJ, Schultz MJ, Juffermans NP. Risk Factors for the Onset of Transfusion Related Acute Lung Injury (TRALI) in Critically Ill Patients: A Retrospective Nested Case Control Study. InC54. ALI/ARDS: DIAGNOSIS AND OUTCOMES. American Thoracic Society. 2009:A4636.
- Hawkins J, Aster RH, Curtis BR. Post-transfusion purpura: Current perspectives. J Blood Med. 2019;10:405.
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205-1208.
- Liu ST, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: A propensity score–matched control study. Nat Med. 2020;26(11):1708-1713.
- Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. Jama. 2020;324(5):460-470.
Citation: Gazitua R, Briones JL, Selman C, Villarroel-Espíndola F, Aguirre-Ducler A, Gonzalez-Steigmaier R, et al. (2021) Convalescent Plasma in COVID-19; Mortality-Safety First Results of the Prospective Multicenter FALP 001-2020 Trial. J Vaccines Vaccin. S12:002.
Copyright: © 2021 Gazitua R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

