Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 3
Consumption of Protein Enriched Yogurt for Nutritional Status Improvement in Patients Treated with Hemodialysis
Dror Ben Noach1,2, Ayelet Grupper2,3, Limor Ben Haim1, Doron Schwartz2,3, Ronit Anbar1 and Orit Kliuk Ben Bassat2,3*2Department of Nephrology and Hypertension, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel
3Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel
Received: 04-Sep-2024, Manuscript No. JNDT-24-26879; Editor assigned: 06-Sep-2024, Pre QC No. JNDT-24-26879(PQ); Reviewed: 20-Sep-2024, QC No. JNDT-24-26879; Revised: 27-Sep-2024, Manuscript No. JNDT-24-26879(R); Published: 04-Oct-2024, DOI: 10.35248/2161-0509.24.14.298
Abstract
Background: Protein Energy Wasting (PEW) is highly prevalent among dialysis patients, leading to significant morbidity and mortality. Protein enriched dairy products may provide a feasible nutritional intervention to overcome the discrepancy between protein requirement and intake. We investigated the impact of an additional daily protein enriched yogurt on nutritional parameters in Hemodialysis (HD) patients with hypoalbuminemia.
Methods: A prospective, single center, cross-over pilot study. Patients treated with HD and hypoalbuminemia, defined as blood albumin below 38 g/L, were considered eligible. After enrollment, all patients started a 4-months control period of strict dietitian surveillance followed by 4-months intervention period that consisted of an additional 20 g protein-enriched yogurt daily. Primary endpoint was increase in protein intake measured by normalized Protein Catabolic Rate (nPCR).
Results: Ten hemodialysis patients were recruited. Mean albumin at baseline was 35 g/dL. Seven patients entered control period, 5 started intervention. Four patients completed intervention period. There was no difference in nPCR between screening to control or intervention period (mean nPCR 1.06, 1.10 and 1.26 in screening, control and intervention period respectively, p=0.273). Albumin level significantly increased from screening (34.5 g/L) to end of control period (38 g/L), p=0.012; with no further increase at the end of intervention period (36.35 g/L, p=0.196 between intervention and control period). The yogurt was well tolerated.
Conclusion: Protein enriched yogurt did not increase nPCR in a limited cohort of dialysis patients with hypoalbuminemia, however it was well tolerated. Larger-scale studies may provide more information about its role in PEW management in this patient population.
Keywords
Protein Energy Wasting (PEW); Malnutrition; Hypoalbuminemia; Dialysis; Protein enriched yogurt
Introduction
Protein Energy Wasting (PEW) represents metabolic and nutritional derangements with reduced body stores of protein and energy, commonly observed in chronic disease conditions, that is highly prevalent in patients with End Stage Kidney Disease (ESKD) treated with dialysis [1,2]. Prevalence of PEW among dialysis patients is high, reported in 28%-54%, with higher prevalence observed with increased dialysis vintage [3-6]. Various factors are proposed as causative agents, including accumulation of uremic toxins, metabolic acidosis, inflammation, dialysis associated catabolism and reduced dietary intake. PEW increases susceptibility to infections, reduces muscle function, induces a pro-inflammatory state, accelerates atherosclerosis is associated with poor clinical outcomes, hospitalization, decreased quality of life and mortality [7,8]. Various clinical and biochemical parameters that correlate with poor nutritional status are associated with increased morbidity and mortality in patients treated with dialysis, including hypoalbuminemia, low normalized Protein Nitrogen Appearance (nPNA), decreased protein and calories intake [9-11]. According to National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) 2020 nutritional guidelines, Subjective Global Assessment (SGA) is the preferred tool for nutritional assessment in dialysis patients [12]. Severe malnutrition, assessed by SGA, correlated with 33% higher mortality risk [13].
Inadequate dietary intake of protein and calories commonly accounts for PEW in patients treated with dialysis and constitutes an important therapeutic target [7]. Therefore, KDOQI 2020 nutritional guidelines recommend prescribing metabolically stable patients on Maintenance Hemodialysis (MHD) a daily protein intake of 1-1.2 g/kg body weight and caloric intake of 25-35 kcal/ kg to maintain adequate nutritional status [12]. Despite these recommendations, when analyzing the baseline characteristics of patients randomized to the Hemodialysis (HEMO) study, 61% of patients had a non-satisfactory dietary protein intake of less than 1.0 g/kg/day [5].
Previous studies were conducted to evaluate strategies to improve dietary intake in patients treated with dialysis, using Oral Nutritional Supplements (ONS) and NKF-KDOQI guidelines recommend a 3-month trial of ONS in patients at risk or with PEW despite dietary counseling [13-15]. However, in practice, patients’ compliance to ONS may be inadequate due to appearance, taste preference, gastrointestinal symptoms as well as other psychological and economic reasons, which emphasizes the need to search for an alternative.
Protein enriched products may provide a feasible and affordable nutritional intervention strategy in disease states when there is a discrepancy between protein requirement and intake [16,17]. We hypothesized that using an oral supplement not regarded by patient as a pharmaceutical product, that is also affordable and accessible, might increase compliance as well as nutritional status. The aim of the study was to assess the effectiveness and tolerance of daily addition of protein enriched yogurt on nutritional parameters in patients on MHD with hypoalbuminemia.
Methodology
This prospective, single center, cross-over pilot study was conducted at an ambulatory dialysis unit in a tertiary hospital. Study protocol was approved by the local ethics committee and was conducted according to GCP requirements. Study registration number MOH_2018-10-31_004728. All patients gave informed consent before participation.
Study population
Patients were considered eligible if they were 18-95 yrs old, treated by MHD at least 6 months before enrollment and had hypoalbuminemia defined as blood albumin below 38 g/L. Exclusion criteria included the use of ONS or Intradialytic Parentral Nutrition (IDPN), lactose intolerance, severe liver disease, active malignancy, a major surgery 6 months before study participation, hospitalization for more than 3 days with in 3 months before study participation, persistent hyperphosphatemia above 7 mg/dL or pregnancy. Patients who were not able to give informed consent or were unable to cooperate with study procedures were also excluded.
Study endpoints
Primary endpoint was increase in protein intake measured by normalized Protein Catabolic Rate (nPCR). Secondary endpoints were nutritional status improvement according to SGA, improvement in nutrition associated biochemical markers, improvement in Protein Catabolic Rate (PCR) not normalized to body weight and patients’ compliance.
Study design
After informed consent, patients entered a screening period to evaluate nutritional status. Thereafter all patients started a 4-months control period of strict dietitian surveillance.
A registered dietitian performed a complete nutritional assessment and adapted to each patient a specified nutritional program according to NKF-KDOQI recommendations at time of enrollment, that included daily 30-35 kcal/kg, protein intake 1.2 -1.3 gram/kg, maximal potassium content of 2400 mg and phosphorus intake of 10-17 mg/kg. During control period, a regular plain yogurt, containing 7 g protein and 100 kcal per serving, was part of the daily menu to avoid bias of increased caloric intake during the subsequent intervention period.
After completion of the control period, participants entered the intervention phase. The regular yogurt was replaced with protein-enriched yogurt, containing 20 g protein per serving, which was given daily for 4-months. Patients served as their own control for calculating the difference between baseline, control and intervention period. Nutritional, clinical and biochemical parameters were assessed according to supplementary table.
Nutritional assessment was performed at baseline, at the end of control period and after completion of intervention phase, using SGA, a validated tool recommended by the KDOQI for nutritional evaluation of patients with Chronic Kidney Disease (CKD) and ESKD. Body Mass Index (BMI) was calculated using weight in kilograms divided by height in meters squared. Muscle strength was evaluated at baseline using Hand Grip Strength (HGS).
Blood samples were taken at baseline and monthly thereafter. Biochemical parameters included creatinine, Blood Urea Nitrogen (BUN), albumin, total cholesterol, hemoglobin, phosphate and potassium.
Transferrin and C-Reactive Protein (CRP) were measured every 3 months. nPCR was used for estimation of daily protein consumption according to urea kinetics calculations [18]. Dialysis adequacy was calculated by single pool Kt/Vurea using daugirdas formula [19].
Intervention period
During intervention period patients consumed protein-enriched yogurt. Content of one cup (200 g) includes 140 kcal, 20 g protein, 7.2 g carbohydrate, 3 g fat, 80 mg sodium, 220 mg calcium, 280 mg phosphor and 460 mg potassium. It contains milk and bacteria and is gluten-free. Each participant received a weekly protein-enriched yogurt supply in a refrigerated bag and returned the empty cups to assess for compliance.
Statistical analysis
We aimed to recruit 50 patients based on sample size calculated for one-sided test, however the study was terminated prematurely as a result of slow recruitment. Due to the low number of participants, data is descriptive, presented as mean+Standard Deviation (SD) for normally distributed parameters and median+Interquartile Range (IQR) for skewed parameters.
Results
Study consort is depicted in Figure 1. Between March 2020-June 2021, 153 maintenance HD patients were screened, 10 patients were recruited. Patients’ baseline characteristics are presented in Table 1. Thirty percent were females, mean age was 75.4 ± 12.2. Although mean albumin at baseline was 35 g/dL, 90% of patients were categorized as SGA A, indicating a good nutritional status.
| N=10 | Parameter |
|---|---|
| 75.4 ± 12.2 | Age, years |
| 3 (30%) | Sex, female (%) |
| 39.3 ± 28.8 | Dialysis vintage, months |
| 7 (70%) | Dialysis access, AVF (%) |
| 1.44 ± 0.3 | spKt/V urea |
| 8 (80%) | Diabetes mellitus, (%) |
| 4 (40%) | Heart failure, (%) |
| Nutritional assessment | |
| 76.1 ±14.9 | Dry weight (Kg) |
| 26.3 ± 3.7 | BMI |
| 9 (90%) | SGA, A (%) |
| 73.2 ± 23.6 | PCR |
| Biochemistry results | |
| 35 ± 1.56 | Albumin (g/L) |
| 59.2±26.5 | BUN (mg/dL) |
| 12.5 ±18.8 | Creatinine (mg/dL) |
| 125.3±27.8 | Cholesterol |
| 5.2 ± 1.4 | Phosphorus (mg/dL) |
| 180.3 ± 53.9 | Transferrin |
Abbreviations: AVF-Arteriovenous Fistula; BMI-Body Mass Index; SGA-Subjective Global Assessment; PCR-Protein Catabolic Rate; BUN-Blood Urea Nitrogen.
Table 1: Patients' baseline characteristics.

Figure 1: Study consort is about maintenance of patients screening and recruitment.
Three patients did not start control period
Two withdrew consent and one died from severe pneumonia shortly after recruitment. Seven patients entered control period, one had recurrent hospitalizations and medical procedures and did not wish to continue with study procedures and one died from septic shock during this period. Five patients started intervention period, however one decided to withdraw participation since he did not want to consume the yogurts. The other 4 patients had a good compliance (above 90%) and tolerability to the protein enriched yogurt. There were no adverse events considered related to study product.
Primary endpoint was not reached. In patients who completed the study, there was no difference in nPCR between screening to control or intervention period (mean nPCR 1.06, 1.10 and 1.26 in screening, control and intervention period respectively, p=0.273) (Figure 2). Mean PCR was 83.6 in screening, 86.4 in control and 98.1 in intervention period (p=0.22).

Figure 2: Change in normalized protein catabolic rate during study periods in patients who completed the study.
Albumin level significantly increased from screening (34.5 g/L) to end of control period (38 g/L), p=0.012. Albumin level at the end of intervention period was 36.35 g/L. The difference between albumin level at end of intervention did not vary significantly compared to screening or control period (p=0.196) between intervention and control period, (Figure 3). Since Albumin is an inflammatory marker, we examined whether improvement in albumin from screening to control period correlates with a reduction in CRP levels between study periods, however in 6 out of 7 patients, CRP level increased at the end of control period compared to screening.

Figure 3: Change in albumin during study periods in patients who completed control period.
Additional biochemical variables assessed did not differ significantly between study periods in patients who completed the study. Creatinine level was 5.95 mg/dL at screening, 6.56 mg/ dL at end of control and 6.26 mg/dL at the end of intervention (p=0.362); BUN was 58 mg/dL, 56.25 mg/dL and 67 mg/dL at screening, control and intervention periods respectively (p=0.517); total cholesterol was 141 mg/dL, 157 mg/dL and 145.5 mg/dL at screening, control and intervention periods respectively (p=0.289) and transferrin level was 168 mg/dL. Yogurt consumption did not result in increased phosphorus level: Phosphorus level was 5.7 mg/ dL at screening, 6.4 mg/dL at end of control period and 5.2 mg/ dL at end of intervention period (p=0.354).
Nine out of 10 patients had SGA A at screening. The patient who started the study with SGA B had SGA B at the end of control period. He decided to withdraw participation when entering the intervention period. One patient with recurrent hospitalizations during control period, entered the study with SGA A that decreased to SGA B at the end of control period. As already depicted, he did not wish to continue with study procedures and did not enter intervention period.
Dry weight did not change between study period in patients who completed the study (Figure 4); mean weight was 81.62 Kg at screening, 82.05 Kg at control and 81.42 Kg at intervention (p=0.636).
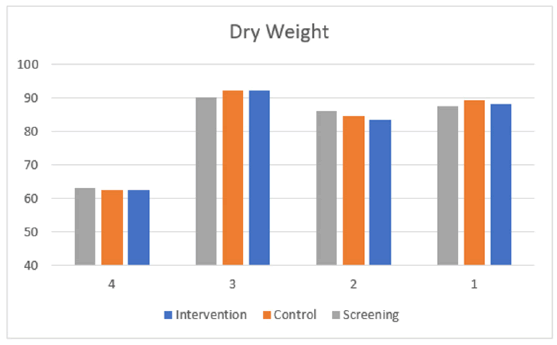
Figure 4: Change in dry weight during study periods in patients who completed the study.
Discussion
In the current study we were unable to demonstrate clinical benefit of protein enriched yogurt on nutritional parameters, however we confirmed the importance of a comprehensive and dedicated nutritional consultation to improve hypoalbuminemia in HD patients.
The study was designed to assess the effectiveness of the addition of protein enriched yogurt daily on nutritional parameters in HD patients with PEW. However, PEW at baseline was defined purely according to serum albumin level, a definition that was probably inappropriate. Although hypoalbuminemia is linked to malnutrition in HD patients, an ongoing debate exists whether albumin is indeed a marker of malnutrition or alternatively serves as a marker of inflammation and significant comorbidities [20-23]. According to the KDOQI clinical guidelines from 2020 serum albumin may be considered as a complementary tool to assess malnutrition, however a position statement released by the American Society for Parenteral and Enteral Nutrition (ASPEN) in 2021 concluded that albumin is a marker of inflammation rather than nutrition status and as such should not be used to monitor efficiency of different nutritional support strategies [24]. Indeed, in the current study, most patients had SGA A at enrollment, indicating a good nutritional status, despite hypoalbuminemia.
There was a trend for increase in nPCR between screening (1.06) and intervention period (1.26), albeit it did not reach statistical significance, even after adjustment of the additional protein consumed per dry weight. In search for an explanation to the insufficient increase, we hypothesized that patients consumed the yogurt instead of a meal and not in-between as they were repeatedly educated [25]. Although protein enriched yogurts did not improve satiety nor altered subsequent meals in a healthy population the impact on satiety in specific patient populations may be different and requires further investigation [26,27].
Albumin level significantly increased from screening to end of control period despite an increase in another inflammatory marker. This may be explained by a strict follow-up by a skilled and committed dietician after enrollment. This finding emphasizes the valuable role of the dietician as part of the multidisciplinary approach in dialysis patients. During intervention period, we did not observe an additional improvement using protein enriched yogurts, although conclusions in this small cohort may be misleading.
ONS is an important tool to improve nutritional status in HD patients, recommended by the NKF-KDOQI nutrition guidelines, however patients may be reluctant to use ONS due to its taste, texture, perception of ONS as a medication and other psychological concerns. We hypothesized that the compliance to the consumption of enriched food such as yogurt will be good and except for one patient who did not wish to consume the yogurts after one week of intervention due to its texture, compliance was satisfactory in other patients. There were no gastrointestinal complains. The economic burden of ONS is another important consideration. In Israel, an ONS specifically designed for dialysis patients that contains 17.8 g protein in 220 ml, costs patients after subsidy 4.45 USD per bottle compared to 1.8 USD for a 200 ml cup of protein enriched yogurt, which contains 20 g protein. When consumed daily, the prices differences translate to 967 USD per patient per year. Therefore, functional foods enriched with protein may serve as a practical and affordable method to increase protein consumption in dialysis patients.
Hypoalbuminemia as a single inclusion criterion for PEW may be inappropriate and should be reconsidered in future studies in this field.
Conclusion
Protein enriched yogurt did not improve nutritional status in a limited cohort of dialysis patients with hypoalbuminemia however it was well tolerated. Further larger studies should be considered to assess effectiveness of integrating functional foods enriched with protein in improving nutritional status in dialysis patients. The study has several limitations. It is a small pilot study. Patients’ recruitment was slow with too strict inclusion and exclusion criteria, that limited enrollment of adequate number of patients. COVID-19 pandemic was also a major drawback as patients were reluctant to participate. Therefore, the number of patients is insufficient for statistical significance. However, this study may encourage further clinical trials in a larger patient population as there were no safety signals and the yogurt was well tolerated.
Ethics Approval and Consent to Participate
The study was approved by Tel Aviv medical center ethics committee and was conducted according to GCP requirements. The trial was registered in the ministry of health clinical research site, registration number MOH_2018-10-31_004728. All patients gave informed consent before participation.
Availability of Data and Materials
The data presented in this manuscript are available in the article and in its supplementary material.
Authors Contributions
Dror Ben-Noach, Limor Ben-Haim, Ronit Anbar and Orit Kliuk Ben Bassat contributed to study design; Dror Ben-Noach, Ayelet Grupper and Orit Kliuk Ben Bassat contributed to data acquisition and analysis, Doron Schwartz, Limor Ben-Haim and Ronit Anbar helped in supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Competing Interests
The authors declare no conflict of interest. All authors approved the manuscript for publication.
References
- Sabatino A, Regolisti G, Karupaiah T, Sahathevan S, Singh BS, Khor BH, et al. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr. 2017;36(3):663-671.
[Crossref] [Google Shcolar] [PubMed]
- Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020;49(1-2):202-211.
[Crossref] [Google Shcolar] [PubMed]
- Kinney R. 2005 annual report: ESRD clinical performance measures project. AJKD. 2006;48:S1-05.
[Crossref] [Google Shoclar] [PubMed]
- Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57(3):1176-1181.
- Rocco MV, Paranandi L, Burrowes JD, Cockram DB, Dwyer JT, Kusek JW, et al. Nutritional status in the HEMO study cohort at baseline. AJKD. 2002;39(2):245-256.
[Crossref] [Google Shcolar] [PubMed]
- Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, et al. Global prevalence of protein-energy wasting in kidney disease: A meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr. 2018;28(6):380-392.
- Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096-1097.
[Crossref] [Google Shcolar] [PubMed]
- Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29(1):3-14) [ Crossref] [
[Google Shcolar] [PubMed]
- Combe C, Chauveau P, Laville M, Fouque D, Azar R, Cano N, et al. Influence of nutritional factors and hemodialysis adequacy on the survival of 1,610 French patients. AJKD. 2001;37(1):S81-8.
[Crossref] [Google Shcolar] [PubMed]
- Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, et al. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. AJKD. 2006;48(1):37-49.
[Crossref] [Google Shcolar] [PubMed]
- de Araújo IC, Kamimura MA, Draibe SA, Canziani ME, Manfredi SR, Avesani CM, et al. Nutritional parameters and mortality in incident hemodialysis patients. J Ren Nutr. 2006;16(1):27-35.
[Crossref] [Google Shcolar] [PubMed]
- Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. AJKD. 2020;76(3):S1-07.
[Crossref] [Google Shcolar] [PubMed]
- Pifer TB, Mccullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62(6):2238-2245.
[Crossref] [Google Shcolar] [PubMed]
- Fouque D, McKenzie J, de Mutsert R, Azar R, Teta D, Plauth M, et al. Use of a renal-specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. NDT. 2008;23(9):2902-2910.
[Crossref] [Google Shcolar] [PubMed]
- Moretti HD, Johnson AM, Keeling-Hathaway TJ. Effects of protein supplementation in chronic hemodialysis and peritoneal dialysis patients. J Ren Nutr. 2009;19(4):298-303.
[Crossref] [Google Shcolar] [PubMed]
- Stelten S, Dekker IM, Ronday EM, Thijs A, Boelsma E, Peppelenbos HW, et al. Protein-enriched ‘regular products’ and their effect on protein intake in acute hospitalized older adults; a randomized controlled trial. Clin Nutr. 2015;34(3):409-414.
[Crossref] [Google Shcolar] [PubMed]
- van Til AJ, Naumann E, Cox-Claessens IJ, Kremer S, Boelsma E, de van der Schueren MA. Effects of the daily consumption of protein enriched bread and protein enriched drinking yoghurt on the total protein intake in older adults in a rehabilitation centre: A single blind randomised controlled trial. J Nutr Health Aging. 2015;19:525-530.
[Crossref] [Google Shcolar] [PubMed]
- Depner TA, Daugirdas JT. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. JASN. 1996;7(5):780-785.
[Crossref] [Google Shcolar] [PubMed]
- Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. JASN. 1993;4(5):1205-1213.
[Crossref] [Google Shcolar] [PubMed]
- Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, et al. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int. 1998;53(3):773-782.
[Crossref] [Google Shcolar] [PubMed]
- Kovesdy CP, Kalantar‐Zadeh K. Accuracy and limitations of the diagnosis of malnutrition in dialysis patients. Semin Dial. 2012;25(4):423-427.
[Crossref] [Google Shcolar] [PubMed]
- Gama-Axelsson T, Heimbürger O, Stenvinkel P, Bárány P, Lindholm B, Qureshi AR. Serum albumin as predictor of nutritional status in patients with ESRD. CJASN. 2012;7(9):1446-1453.
- de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW, et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127-135.
[Crossref] [Google Shcolar] [PubMed]
- Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P,et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. NCP. 2021;36(1):22-28.
[Crossref] [Gogle Shcolar] [PubMed]
- Veldhorst M, Smeets AJ, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008 23;94(2):300-307.
[Crossref] [Google Shcolar] [PubMed]
- Ortinau LC, Culp JM, Hoertel HA, Douglas SM, Leidy HJ. The effects of increased dietary protein yogurt snack in the afternoon on appetite control and eating initiation in healthy women. Nutr J. 2013;12:1-6.
[Crossref] [Google Shcolar] [PubMed]
- Ikizler TA, Franch HA, Kalantar-Zadeh K, ter Wee PM, Wanner C. Time to revisit the role of renal dietitian in the dialysis unit. J Ren Nutr. 2014;24(1):58-60.
[Crossref] [Google Shcolar] [PubMed]
Citation: Noach DB, Grupper A, Haim LB, Schwartz D, Anbar R, Bassat OKB (2024). Consumption of Protein Enriched Yogurt for Nutritional Status Improvement in Patients Treated with Hemodialysis J Nutr Disorders Ther. 14:298.
Copyright: © 2024 Noach DB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.